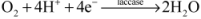Modeling and Simulation of Enzymatic Biofuel Cells with Three-Dimensional Microelectrodes
Abstract
:1. Introduction

2. Mechanism of EBFCs
- Anode:
- Cathode:


3. Simulation Modeling



| Boundary | Diffusion | Potential |
|---|---|---|
| Top boundary of bulk domain | ||
| Bulk-enzyme interface | ||
| Enzyme-electrode interface | ||
| Side and bottom boundaries of bulk domain |
| Constant | Ref. Value | Reference |
|---|---|---|
| R | 8.314 J·mol·K−1 | - |
| T | 300 K | - |
| F | 96,485 C·mol−1 | - |
| Dglucose | 7 × 10−10 m2·s−1 | [42,43,44] |
| Doxygen | 1.74 × 10−9 m2·s−1 | [45,46] |
| KM_GDH | 17.4 mM | [47] |
| KM_laccase | 133.4 mM | [48] |
| kcat_GDH | 360 s−1 | [47] |
| kcat_laccase | 117 s−1 | [48] |
| ø oA | −0.32 V | [49] |
| ø oC | 0.585 V | [49] |
| σcarbon | 8000 S·m−1 | [50] |
| σsubstrate | 4 S·m−1 | - |
- (1)
- 2-D simulation is used to simplify the 3-D microelectrode design.
- (2)
- The DET between enzyme and electrode is assumed.
- (3)
- The enzyme kinetics constant is obtained from the literatures based on immobilized enzymes.
- (4)
- The enzyme is uniformly distributed in the enzyme layer.
- (5)
- Negligible change in heat transfer is assumed between enzyme layer and electrode interface.
- (6)
- Temperature distribution around the EBFCs is assumed to be uniform.
4. Results and Discussions
4.1. The Steady State Response

4.2. Impact of Mass Transport and Reaction Rate
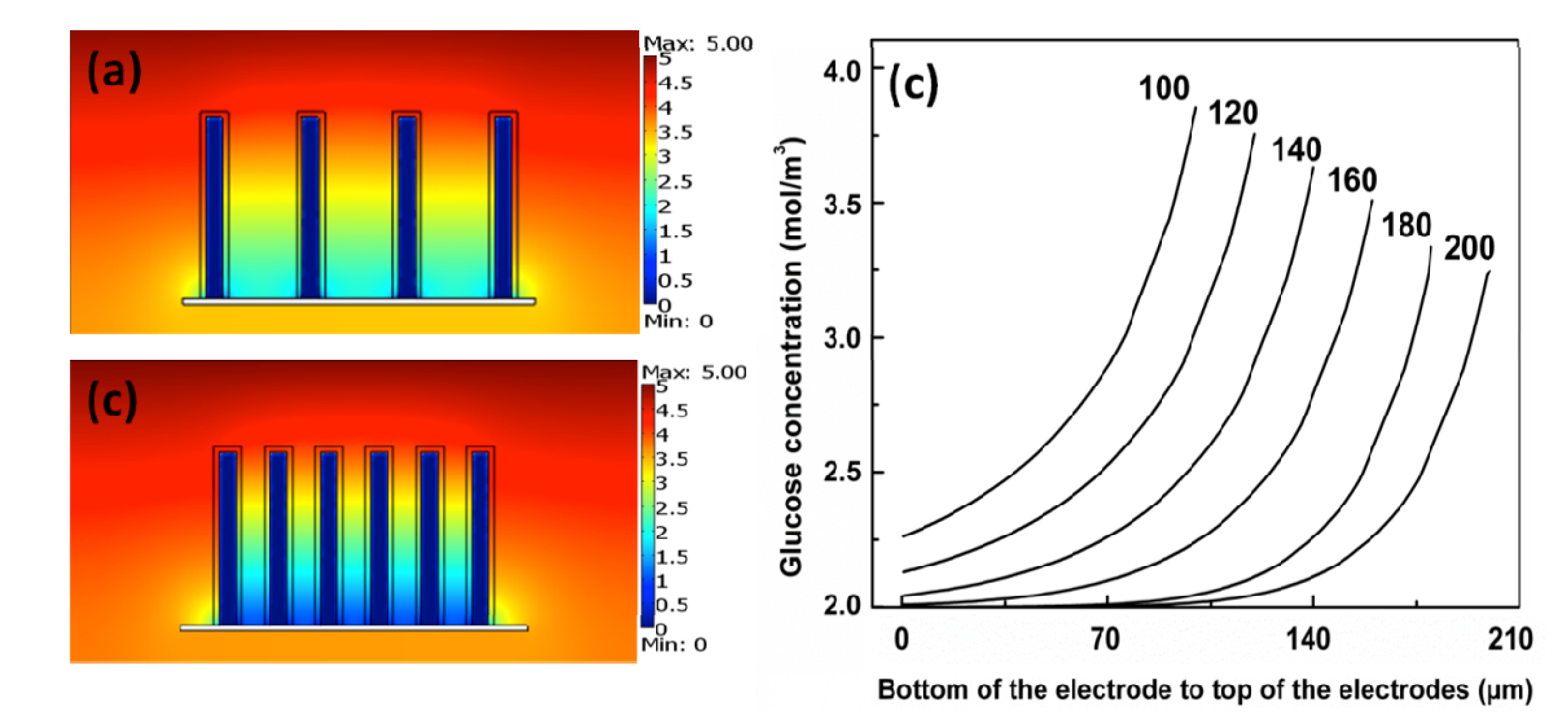

4.3. The Cell Performance of EBFCs

4.4. Geometry of the Electrodes

| Geometry | Side Edges | Top Edge | Corners | |||
|---|---|---|---|---|---|---|
| Property | CD (μA·cm−2) | RH (μW·cm−3) | CD (μA·cm−2) | RH (μW·cm−3) | CD (μA·cm−2) | RH (μW·cm−3) |
| Rectangular | 170 | 4 | 100 | 1.5 | 410 | 23 |
| Triangular | 90–225 | 1–8 | - | - | 270(top) 230(bottom) | 12(top) 7(bottom) |
| Tapered | 130–210 | 2.5–5 | 125–135 | 1–3 | 250 | 8.5 |
| Semi-elliptical | 45–90 | 0.05–0.17 | - | - | 105(top) 47(bottom) | 0.27(top) 0.05(bottom) |

5. Conclusions
Author Contributions
Nomenclature
| [i] | concentration of component i (mol·m−3) |
| c | concentration |
| D | diffusion coefficient (m2·s−1) |
| F | Faraday’s constant (C·mol−1) |
| JJ | current density (mA/cm2) |
| k | rate constant for enzyme complex (s−1) |
| kcat | catalytic rate constant (s−1) |
| KM | Michaelis Mention constant (mM) |
| N | flux (mol·m−2·s−1) |
| R | universal gas constant (J·mol·K−1) |
| T | room temperature (K) |
| v | enzyme reaction (mol·m−3·s−1) |
| z | number of electron transferred |
Greek
| σ | electric conductivity (S·m−1) |
| ø | electric potential (V) |
Conflicts of Interest
References
- Mano, N.; Heller, A. Biofuel cells and their development. J. Electrochem. Soc. 2013, 150, A1136–A1138. [Google Scholar] [CrossRef]
- Palmore, G.; Whitesides, G.M. Microbial and enzymatic biofuel cells. In Enzymatic Conversion of Biomass for Fuels Production; Himmel, E., Ed.; American Chemical Society: Washington, DC, USA, 1994; Volume 566, pp. 271–290. [Google Scholar]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar] [CrossRef]
- Katz, E.; Shipway, A.N.; Willner, I. Handbook of Fuel Cells—Fundamentals, Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 355–381. [Google Scholar]
- Heller, A. Miniature biofuel cells. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Biofuel cell based on direct bioelectrocatalysis. Biosens. Bioelectron. 2005, 20, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Halamkova, L.; Halamek, J.; Bocharova, V.; Szczupak, A.; Alfonta, L.; Katz, E. Implantable biofuel cell operating in a living snail. J. Am. Chem. Soc. 2012, 134, 5040–5043. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Ludwig, R.; Harreither, W.; Haltrich, D.; Gorton, L.; Ruzgas, T.; Shleev, S. A direct electron transfer-based glucose/oxygen biofuel cell operating in human serum. Fuel. Cells 2010, 10, 9–16. [Google Scholar]
- Southcott, M.; MacVittie, K.; Halámek, J.; Halámková, L.; Jemison, WD.; Lobel, R.; Katz, E. Pacemaker powered by implantable biofuel cell operating under conditions mimicking human blood circulatory system–battery not included. Phys. Chem. Chem. Phys. 2013, 15, 6278–6283. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, B.R.; Henderson, E.; Wu, P.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.; Jones-meehan, J. High power density from a miniature microbial fuel cell using shewanella oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Long, J.W.; Dunn, B.; Rolison, D.; White, H.S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Long, J.W.; Rolison, D. Rethinking multifunction in three dimensions for miniaturizing electrical energy storage. Interface 2008, 17, 49–53. [Google Scholar]
- Anandan, V.; Yang, X.; Kim, E.; Rao, Y.; Zhang, G. Role of reaction kinetics and mass transport in glucose sensing with nanopillar array electrodes. J. Biol. Eng. 2007, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Godino, N.; Borrise, X.; Munoz, F.X.; Campo, F.J.; Compton, R. Mass transport to nanoelectrode arrays and limitations of diffusion domain approach: Theory and experiment. J. Phys. Chem. 2009, 113, 11119–11125. [Google Scholar]
- Wang, C.; Taherabadi, L.; Jia, G.; Madou, M. C-MEMS for the manufacture of 3D microbatteries. Electrochem. Solid State Lett. 2004, 7, A435–A438. [Google Scholar] [CrossRef]
- Wang, C.; Jia, G.; Taherabadi, L.; Madou, M.J. A novel method for the fabrication of high aspect ratio C-MEMS structures. Microelectromechanical Syst. 2005, 14, 348–358. [Google Scholar] [CrossRef]
- Wang, C.; Madou, M. From MEMS to NEMS with carbon. Biosens. Bioelectron. 2005, 20, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Taherabadi, L.; Wang, C.; Zoval, J.; Madou, M. Morphological and electrical properties of carbon films of various thinknesses carbonized from photoresist and the implications for C-MEMS devices in conductive media. J. Electrochem. Soc. 2005, 152, J136–J143. [Google Scholar] [CrossRef]
- Malladi, K.; Wang, C.; Madou, M. Microfabrication of suspended C-MEMS structures by EB writer and pyrolysis. Carbon 2006, 44, 2602–2607. [Google Scholar] [CrossRef]
- Park, B.; Zaouk, Y.R.; Wang, C.; Madou, M. Fractal C-MEMS architectures for 3D miniature power and sensor applications. ECS Trans. 2006, 1, 1–11. [Google Scholar]
- Park, B.Y.; Zaouk, R.; Wang, C.; Zoval, J.; Madou, M. Fractal C-MEMS electrodes: Theory and preliminary fabrication. ECS Trans. 2007, 4, 83–92. [Google Scholar]
- Wang, C.; Zaouk, R.B.; Park, Y.; Madou, M. Carbon as a MEMS material: Micro and nano fabrication of pyrolyzed photoresist carbon. Int. J. Manuf. Tech. Manag. 2008, 13, 360–375. [Google Scholar]
- Min, H.; Park, B.Y.; Taherabadi, L.; Wang, C.; Yeh, Y.; Zaouk, R.; Madou, M.; Dunn, B. Fabrication and properties of a carbon/polypyrrole three-dimensional microbattery. J. Power Sour. 2008, 178, 795–800. [Google Scholar] [CrossRef]
- Yang, J.H.; Penmatsa, V.; Tajima, S.; Kawarada, H.; Wang, C. Direct amination on 3D pyrolyzed carbon micropattern surface for DNA detection. Mater. Lett. 2009, 63, 2680–2683. [Google Scholar] [CrossRef]
- Chen, W.; Beidaghi, M.; Penmatsa, V.; Li, W.; Wang, C. Integration of carbon nanotubes to C-MEMS for on-chip supercapacitors. IEEE Nanotech 2010, 9, 1222–1225. [Google Scholar]
- Penmatsa, V.; Yang, J.H.; Yu, Y.; Wang, C. Fabrication of porous carbon micropillars using a block copolymer as porogen. Carbon 2010, 48, 4109–4115. [Google Scholar] [CrossRef]
- Penmatsa, V.; Kawarada, H.; Wang, C. Fabrication of carbon nanostructures using photo nanoimprint lithography and pyrolysis. J. Micromech. Microeng. 2012, 22, 045024. [Google Scholar] [CrossRef]
- Penmatsa, V.; Kim, T.; Beidaghi, M.; Kawarada, H.; Wang, Z.; Gu, L.; Wang, C. Three-dimensional graphene nanosheets encrusted carbon micropillar arrays for electrochemical sensing. Nanoscale 2012, 4, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Penmatsa, V.; Ruslinda, R.; Beidaghi, M.; Kawarada, H.; Wang, C. Platelet-derived growth factor oncoprotein detection using three-dimensional carbon microarrays. Biosens. Bioelectron. 2013, 39, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Penmatsa, V.; Ruslinda, R.; Beidaghi, M.; Kawarada, H.; Wang, C. Functionalized three-dimensional carbon microarrays for cancer biomarker detection. ECS Trans. 2013, 45, 7–14. [Google Scholar] [CrossRef]
- Penmatsa, V. Functionalized carbon micro/nanostructures for biomolecular detection. Florida International University, ProQuest, UMI Dissertations Publishing, 2012; 3554197. [Google Scholar]
- Miyake, T.; Yoshino, S.; Yamada, T.; Hata, K.; Nishizawa, M. Self-regulating enzyme-nanotube ensemble films and their application as flexible electrodes for biofuel cells. J. Am. Chem. Soc. 2011, 133, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Filip, J.; Sefcovicova, J.; Gemeiner, P.; Tkac, J. Electrochemistry of bilirubin oxidase and its use in preparation of a low cost enzymatic biofuel cell based on a renewable composite binder chitosan. Electrochim. Acta 2013, 87, 366–374. [Google Scholar] [CrossRef]
- Krishnan, S.; Armstrong, F.A. Order-of-magnitude enhancement of an enzymatic hydrogen-air fuel cell based on pyrenyl carbon nanostructures. Chem. Sci. 2012, 3, 1015–1023. [Google Scholar] [CrossRef]
- Miyake, T.; Haneda, K.; Yoshino, S.; Nishizawa, M. Flexible, layered biofuel cell. Biosens. Bioelectron. 2013, 40, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Harreither, W.; Ludwig, R.; Gooding, J.J.; Gorton, L. Cellobiose dehydrogenase aryl diazoniunn modified single walled carbon nanotubes: Enhanced direct electron transfer through a positively charged surface. Anal. Chem. 2011, 83, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.M.; Su, L.; Mao, L.Q. Multi-walled carbon nanotube-based glucose/O2 biofuel cell with glucose oxidase and laccase as biocatalysts. J. Nanosci. Nanotechnol. 2007, 7, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Lesniewski, A.; Paszewski, M.; Opallo, M. Gold-carbon three dimensional film electrode prepared from oppositely charged conductive nanoparticles by layer-by-layer approach. Electrochem. Commun. 2010, 12, 435–437. [Google Scholar] [CrossRef]
- Liang, B.; Fang, L.; Yang, G; Hu, Y.C.; Guo, X.; Ye, X. Direct electron transfer glucose biosensor based on glucose oxidase self-assembled on electrochemically reduced carboxyl graphene. Biosens. Bioelectron 2013, 43, 131–136. [Google Scholar]
- Guo, C.X.; Hu, F.P.; Lou, X.W.; Li, C.M. High-performance biofuel cell made with hydrophilic ordered mesoporous carbon as electrode material. J. Power Sour. 2010, 195, 4090–4097. [Google Scholar] [CrossRef]
- Parikh, Y.; Yang, J.H.; Wang, C. Optimizing the mass transport phenomenon around micro-electrodes of an enzymatic biofuel cell inside a blood artery via finite element analysis method. J. Power Sour. 2009, 195, 4685–4694. [Google Scholar] [CrossRef]
- Picioreanu, C.; Head, I.M.; Katuri, K.P.; Loosdrecht, M.C.M.V.; Scott, K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007, 41, 2921–2940. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, P.V. Enzyme electrochemistry-biocatalysis on an electrode. Aust. J. Chem. 2006, 59, 233–256. [Google Scholar] [CrossRef]
- Hamann, C.H.; Hamnett, A.; Vielstich, W. Electrochemistry, 2nd ed.; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Li, H.; Luo, R.; Birgersson, E.; Lam, K.Y. A chemo-electro-mechanical model for simulation of responsive deformation of glucose-sensitive hydrogels with the effect of enzyme catalysis. J. Mech. Phys. Solids 2009, 57, 369–382. [Google Scholar] [CrossRef]
- Kouassi, G.; Irudayaraj, J.; McCarty, G. Activity of glucose oxidase functionalized onto magnetic nanoparticles. Biomagn. Res. Technol. 2005, 3, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, M.; Beden, N.; Leech, D.; Sygmund, C.; Ludwig, R.; Gorton, L. Characterization of different FAD-dependent glucosedehydrogenases for possible use in glucose-based biosensors and biofuel cells. Anal. Bioanal. Chem. 2012, 402, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Rekuća, A.; Bryjaka, J.; Szymańskab, K.; Jarzębski, A. Laccase immobilization on mesostructured cellular foams affords preparations with ultra high activity. Proc. Biochem. 2009, 44, 191–198. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Zimmerman, A.R.; Martínez, C.E.; Archibald, D.D.; Bollag, J.M.; Dec, J. Characteristics of Trametes villosa laccase adsorbed on aluminum hydroxide. Enzym. Microb. Technol. 2007, 41, 141–148. [Google Scholar] [CrossRef]
- Spaeth, E.E.; Friedlander, S.K. The Diffusion of Oxygen, Carbon dioxide and inert gas in flowing blood. Biophys. J. 1967, 7, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Prévoteau, A.; Mano, N. Oxygen reduction on redox mediators may affect glucose biosensors based on “wired enzymes”. Electrochim. Acta 2012, 68, 128–133. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, Y.; Penmatsa, V.; Wang, C. Modeling and Simulation of Enzymatic Biofuel Cells with Three-Dimensional Microelectrodes. Energies 2014, 7, 4694-4709. https://doi.org/10.3390/en7074694
Song Y, Penmatsa V, Wang C. Modeling and Simulation of Enzymatic Biofuel Cells with Three-Dimensional Microelectrodes. Energies. 2014; 7(7):4694-4709. https://doi.org/10.3390/en7074694
Chicago/Turabian StyleSong, Yin, Varun Penmatsa, and Chunlei Wang. 2014. "Modeling and Simulation of Enzymatic Biofuel Cells with Three-Dimensional Microelectrodes" Energies 7, no. 7: 4694-4709. https://doi.org/10.3390/en7074694