Research on Cellular Instabilities of Lean Premixed Syngas Flames under Various Hydrogen Fractions Using a Constant Volume Vessel
Abstract
:1. Introduction
2. Experimental and Computational Specifications
2.1. Experimental Setups and Procedure

2.2. Laminar Burning Velocity




 is the unstretched flame propagation speed and can be considered the flame propagation speed without any stretch effect on the flame front; thus,
is the unstretched flame propagation speed and can be considered the flame propagation speed without any stretch effect on the flame front; thus,  can be obtained as the intercept value at K = 0.
can be obtained as the intercept value at K = 0. can be calculated from the mass conservation across the thin flame:
can be calculated from the mass conservation across the thin flame:

2.3. Thermal Expansion Ratio and Flame Thickness

2.4. Lewis Number


3. Results and Discussion
3.1. System Validation and Laminar Burning Velocity


3.2. Flame Morphology







3.3. Critical Flame Radius and Critical Peclet Number

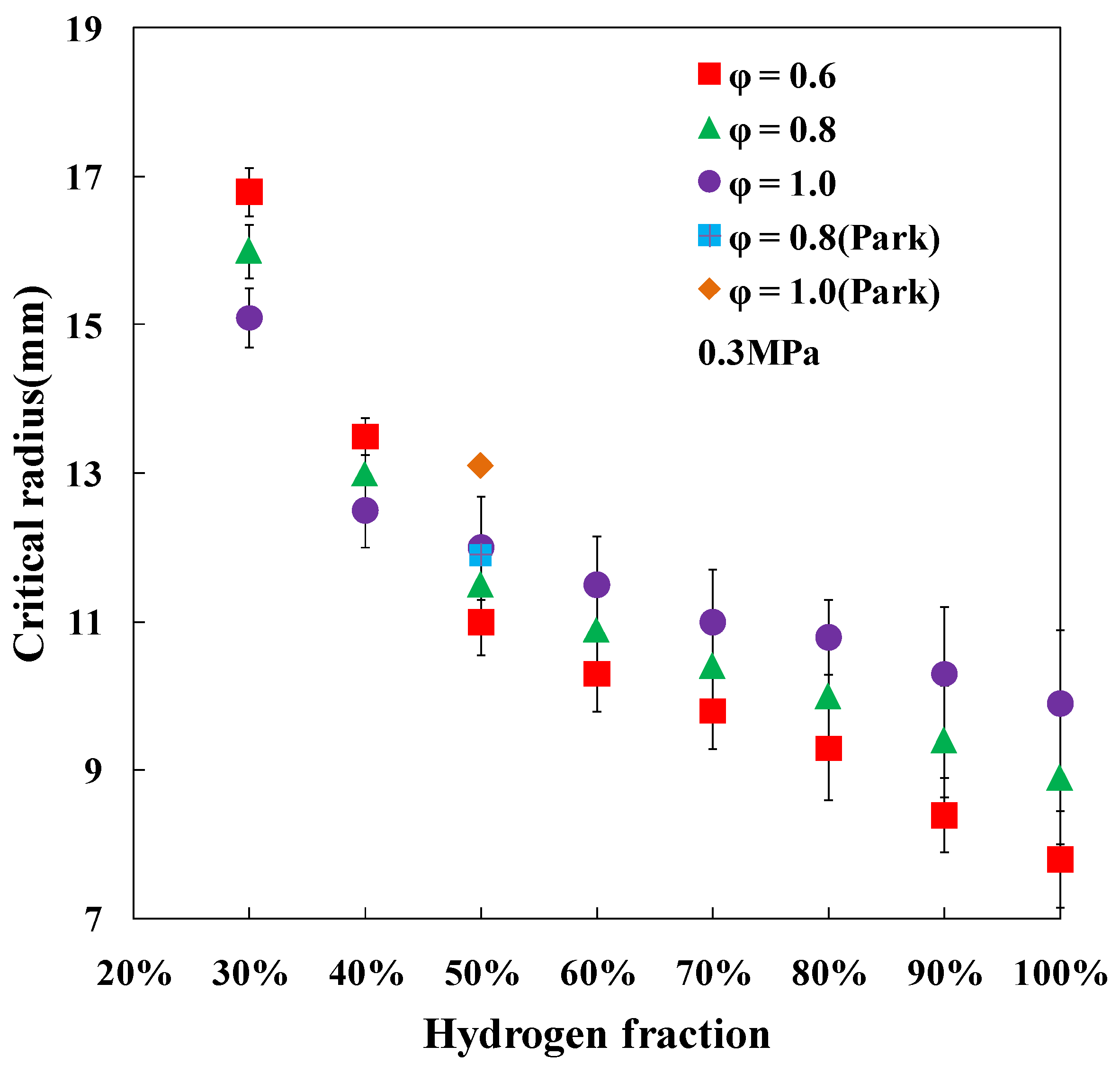

4. Conclusions
- (1)
- When hydrogen fractions are above 50%, the flame tends to be more stable as the equivalence ratio increases; however, the instability increases for flames of lower hydrogen fractions.
- (2)
- For the premixed syngas flames with hydrogen fractions lower than 50%, the effects of equivalence ratio on the variation in the Lewis number can be neglected. With the increase in equivalence ratio, the cellular instabilities become more evident because the enhanced hydrodynamic instabilities become the dominant effect.
- (3)
- For the premixed syngas flame with hydrogen fractions greater than 50%, the decline in cellular instabilities induced by the increase in equivalence ratio can be attributed to the reducing diffusive-thermal instabilities rather than the increasing hydrodynamic instabilities.
- (4)
- For the premixed syngas flame, the enhancement of cellular instabilities induced by the increase in hydrogen fraction is the result of both increasing diffusive-thermal and increasing hydrodynamic instabilities.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, C.; Wang, S. Combustion and emissions performance of a hybrid hydrogen–gasoline engine at idle and lean conditions. Int. J. Hydrog. Energy 2010, 35, 346–355. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Tang, C.; Zheng, J. Effect of hydrogen addition on early flame growth of lean burn natural gas–air mixtures. Int. J. Hydrog. Energy 2010, 35, 7246–7252. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Wang, L.; He, M.; Cao, Y. Research on optimal control to resolve the contradictions between restricting abnormal combustion and improving power output in hydrogen fueled engines. Int. J. Hydrog. Energy 2012, 37, 774–782. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S.; Zhang, B. Combustion and emissions characteristics of a hybrid hydrogen–gasoline engine under various loads and lean conditions. Int. J. Hydrog. Energy 2010, 35, 5714–5422. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Zhang, Q.; Meng, Y.; Pei, P. Research on optimum method to eliminate backfire of hydrogen internal combustion engines based on combining postponing ignition timing with water injection of intake manifold. Int. J. Hydrog. Energy 2012, 37, 12868–12878. [Google Scholar] [CrossRef]
- Burluka, A.A.; Ei-Dein Hussin, A.M.T.; Sheppard, C.G.W.; Liu, K.; Sanderson, V. Turbulent combustion of hydrogen-CO mixtures. Flow Turbul. Combust. 2011, 86, 735–749. [Google Scholar] [CrossRef]
- Fu, J.; Tang, C.L.; Jin, W.; Thi, L.D.; Huang, Z.; Zhang, Y. Study on laminar flame speed and flame structure of syngas with varied compositions using OH-PLIF and spectrograph. Int. J. Hydrog. Energy 2013, 38, 1636–1643. [Google Scholar] [CrossRef]
- Cormos, C.C. Evaluation of energy integration aspects for IGCC-based hydrogen and electricity co-production with carbon capture and storage. Int. J. Hydrog. Energy 2010, 35, 7485–7497. [Google Scholar] [CrossRef]
- Prathap, C.; Ray, A.; Ravi, M.R. Effects of dilution with carbon dioxide on the laminar burning velocity and flame stability of H2-CO mixtures at atmospheric condition. Combust. Flame 2012, 159, 482–492. [Google Scholar] [CrossRef]
- Bradley, D.; Sheppart, C.G.W.; Woolley, R.; Greenhalgh, D.A.; Lockett, R.D. The development and structure of flame instabilities and cellularity at low Markstein numbers in explosions. Combust. Flame 2000, 122, 195–209. [Google Scholar] [CrossRef]
- Williams, F.A. Combustion Theory, 2nd ed.; Addison-Wesley: Redwood, CA, USA, 1985; p. 349. [Google Scholar]
- Darrieus, G. Propagation d’un front de flame. Tech. Mod. 1938, 30, 18. [Google Scholar]
- Landau, L.D. On the theory of slow combustion. Acta Physicochim. URSS 1944, 19, 77–88. [Google Scholar]
- Mazaheri, K.; Mahmoudi, Y.; Radulescu, M.I. Diffusion and hydrodynamic instabilities in gaseous detonations. Combust. Flame 2012, 159, 2138–2154. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Zheng, J.; Miao, H. Measurements of laminar burning velocities and onset of cellular instabilities of methane-hydrogen-air flames at elevated pressures and temperatures. Int. J. Hydrog. Energy 2009, 34, 5574–5584. [Google Scholar] [CrossRef]
- Mukaiyama, K.; Shibayama, S.; Kuwana, K. Fractal structures of hydrodynamically unstable and diffusive-thermally unstable flames. Combust. Flame 2013, 160, 2471–2475. [Google Scholar] [CrossRef]
- Markstein, G.H. Cell structure of propane flames burning in tubes. J. Chem. Phys. 1949, 17, 428–429. [Google Scholar] [CrossRef]
- Manton, J.; von Elbe, G.; Lewis, B. Nonisotropic propagation of combustion waves in explosive gas mixtures and the development of cellular flames. J. Chem. Phys. 1952, 20, 153–157. [Google Scholar] [CrossRef]
- Kadowaki, S. The effects of heat loss on the burning velocity of cellular premixed flames generated by hydrodynamic and diffusive-thermal instabilities. Combust. Flame 2005, 143, 174–182. [Google Scholar] [CrossRef]
- Tang, C.L.; Huang, Z.H.; Jin, C.; He, J.; Wang, J.; Wang, X.; Miao, H. Laminar burning velocities and combustion characteristics of propane-hydrogen-air premixed flames. Int. J. Hydrog. Energy 2008, 33, 4906–4914. [Google Scholar] [CrossRef]
- Muppala, S.P.R.; Nakahara, M.; Aluri, N.K.; Kido, H.; Wen, J.X.; Papalexandris, M.V. Experimental and analytical investigation of the turbulent burning velocity of two-component fuel mixtures of hydrogen, methane and propane. Int. J. Hydrog. Energy 2009, 34, 9258–9265. [Google Scholar] [CrossRef]
- Bouvet, N.; Halter, F.; Chauveau, C.; Yoon, Y. On the effective Lewis number formulations for lean hydrogen/hydrocarbon/air mixtures. Int. J. Hydrog. Energy 2013, 38, 5949–5960. [Google Scholar] [CrossRef]
- Markstein, B.J. Nonsteady Flame Propagation; Pergamon: Oxford, UK, 1964; p. 49. [Google Scholar]
- Kadowaki, S. The body-force effect on the cell formation of premixed flames. Combust. Flame 2001, 124, 409–421. [Google Scholar] [CrossRef]
- Tse, S.D.; Zhu, D.L.; Law, C.K. Morphology and burning rates of expanding spherical flames in H2/O2/inert mixtures up to 60 atmospheres. Proc. Combust. Inst. 2000, 28, 1793–1800. [Google Scholar] [CrossRef]
- Kwon, O.C.; Rozenchan, G.; Law, C.K. Cellular instabilities and self-acceleration of outwardly propagating spherical flames. Proc. Combust. Inst. 2002, 20, 1775–1789. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Liu, F.S.; Bao, X.C.; Liu, X.H. Research on cellular instabilities in outwardly propagating spherical hydrogen-air flames. Int. J. Hydrog. Energy 2012, 37, 7889–7899. [Google Scholar] [CrossRef]
- Qiao, L.; Gu, Y.; Dahm, W.J.A.; Dahm, E.S.O.; Faeth, G.M. A study of the effects of diluents on near-limit H2-air flames in microgravity at normal and reduced pressures. Combust. Flame 2007, 151, 196–208. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Miao, H. Experimental and numerical study on laminar burning velocities and flame instabilities of hydrogen–air mixtures at elevated pressures and temperatures. Int. J. Hydrog. Energy 2009, 34, 8741–8755. [Google Scholar] [CrossRef]
- Law, C.K.; Jomaas, G.; Bechtold, J.K. Cellular instabilities of expanding hydrogen/propane spherical flames at elevated pressures: Theory and experiment. Proc. Combust. Inst. 2005, 30, 159–167. [Google Scholar] [CrossRef]
- Law, C.K.; Kwon, O.C. Effects of hydrocarbon substitution on atmospheric hydrogen-air flame propagation. Int. J. Hydrog. Energy 2004, 29, 867–879. [Google Scholar] [CrossRef]
- Okafor, E.C.; Hayakawa, A.; Nagano, Y.; Kitagawa, T. Effects of hydrogen concentration on premixed laminar flames of hydrogen-methane-air. Int. J. Hydrog. Energy 2014, 39, 2409–2417. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Z.; Wang, J.; Zheng, J. Effects of hydrogen addition on cellular instabilities of the spherically expanding propane flames. Int. J. Hydrog. Energy 2009, 34, 2483–2487. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Wang, X.; Jin, C.; Tang, C.; Wei, L.; Law, C.K. Laminar burning velocities and flame instabilities of 2,5-dimethylfuran-air mixtures at elevated pressures. Combust. Flame 2011, 158, 539–546. [Google Scholar] [CrossRef]
- Miao, H.; Jiao, Q.; Huang, Z.; Jiang, D. Effect of initial pressure on laminar combustion characteristics of hydrogen enriched natural gas. Int. J. Hydrog. Energy 2008, 33, 3876–3885. [Google Scholar] [CrossRef]
- Song, W.S.; Jung, S.W.; Par, J.; Kwon, O.B; Kim, Y.J.; Kim, T.H.; Yun, J.H.; Keel, S.I. Effects of syngas addition on flame propagation and stability in outwardly propagating spherical dimethyl ether-air premixed flames. Int. J. Hydrog. Energy 2013, 38, 14102–14114. [Google Scholar] [CrossRef]
- Vu, T.M.; Park, J.; Kwon, O.B.; Kim, J.S. Effects of hydrocarbon addition on cellular instabilities in expanding syngas–air spherical premixed flames. Int. J. Hydrog. Energy 2009, 34, 6961–6969. [Google Scholar] [CrossRef]
- Hu, E.; Fu, J.; Pan, L.; Jiang, X.; Huang, Z.; Zhang, Y. Experimental and numerical study on the effect of composition on laminar burning velocities of H2/CO/N2/CO2/air mixtures. Int. J. Hydrog. Energy 2012, 37, 18509–18519. [Google Scholar] [CrossRef]
- Vu, T.M.; Park, J.; Kwon, O.B.; Bae, D.S.; Yun, J.H.; Keel, S.I. Effects of diluents on cellular instabilities in outwardly propagating spherical syngas-air premixed flames. Int. J. Hydrog. Energy 2010, 35, 3868–3880. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Kobayashi, H.; Ogami, Y. Laminar burning velocities and flame characteristics of CO-H2-CO2-O2 mixtures. Int. J. Hydrog. Energy 2012, 37, 19158–19167. [Google Scholar] [CrossRef]
- Liu, C.C.; Shy, S.S; Chiu, C.W.; Peng, M.W.; Chung, H.J. Hydrogen/carbon monoxide syngas burning rates measurements in high-pressure quiescent and turbulent environment. Int. J. Hydrog. Energy 2011, 36, 8595–8603. [Google Scholar] [CrossRef]
- Burke, M.P.; Chen, Z.; Ju, Y.; Dryer, F.L. Effect of cylindrical confinement on the determination of laminar flame speeds using outwardly propagating flames. Combust. Flame 2009, 156, 771–779. [Google Scholar] [CrossRef]
- Bradley, D.; Hicks, R.A.; Lawes, M.; Sheppard, C.G.W.; Woolley, R. The measurement of laminar burning velocities and Markstein numbers for iso-octane–air and iso-octane–n-heptane–air mixtures at elevated temperatures and pressures in an explosion bomb. Combust. Flame 1998, 115, 126–144. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M. GRI3.0 Mesh. Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 10 November 2013).
- Liu, K.; Burluka, A.A.; Sheppard, C.G.W. Turbulent flame and mass burning rate in a spark ignition engine. Fuel 2013, 107, 202–208. [Google Scholar] [CrossRef]
- Moccia, V.; D’Alessio, J. Burning behaviour of high-pressure CH4-H2-air mixtures. Energies 2013, 6, 97–116. [Google Scholar] [CrossRef]
- Hassan, M.I.; Aung, K.T.; Faeth, G.M. Properties of laminar premixed CO/H/air flames at various pressures. J. Propuls. Power 1997, 13, 239–245. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.I.; Jomaas, G.; Law, C.K. High-pressure laminar flame speeds and kinetic modeling of carbon monoxide/hydrogen combustion. Proc. Combust. Inst. 2007, 31, 439–446. [Google Scholar] [CrossRef]
- Krejci, M.C.; Mathieu, O.; Vissotski, A.J.; Ravi, S.; Sikes, T.G.; Petersen, E.L. Laminar flame speed and ignition delay time data for the kinetic modeling of hydrogen and syngas fuel blends. J. Eng. Gas Turbines Power 2013, 135, 021503. [Google Scholar] [CrossRef]
- Prathap, C.; Ray, A.; Ravi, M.R. Investigation of nitrogen dilution effects on the laminar burning velocity and flame stability of syngas fuel at atmospheric condition. Combust. Flame 2008, 155, 145–160. [Google Scholar] [CrossRef]
- McLean, I.C.; Smith, D.B.; Taylor, S.C. The use of carbon monoxide/hydrogen burning velocities to examine the rate of the CO + OH reaction. Symp. (Int.) Combust. 1994, 25, 749–757. [Google Scholar] [CrossRef]
- Bouvet, N.; Chauveau, C.; Gökalp, I.; Halter, F. Experimental studies of the fundamental flame speeds of syngas (H2/CO)/air mixtures. Proc. Combust. Inst. 2011, 33, 913–920. [Google Scholar] [CrossRef]
- Gu, X.J.; Haq, M.Z.; Lawes, M. Laminar burning velocity and Markstein lengths of Methane-air mixtures. Combust. Flame 2000, 121, 54–58. [Google Scholar]
- Zamashchikov, V.V.; Alekseev, V.A.; Konnov, A.A. Laminar burning velocities of rich near-limiting flames of hydrogen. Int. J. Hydrog. Energy 2014, 39, 1874–1881. [Google Scholar] [CrossRef]
- Bechtold, J.K.; Matalon, M. Hydrodynamic and diffusion effects on the stability of spherically expanding flames. Combust. Flame 1987, 67, 77–90. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.-M.; Li, G.-X.; Sun, Z.-Y.; Zhai, Y.; Zhou, Z.-H. Research on Cellular Instabilities of Lean Premixed Syngas Flames under Various Hydrogen Fractions Using a Constant Volume Vessel. Energies 2014, 7, 4710-4726. https://doi.org/10.3390/en7074710
Li H-M, Li G-X, Sun Z-Y, Zhai Y, Zhou Z-H. Research on Cellular Instabilities of Lean Premixed Syngas Flames under Various Hydrogen Fractions Using a Constant Volume Vessel. Energies. 2014; 7(7):4710-4726. https://doi.org/10.3390/en7074710
Chicago/Turabian StyleLi, Hong-Meng, Guo-Xiu Li, Zuo-Yu Sun, Yue Zhai, and Zi-Hang Zhou. 2014. "Research on Cellular Instabilities of Lean Premixed Syngas Flames under Various Hydrogen Fractions Using a Constant Volume Vessel" Energies 7, no. 7: 4710-4726. https://doi.org/10.3390/en7074710



