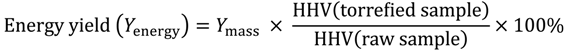Effect of Torrefaction on the Properties of Corn Stalk to Enhance Solid Fuel Qualities
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Elements (wt%) | C | 44.79 |
| H | 5.48 | |
| N | 0.34 | |
| O | 44.3 | |
| S | 0.04 | |
| Cl | 0.06 | |
| Others | 4.99 | |
| Moisture (%) | 13.2 | |
| Volatile fraction (%) | 84.7 | |
| Ash (%) | 2.1 | |
| HHV (MJ/kg) | 18.49 | |
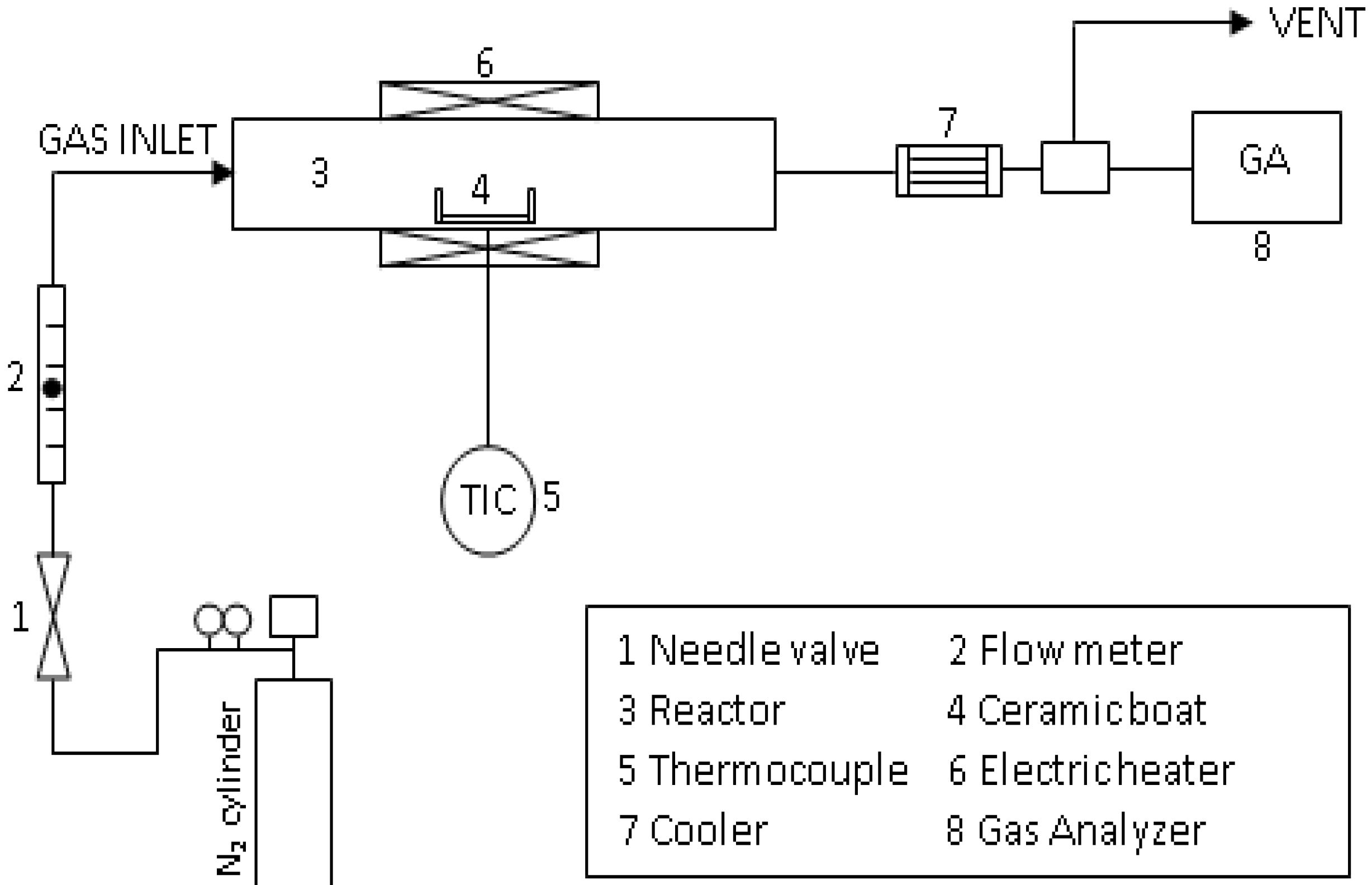
2.2. Experimental Device and Procedure
2.3. Kinetic Analysis Methods








 versus θ in Equation (9) and Y = ln[−ln(1 − α)] versus θ in Equation (10), we can obtain the activation energy, E, from the slope of the line.
versus θ in Equation (9) and Y = ln[−ln(1 − α)] versus θ in Equation (10), we can obtain the activation energy, E, from the slope of the line.
3. Results and Discussion
3.1. Thermogravimetric Analysis

| Methods | Apparent activation energy (kJ/mol) | |
|---|---|---|
| Equation (4) | 193.0 | |
| Equation (9) or (10) | Heating rate: 10 °C/min | 88.2 |
| Heating rate: 20 °C/min | 72.9 | |
| Heating rate: 30 °C/min | 69.0 | |
| Average | 76.7 | |
| Equation (11) | 184.1 | |
3.2. Elemental Analysis


3.3. Energy Yield, Mass Yield, Higher Heating Value, Volatile Fraction, and Ash Content

3.4. Effect of the Torrefaction Holding Time


3.5. Composition of the Gaseous Products


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Werther, J.; Saenger, M.; Hartge, E.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of oil palm wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Lu, J.J.; Chen, W.H. Product yields and characteristics of corncob waste under various torrefaction atmospheres. Energies 2014, 7, 13–27. [Google Scholar]
- Goyal, H.; Seal, D.; Saxena, R. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandre, J. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.; Rubiera, F.; Pis, J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Kuang, J.; Zhang, Y.; Luo, Y. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis. 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Legros, R.; Bi, X.T.; Lim, C.; Sokhansanj, S. Torrefaction of sawdust in a fluidized bed reactor. Bioresour. Technol. 2012, 103, 453–458. [Google Scholar] [CrossRef]
- Chen, W.; Kuo, P. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Bridgeman, T.; Jones, J.; Shield, I.; Williams, P. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash inBiomass; Report No. TP-510-42622; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. Torrefaction of wood: Part 2. Analysis of products. J. Anal. Appl. Pyrol. 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2011, 91, 147–154. [Google Scholar]
- Medic, D.; Darr, M.; Shah, A.; Rahn, S. The effects of particle size, different corn stover components, and gas torrefaction time on torrefaction of corn stover. Energies 2012, 5, 1199–1214. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.; Othman, N.A.; Yusup, S.; Tsutsui, T. Torrefaction of oil palm EFB in the presence of oxygen. Fuel 2013, 103, 156–160. [Google Scholar] [CrossRef]
- Sadaka, S.; Sharara, M.A.; Ashworth, A.; Keyser, P.; Allen, F.; Wright, A. Characterization of biochar from Switch grass carbonization. Energies 2014, 7, 548–567. [Google Scholar] [CrossRef]
- Bergman, P.; Boersma, A.; Zwart, R.; Kiel, J. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations; Report No. ECN-C-05-013; Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2005. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Poudel, J.; Oh, S.C. Effect of Torrefaction on the Properties of Corn Stalk to Enhance Solid Fuel Qualities. Energies 2014, 7, 5586-5600. https://doi.org/10.3390/en7095586
Poudel J, Oh SC. Effect of Torrefaction on the Properties of Corn Stalk to Enhance Solid Fuel Qualities. Energies. 2014; 7(9):5586-5600. https://doi.org/10.3390/en7095586
Chicago/Turabian StylePoudel, Jeeban, and Sea Cheon Oh. 2014. "Effect of Torrefaction on the Properties of Corn Stalk to Enhance Solid Fuel Qualities" Energies 7, no. 9: 5586-5600. https://doi.org/10.3390/en7095586