Performance of a Small-Scale, Variable Temperature Fixed Dome Digester in a Temperate Climate
Abstract
:1. Introduction
2. Results
2.1. Temperature and Biogas Production


2.2. VS Reduction
 ± θ). Statistical differences of operational data among stages were conducted through unbalanced one-way Anova, and Tukey–Kramer multicomparison tests were used for comparisons among stages.
± θ). Statistical differences of operational data among stages were conducted through unbalanced one-way Anova, and Tukey–Kramer multicomparison tests were used for comparisons among stages.
| Parameter | Five stages of operation d | ||||
| 1. Start up | 2. Initial Rest | 3. Optimal Operation | 4. Poor Operation | 5. Final Rest | |
| 8 March 2010–30 April 2010 | 1 May 2010–30 June 2010 | 1 July 2010–10 November 2010 | 11 November 2010–10 January 2011 | 11 January 2011–11 March 2011 | |
| OLR a, kg VS/m3/day | 0.83 ± 0.08 I | 0.00 | 2.43 ± 0.22 II | 1.58 ± 0.4 III | 0.00 |
| Temperature, °C | 12.7 ± 1.89 I | 22.74 ± 1.37 II | 25.04 ± 0.60 III | 13.42 ± 1.44 IV | 7.05 ± 0.38 V |
| Total Solids influent, mg/L | 74,000 ± 8,000 I | – | 81,000 ± 4,000 I | 80,000 ± 6,000 I | – |
| Volatile Solids influent, mg/L | 61,000 ± 6,000 I | – | 67,000 ± 3,000 II | 63,000 ± 5,000 I,II | – |
| Total Solids effluent, mg/L | 42,000 ± 2,000 I | – | 58,000 ± 4,000 II | 60,000 ± 5,000 II | – |
| Volatile Solids effluent, mg/L | 36,000 ± 1,700 I | – | 44,000 ± 2,500 II | 46,000 ± 2,000 II | – |
| Volatile Solids reduction, % | 40.1 ± 5.1 I | – | 35.8 ± 4.6 I | 25.6 ± 6.7 II | – |
| Biogas, liters/day | 18.66 ± 8.6 I | 268 ± 86 II | 914 ± 63 III | 302 ± 91 IV | 39 ± 11 I |
| Methane, % | 24.6 ± 2.25 I | 49.6 ± 5.5 II | 51.55 ± 2.39 II | 37.24 ± 6.30 III | 27.43 ± 5.34 I |
| Methane Yield, m3 of CH4/kg VS added | 0.005 ± 0.002 | – | 0.176 ± 0.005 | 0.06 ± 0.013 | – |
| TVFAs b, mg HAc/L | – | – | 2222 ± 276 I | 4074 ± 980 II | 6917 ± 301 III |
| TIC c, mg CaCO3/L | – | – | 8904 ± 652 I | 8544 ± 350 I | 6085 ± 272 II |
| TVFAs/TIC | – | – | 0.25 ± 0.03 I | 0.48 ± 0.13 I | 1.15 ± 0.08 II |
| pH | – | – | 7.39 ± 0.03 I | 7.01 ± 0.08 II | 6.81 ± 0.04 III |
 —mean; x ± θ —lower and upper endpoints of confidence interval; a OLR—Organic loading rate; b TVFAs—Total volatile fatty acids; c TIC—Total inorganic carbonate alkalinity; I, II, III, IV and V superscripts are used to show multiple comparisons of group means. Different superscripts between operational stages indicate marginal means are significantly different
—mean; x ± θ —lower and upper endpoints of confidence interval; a OLR—Organic loading rate; b TVFAs—Total volatile fatty acids; c TIC—Total inorganic carbonate alkalinity; I, II, III, IV and V superscripts are used to show multiple comparisons of group means. Different superscripts between operational stages indicate marginal means are significantly different3. Discussion
 ) Data from this study; (
) Data from this study; (  ) Data from other small-scale digesters with no controlled temperature [27,38]; (
) Data from other small-scale digesters with no controlled temperature [27,38]; (  ) Data from lab experiments where digesters had controlled temperature [30,34,37,39]; (•) Large farm digester with controlled temperature [29].
) Data from lab experiments where digesters had controlled temperature [30,34,37,39]; (•) Large farm digester with controlled temperature [29].
 ) Data from this study; (
) Data from this study; (  ) Data from other small-scale digesters with no controlled temperature [27,38]; (
) Data from other small-scale digesters with no controlled temperature [27,38]; (  ) Data from lab experiments where digesters had controlled temperature [30,34,37,39]; (•) Large farm digester with controlled temperature [29].
) Data from lab experiments where digesters had controlled temperature [30,34,37,39]; (•) Large farm digester with controlled temperature [29].
4. Experimental Section
4.1. Study Site
4.2. Modified Fixed Domed Digester
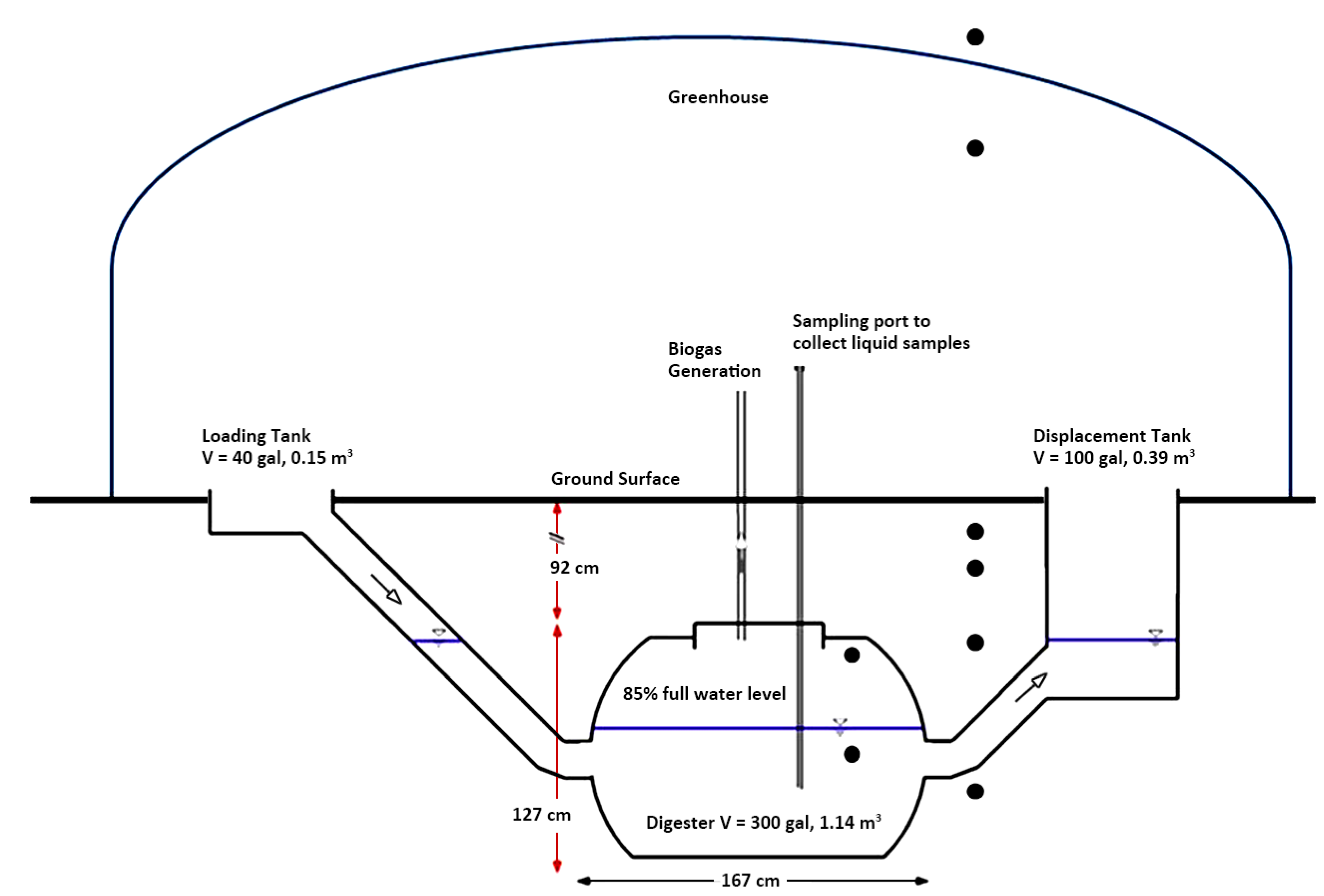
4.3. Digester—Instrumentation
4.4. Digester Start-up and Feeding

- Q = m3 of diluted manure per fed (This volume ranged from 0.018 to 0.087 m3 per load);
- OLR = Organic Loading rate, kg VS/m3/day;
- V = 0.91 m3 (240—digester working volume;
- C = VS concentration, mg/L;
- 1000 × 7/3 = conversion factor to calculate the loading in m3. The weekly organic loading rate was delivered three times per week.
4.5. Analytical Methods
- TIC = Total inorganic carbonate alkalinity, mg CaCO3/L;
- TVAS = Total volatile fatty acids, mg HAc/L;
- Vt = Total volume of H2SO4 0.1 N used, mL;
- V1 = Volume added from start to pH 5, mL;
- Vt − V1 = Volume added from pH 5 to pH 4.4, mL.
4.6. Methane Yield

= avg. biogas production, L;
= avg. methane concentration, %;
- V = Digester liquid volume, m3;
= Avg. organic loading rate, kg VS/m3/day.
4.7. Statistical Analysis
| Stage | Period | Justification | OLR, kg VS/m3/day Mean (SD) |
|---|---|---|---|
| (1) Start up | 8 March 2010–30 April 2010 | Period after inoculation when the digester was first loaded | 0.83 (0.12) |
| (2) Initial Rest | 1 May 2010–30 June 2010 | Due to low gas production, the loading was stopped | 0 |
| (3) Optimal operation | 1 July 2010–10 November 2010 | Operation with highest OLR and maximum biogas and methane production | 2.43 (0.5) |
| (4) Poor Operation | 11 November 2010–10 January 2011 | Operation with a lower OLR, the system showed signs of unstable performance in terms of decreasing total inorganic alkalinity and an increasing total volatile fatty acids content | 1.58 (0.6) |
| (5) Final Rest | 11 January 2011–11 March 2011 | Digester soured, loading was stopped | 0 |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karim, K.; Klasson, K.T.; Drescher, S.R.; Ridenour, W.; Borole, A.P.; Al-Dahhan, M.H. Mesophilic digestion kinetics of manure slurry. Appl. Biochem. Biotechnol. 2007, 142, 231–242. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Anaerobic Digestion Capital Cost for Dairy Farms. 2010. Available online: http://www.epa.gov/agstar/documents/digester_cost_fs.pdf (accessed on 6 January 2011). [Google Scholar]
- Demirer, G.; Chen, S. Two-phase anaerobic digestion of unscreened dairy manure. Process Biochem. 2005, 40, 3542–3549. [Google Scholar] [CrossRef]
- Parawira, W.; Read, J.S.; Mattiasson, B.; Björnsson, L. Energy production from agricultural residues: High methane yields in pilot-scale two-stage anaerobic digestion. Biomass Bioenergy 2008, 32, 44–50. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Operating Anaerobic Digester Projects. 2011. Available online: http://www.epa.gov/agstar/projects/index.html (accessed on 6 January 2011). [Google Scholar]
- U.S. Department of Agriculture (USDA). Overview of the United States Dairy Industry. 2010. Available online: http://usda.mannlib.cornell.edu/usda/current/USDairyIndus/USDairyIndus-09-22-2010.pdf (accessed on 6 November 2011).
- Daxiong, Q.; Shuhua, G.; Baofen, L.; Gehua, W. Diffusion and innovation in the Chinese biogas program. World Dev. 1990, 18, 555–563. [Google Scholar] [CrossRef]
- Khoiyangbam, R.S.; Kumar, S.; Jain, M.C.; Gupta, N.; Kumar, A.; Kumar, V. Methane emission from fixed dome biogas plants in hilly and plain regions of northern India. Bioresour. Technol. 2004, 95, 35–39. [Google Scholar] [CrossRef]
- Ciotola, R.J.; Martin, J.F.; Tamkin, A.; Castańo, J.M.; Rosenblum, J.; Bisesi, M.S.; Lee, J. The Influence of Loading Rate and Variable Temperatures on Microbial Communities in Anaerobic Digesters. Energies 2014, 7, 785–803. [Google Scholar]
- He, P.J. Anaerobic digestion: An intriguing long history in China. Waste Manag. 2010, 30, 549–550. [Google Scholar]
- Nianguo, L. Biogas in China. Trends Biotechnol. 1984, 2, 77–79. [Google Scholar] [CrossRef]
- An, B.X.; Preston, T. Gas production from pig manure fed at different loading rates to polyethylene tubular biodigesters. Livest. Res. Rural Dev. 1999, 11. Available online: http://www.lrrd.org/lrrd11/1/an111.htm (accessed on 20 August 2014).
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef]
- McKeown, R.M.; Scully, C.; Enright, A.-M.; Chinalia, F.A.; Lee, C.; Mahony, T.; O’Flaherty, V.; Collins, G. Psychrophilic methanogenic community development during long-term cultivation of anaerobic granular biofilms. ISME J. 2009, 3, 1231–1242. [Google Scholar] [CrossRef]
- Lettinga, G.; Rebac, S.; Parshina, S.; Nozhevnikova, A.; van Lier, J.B.; Stams, A.J. High-rate anaerobic treatment of wastewater at low temperatures. Appl. Environ. Microbiol. 1999, 65, 1696–1702. [Google Scholar]
- Rebac, S. Kinetics of fatty acid degradation by psychrophilically grown anaerobic granular sludge. Bioresour. Technol. 1999, 69, 241–248. [Google Scholar] [CrossRef]
- Rebac, S.; Lier, J.B.; Lens, P.; Cappellen, J.; Vermeulen, M.; Stams, A.J.M.; Lettinga, G.; Dekkers, F.; Swinkels, K.T.M. Psychrophilic (6–15 °C) high-rate anaerobic treatment of malting wastewater in a two-module expanded granular sludge bed system. Biotechnol. Prog. 1998, 14, 856–864. [Google Scholar]
- Rebac, S.; Ruskova, J.; Gerbens, S.; Lier, J.B.; Stams, A.J.M.; Lettinga, G. High-Rate Anaerobic Treatment of Wastewater under Psychrophilic Conditions. J. Ferment. Bioeng. 1995, 80, 499–506. [Google Scholar] [CrossRef]
- Balasubramaniyam, U.; Zisengwe, L.S.; Meriggi, N.; Buysman, E. Biogas Production in Climates with Long Cold Winters; Wageningen University: Wageningen, The Netherlands, 2008. Available online: http://www.wecf.eu/english/publications/2008/biogas-coldclimates.php (accessed on 20 April 2010).
- Hill, D.T.; Taylor, S.E.; Grift, T.E. Simulation of low temperature anaerobic digestion of dairy and swine manure. Bioresour. Technol. 2001, 78, 127–131. [Google Scholar] [CrossRef]
- Alvarez, R.; Lidén, G. Low temperature anaerobic digestion of mixtures of llama, cow and sheep manure for improved methane production. Biomass Bioenergy 2009, 33, 527–533. [Google Scholar] [CrossRef]
- Colleran, E.; Concannon, F.; Golden, T.; Geoghegan, F.; Crumlish, B.; Killilea, E.; Henry, M.; Coates, J. Use of methanogenic activity tests to characterize anaerobic sludges, screen for anaerobic biodegradability and determine toxicity thresholds against individual anaerobic trophic groups and species. Water Sci. Technol. 1992, 25, 31–40. [Google Scholar]
- Ho, R. Handbook of Univariate and Multivariate Data Analysis and Interpretation with SPSS; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lossie, U.; Pütz, P. Targeted control of biogas plants with the help of FOS/TAC. In Practice Report, Laboratory Analysis, Titration FOS/TAC; Hach Lange Ltd.: Salford, UK, 2001. [Google Scholar]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Gupta, R.K.; Guleri, R.L.; Singh, S.P. Performance evaluation of a 1 m3 modified, fixed-dome Deenbandhu biogas plant under hilly conditions. Bioresour. Technol. 1994, 50, 239–241. [Google Scholar] [CrossRef]
- Kalia, A.; Kanwar, S. Long-term evaluation of a fixed dome Janata biogas plant in hilly conditions. Bioresour. Technol. 1998, 65, 61–63. [Google Scholar] [CrossRef]
- Martin, J., Jr.; Wright, P.; Inglis, S.; Roos, K. Evaluation of the performance of a 550 cow plug-flow anaerobic digester under steady-state conditions. In Animal, Agricultural, and Food Processing Wastes IX; Joseph, St., Ed.; American Society of Agricultural Engineers: Triangle Park, NC, USA, 2003. [Google Scholar]
- Callaghan, F.; Wase, D.; Thayanithy, K.; Forster, C. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Schoen, M.A.; Sperl, D.; Gadermaier, M.; Goberna, M.; Franke-Whittle, I.; Insam, H.; Ablinger, J.; Wett, B. Population dynamics at digester overload conditions. Bioresour. Technol. 2009, 100, 5648–5655. [Google Scholar]
- Safley, L., Jr.; Westerman, P. Low-temperature digestion of dairy and swine manure. Bioresour. Technol. 1994, 47, 165–171. [Google Scholar] [CrossRef]
- Van Horn, H.H.; Wilkie, A.C.; Powers, W.J.; Nordstedt, R.A. Components of Dairy Manure Management Systems. J. Dairy Sci. 1994, 77, 2008–2030. [Google Scholar] [CrossRef]
- Kaparaju, P.L.N.; Rintala, J. The effects of post-treatments and temperature on recovering the methane potential of >2 mm solid fraction of digested cow manure. Environ. Technol. 2005, 26, 625–632. [Google Scholar]
- Guo, J.; Li, X.; Xu, P.; Dong, R.; Clemens, J. Process control and fluxes of medium size agricultural biogas plants management at ambient temperature: A case study in Beijing. Available online: http://www.ramiran.net/ramiran2010/docs/Ramiran2010_0279_final.pdf (accessed on 6 July 2011).
- Hashimoto, A.; Varel, V.; Chen, Y. Ultimate methane yield from beef cattle manure: Effect of temperature, ration constituents, antibiotics and manure age. Agric. Wastes 1981, 3, 241–256. [Google Scholar] [CrossRef]
- Kalia, A. Development and evaluation of a fixed dome plug flow anaerobic digester. Biomass 1988, 16, 225–235. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Huttunen, S.; Rintala, J.A. Laboratory investigations on co-digestion of energy crops and crop residues with cow manure for methane production: Effect of crop to manure ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Water Lifting. FAO Irrigation and Drainage. Paper 43. 1986. Available online: http://www.fao.org/docrep/010/ah810e/AH810E13.htm (accessed on 20 January 2012).
- Ciotola, R.J.; Martin, J.F.; Castańo, J.M.; Lee, J.; Michel, F. Microbial Community Response to Seasonal Temperature Variation in a Small-Scale Anaerobic Digester. Energies 2013, 6, 5182–5199. [Google Scholar] [CrossRef]
- Desai, S.R.; Palled, V. Performance evaluation of fixed dome type biogas plant using solid state digestion of cattle dung. Environ. Ecol. 2013, 31, 435–439. [Google Scholar]
- Rajendran, K.; Aslanzadeh, S.; Taherzadeh, M.J. Household biogas digesters—A review. Energies 2012, 5, 2911–2942. [Google Scholar] [CrossRef]
- Lorimer, J.; Powers, W.; Sutton, A. Manure Characteristics; Midwest Plan Service: Ames, IA, USA, 2004. [Google Scholar]
- American Public Health Association. 2005 Standard Methods for the Examination of Water and Wastewater, 21 Har/Cdr ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Ramsey, F.L.; Schafer, D.W. The Statistical Sleuth: A Course in Methods of Data Analysis; Duxbury Press: Belmont, CA, USA, 1997; pp. 100–150. [Google Scholar]
- Zeeman, G.; Sutter, K.; Vens, T.; Koster, M.; Wellinger, A. Psychrophilic digestion of dairy cattle and pig manure: Start-up procedures of batch, fed-batch and CSTR-type digesters. Biol. Wastes 1988, 26, 15–31. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castano, J.M.; Martin, J.F.; Ciotola, R. Performance of a Small-Scale, Variable Temperature Fixed Dome Digester in a Temperate Climate. Energies 2014, 7, 5701-5716. https://doi.org/10.3390/en7095701
Castano JM, Martin JF, Ciotola R. Performance of a Small-Scale, Variable Temperature Fixed Dome Digester in a Temperate Climate. Energies. 2014; 7(9):5701-5716. https://doi.org/10.3390/en7095701
Chicago/Turabian StyleCastano, Juan M., Jay F. Martin, and Richard Ciotola. 2014. "Performance of a Small-Scale, Variable Temperature Fixed Dome Digester in a Temperate Climate" Energies 7, no. 9: 5701-5716. https://doi.org/10.3390/en7095701