Analysis on Storage Off-Gas Emissions from Woody, Herbaceous, and Torrefied Biomass
Abstract
:1. Introduction
Objective
2. Material and Methods
2.1. Raw Material and Properties
2.2. Particle Density and Porosity
- P1 = pressure reading after pressurizing the reference cell (kPa)
- P2 = pressure after connecting the reference cell to the sample cell
- Vc = sample volume
- VR = reference volume.
- ρb = bulk density
- ρs = particle density.
2.3. Off-Gas Measurement

2.4. Emission Factor Calculation
- R = gas constant (8.31 J/mol∙K)
- T = temperature (K)
- Mwt = gas molecular weight (g/mole)
- M = mass of material in the container (kg)
- Vg = gas volume in the container (m3)
- Pa = absolute pressure of the container (generally, there is minimal change in Pa at relatively low temperatures associated with the observed tests [7]). In their study, pressure in the storage container was measured using a pressure transducer (PX143-01BD5V, 91 Pa, Omega, Laval, QC, Canada) and the data was logged into a computer using LabVIEW software
- Ci = volumetric concentration of a particular off-gassing measure by GC (expressed as fraction volume/volume).
| S. No. | Equation Parameters | Value |
|---|---|---|
| 1 | Molecular weight of CO, CO2, and CH4 (Mwt) | 28, 44, and 16 |
| 2 | Volumetric concentration of particular off gas (m3) | Gas concentrations measured using GC (ppmv/1,000,000) |
| 3 | Temperature (K) | 293 and 313 |
| 4 | Pressure (Pa) | 101,300 |
| 5 | Gas constant (R) (J/mol·K) | 8.31 |
| 6 | Mass of the biomass stored in the container (mw) (kg) | Ground switchgrass: 0.36 Wood pellets: 1.60 Wood chips: 0.64 Torrefied wood chips: 0.54 |
| 7 | Volume of the gas in the container, including headspace of 5% (m3) | Ground switchgrass: 0.0021 Wood pellets: 0.00119 Wood chips: 0.00152 Torrefied wood chips: 0.00154 |
3. Results and Discussion
3.1. Physical Properties
| S. No. | Biomass Feedstock | Moisture Content (%, w.b.) | Bulk Density (kg/m3) | Particle Density (kg/m3) | Porosity |
|---|---|---|---|---|---|
| 1 | Ground switchgrass | 10.21 ± 0.21 | 151 ± 9 | 650 ± 21 | 0.76 |
| 2 | Wood pellets | 5.02 ± 0.16 | 710 ± 19 | 1210 ± 8 | 0.41 |
| 3 | Wood chips | 12.12 ± 0.12 | 265 ± 12 | 580 ± 17 | 0.54 |
| 4 | Torrefied wood chips | 1.8 ± 0.19 | 225 ± 23 | 505 ± 11 | 0.55 |
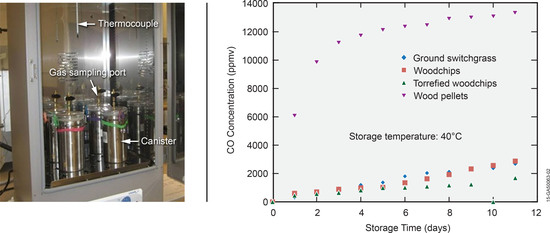
3.2. Off-Gas Concentrations






3.3. Emission Factor (mg/kg)
3.4. Discussion
4. Conclusions
Acknowledgments
Author Contributions
U.S. Department of Energy Disclaimer
Conflicts of Interest
References
- Thoernquisit, T.; Lundstroem, H. Health hazards caused by fungi in stored wood chips. For. Prod. J. 1982, 32, 11–12. [Google Scholar]
- Isenberg, I.H. Pulpwoods of the US and Canada, Volume II: Hardwoods, 3rd ed.; The Institute of Paper Chemistry: Appleton, WI, USA, 1981. [Google Scholar]
- Feist, W.C.; Springer, E.L.; Hajny, G.J. Spontaneous heating in piled wood chips‑contribution of bacteria. TAPPI 1973, 56, 148–151. [Google Scholar]
- Zoch, L.L., Jr.; Springer, E.L.; Hajny, D.J. Storage of aspen whole‑tree chips under laboratory conditions. In USDA Forest Service Research Paper; FPL 288; Forest Products Laboratory: Madison, WI, USA, 1976. [Google Scholar]
- Moran, J.S. Kraft mill experience with whole-tree chips. In TAPPI Annual Meeting, NY, February 1975; TAPPI: Atlanta, GA, USA, 1975. [Google Scholar]
- Johansson, L.S.; Leckner, B.; Gustavsson, L.; Cooper, D.; Tullin, C.; Potter, A. Emission characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 2004, 38, 4183–4195. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Kuang, X.; Sokhansanj, S.; Lim, C.J.; Bi, X.T.; Melin, S. Development of laboratory studies on the off‑gassing of wood pellets. Can. Biosyst .Eng. 2010, 52, 8.1–8.9. [Google Scholar]
- Kuang, X.; Shankar, T.J.; Bi, X.T.; Sokhansanj, S.; Lim, C.J.; Melin, S. Characterization and kinetics study of off-gas emission from stored wood pellets. Ann. Occup. Hyg. 2008, 52, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Shankar, T.J.; Bi, X.T.; Lim, C.J.; Sokhansanj, S.; Melin, S. Rate and peak concentrations of off-gas emissions in stored wood pellets-sensitivities to temperature, relative humidity, and headspace volume. Ann. Occup. Hyg. 2009, 53, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Shankar, T.J.; Sokhansanj, S.; Lim, C.J.; Bi, T.X.; Melin, S. Effects of headspace volume ratio and oxygen level on off‑gas emissions from stored wood pellets. Ann. Occup. Hyg. 2009, 53, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, U.R.A.; Hogberg, H.E.; Hogberg, J.; Galle, B. Emission of hexanal and carbon monoxide from storage of wood pellets, a potential occupational and domestic health hazard. Ann. Occup. Hyg. 2004, 48, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, U.; Samuelsson, J.; Melin, S. Hazardous off-gassing of carbon monoxide and oxygen depletion during ocean transportation of wood pellets. Ann. Occup. Hyg. 2008, 52, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tumuluru, J.S.; Sokhansanj, S.; Lim, C.J.; Bi, X.T.; Kuang, X.; Melin, S. Effect of storage temperatures on headspace gas composition and physical properties of wood pellets. Int. Wood Prod. J. 2012, 4, 207–216. [Google Scholar]
- Back, E.L.; Allen, L.H. Pitch Control, Wood Resin and Deresination; TAPPI Press: Atlanta, GA, USA, 2000. [Google Scholar]
- Höll, W.; Pieczonka, K. Lipids in sap and heartwood of Picea abies (L.) Karst. Z. Pflanzenphysiol. 1978, 87, 191–198. [Google Scholar] [CrossRef]
- Piispanen, R.; Saranpää, P. Neutral lipids and phospholipids in Scots pine (Pinus sylvestris) sapwood and heartwood. Tree Physiol. 2002, 22, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Emery, I.R.; Mosier, N.S. The impact of dry matter loss during herbaceous biomass storage on net greenhouse gas emissions from biofuels production. Biomass Bioenergy 2012, 39, 237–246. [Google Scholar] [CrossRef]
- Yancey, N.A.; Tumuluru, J.S.; Wright, C.T. Drying, grinding and pelletization studies on raw and formulated biomass feedstock’s for bioenergy applications. J. Biobased Mater. Bioenergy 2013, 7, 549–558. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Hess, J.R.; Boardman, R.D.; Wright, C.T.; Westover, T.L. Formulation, pretreatment, and densification options to improve biomass specifications for co-firing high percentages with coal. Ind. Biotechnol. 2012, 8, 113–132. [Google Scholar] [CrossRef]
- Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Patil, K.N.; Bellmer, D.D. Gasification performance of switchgrass pretreated with torrefaction and densification. Appl. Energy 2014, 127, 194–201. [Google Scholar] [CrossRef]
- Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Patil, K.N.; Bellmer, D.D. Thermal devolatilization kinetics of switchgrass pretreated with torrefaction and densification. Trans. ASABE 2014, 57, 1199–1210. [Google Scholar]
- Yang, Z.; Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Huhnke, R.L. Effects of torrefaction and densification on switchgrass pyrolysis products. Bioresource Technol. 2014, 174, 266–273. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Boardman, R.D.; Wright, C.T. Response surface analysis of elemental composition and energy properties of corn stover during torrefaction. J. Biobased. Mater. Biol. 2012, 6, 25–35. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Boardman, R.D.; Wright, C.T.; Hess, J.R. Some chemical compositional changes in miscanthus and white oak sawdust samples during torrefaction. Energies 2012, 5, 3928–3947. [Google Scholar] [CrossRef]
- Bourgois, J.P.; Doat, J. Torrefied wood from temperate and tropical species, advantages and prospects. Bioenergy 1984, 84, 153–159. [Google Scholar]
- Bourgois, J.P.; Guyonnet, R. Characterization and analysis of torrefied wood. Wood Sci. Technol. 1988, 22, 143–155. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Peng, J.; Lim, C.J.; Bi, X.T.; Wang, L.; Lam, P.S.; Hoi, J.P.; Melin, S.; Tumuluru, J.S.; Wright, C.T. Optimum torrefaction and pelletization of biomass feedstock. In Proceedings of the TCS 2010 Symposium on Thermal and Catalytic Sciences for Biofuels and Biobased Products, Iowa State University, Ames, IA, USA, 21–23 September 2010.
- Association of Official Analytical Chemist. AOAC: Official Methods of Analysis, 12th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Cubes, Pellets and Crumbles‑Definitions and Methods for Determining Density, Durability and Moisture Content; ASAE Standards S269.4; The American Society of Agricultural and Biological Engineers (ASAE): St. Joseph, MI, USA, 1992.
- Tumuluru, J.S.; Tabil, L.G.; Song, Y.; Iroba, K.L.; Meda, V. Grinding energy and physical properties of chopped and hammer‑milled barley, wheat, oat, and canola straws. Biomass Bioenergy 2014, 60, 58–67. [Google Scholar] [CrossRef]
- Yazdanpanah, F.; Sokhansanj, S.; Lim, C.J.; Bi, X.T.; Melin, S. Stratification of off‑gases in stored wood pellets. Biomass Bioenergy 2014, 71, 1–11. [Google Scholar] [CrossRef]
- Yazdanpanah, F. Evolution and Stratification of Off-Gasses in Stored Wood Pellets. Ph.D. Thesis, University of British Columbia, Vancouver/Kelowna, BC, Canada, 2013. [Google Scholar]
- Springer, E.L.; Hajny, G.J. Spontaneous heating in piled wood chips. TAPPI 1970, 53, 85–86. [Google Scholar]
- Kuber, H.; Wang, Y.R.; Barkalow, D. Generation of heat in wood between 80° and 130 °C. Holzforschung 1985, 39, 85–89. [Google Scholar] [CrossRef]
- Kuber, H. Air convection in self‑heating piles of wood chips. TAPPI 1982, 65, 79–83. [Google Scholar]
- Kuber, H. Heat generating processes as cause of spontaneous ignition in forest products. For. Prod. Abstr. 1987, 10, 299–327. [Google Scholar]
- Levitt, M.D.; Ellis, C.; Springfield, J.; Engel, R.R. Carbon monoxide generation from hydrocarbons at ambient and physiological temperature: A sensitive indicator of oxidant damage? J. Chromatogr. 1995, 695, 324–328. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A review of biomass densification systems to develop uniform feedstock commodities for bioenergy applications. Biofuels Bioprod. Biorefin. 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Tabil, L.G.; Song, Y.; Iroba, K.L.; Meda, V. Impact of process conditions on the density and durability of wheat, oat, canola and barley straw briquettes. Bioenergy Res. 2014. [Google Scholar] [CrossRef]
- Steele, J.L.; Saul, R.A.; Hukill, W.V. Deterioration of shelled corn as measured by carbon dioxide production. ASABE 1969, 12, 685–689. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumuluru, J.S.; Lim, C.J.; Bi, X.T.; Kuang, X.; Melin, S.; Yazdanpanah, F.; Sokhansanj, S. Analysis on Storage Off-Gas Emissions from Woody, Herbaceous, and Torrefied Biomass. Energies 2015, 8, 1745-1759. https://doi.org/10.3390/en8031745
Tumuluru JS, Lim CJ, Bi XT, Kuang X, Melin S, Yazdanpanah F, Sokhansanj S. Analysis on Storage Off-Gas Emissions from Woody, Herbaceous, and Torrefied Biomass. Energies. 2015; 8(3):1745-1759. https://doi.org/10.3390/en8031745
Chicago/Turabian StyleTumuluru, Jaya Shankar, C. Jim Lim, Xiaotao T. Bi, Xingya Kuang, Staffan Melin, Fahimeh Yazdanpanah, and Shahab Sokhansanj. 2015. "Analysis on Storage Off-Gas Emissions from Woody, Herbaceous, and Torrefied Biomass" Energies 8, no. 3: 1745-1759. https://doi.org/10.3390/en8031745