Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yeast Cultivation for Lipid Production
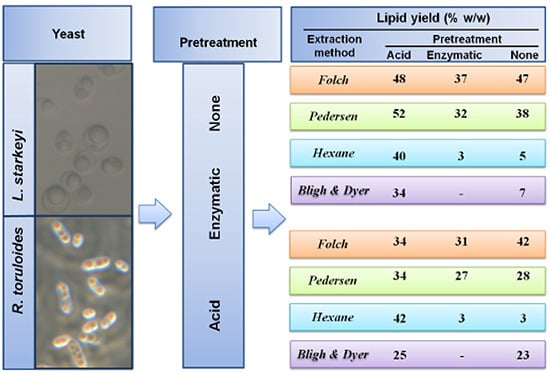
2.2. Lipid Extraction from Intact and Pretreated R. toruloides and L. starkeyi Cells
| Solvent | PT | R. toruloides | L. starkeyi |
|---|---|---|---|
| Lipid Yield (% w/w) | Lipid Yield (% w/w) | ||
| C:M (2:1) Folch et al. [23] | acid | 34 ± 15 * | 48 ± 3 ** |
| enz | 31 ± 2 ** | 37 ± 0 ** | |
| none | 42 ± 1 ** | 47 ± 1 ** | |
| C:M (1:1) Pedersen [16] | acid | 34 ± 7 * | 52 ± 7 *** |
| enz | 27 ± 7 *** | 32 ± 0 ** | |
| none | 28 ± 2 ** | 38 ± 0 ** | |
| Hexane | acid | 42 ± 2 ** | 40 ± 0 ** |
| enz | 3 ± 0 ** | 3 ± 0 ** | |
| none | 3 ± 0 ** | 5 ± 1 ** | |
| C:M:W (2:2:1.8) Bligh and Dyer [24] | acid | 25 ± 0 ** | 34 ± 7 *** |
| none | 23 ± 2 ** | 7 ± 1 ** |
2.3. FAME Composition and Estimated Properties of Biodiesel from the Oil Extracted from R. toruloides and L. starkeyi

3. Experimental Section
3.1. Microorganisms, Media, and Chemicals
3.2. Yeast Cultivation for Lipid Production
3.3. Pretreatments
3.4. Lipid Extraction
3.5. Analytical Methods
3.6. Prediction of Biodiesel Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meng, X.; Yang, J.M.; Xu, X.; Zhang, L.; Nie, Q.J.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- European Commission. EU Energy and Transport in Figures 2010. Available online: http://ec.europa.eu/energy/publications/doc/2010_energy_transport_figures.pdf (accessed on 10 July 2014).
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Y.; Chen, L.; Zong, M. Production of microbial oil with high oleic acid content by Trichosporon capitatum. Appl. Energy 2011, 88, 138–142. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Z.K. Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol. 2007, 82, 775–780. [Google Scholar] [CrossRef]
- Ratledge, C. Single cell oil. Enzyme Microb. Technol. 1982, 4, 58–60. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Yen, H.W.; Zhang, Z.Y. Effects of dissolved oxygen level on cell growth and total lipid accumulation in the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2011, 112, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; Souza, L.C.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Christophe, G.; Kumar, V.; Nouaille, R.; Gaudet, G.; Fontanille, P.; Pandey, A.; Soccol, C.R.; Larroche, C. Recent developments in microbial oils production: A possible alternative to vegetable oils for biodiesel without competition with human food? Braz. Arch. Biol. Technol. 2012, 55, 29–46. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.A. Lipid formation in Cryptococcus terricolus 3. Extraction and purification of lipids. Acta Chem. Scand. 1962, 16, 374–382. [Google Scholar] [CrossRef]
- Jacob, Z. Yeast lipids—Extraction, quality analysis, and acceptability. Crit. Rev. Biotechnol. 1992, 12, 463–491. [Google Scholar] [CrossRef]
- Jin, G.; Yang, F.; Hu, C.; Shen, H.; Zhao, Z.K. Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2012, 111, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takigawa, A.; Hasegawa, K. Lipid extraction methods for Lipomyces-starkeyi. Agric. Biol. Chem. 1973, 37, 2653–2656. [Google Scholar] [CrossRef]
- Sobus, M.T.; Holmlund, C.E. Extraction of lipids from yeast. Lipids 1976, 11, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Somashekar, D.; Venkateshwaran, G.; Srividya, C.; Sambaiah, K.; Lokesh, B.R. Efficacy of extraction methods for lipid and fatty acid composition from fungal cultures. World J. Microbiol. Biotechnol. 2001, 17, 317–320. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, B.D.; Oh, H.M. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech. 1998, 12, 553–556. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Yu, X.; Dong, T.; Zheng, Y.; Miao, C.; Chen, S. Investigations on cell disruption of oleaginous microorganisms: Hydrochloric acid digestion is an effective method for lipid extraction. Eur. J. Lipid Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Gonzalez, I.; Parashar, A.; Bressler, D.C. Hydrothermal treatment of oleaginous yeast for the recovery of free fatty acids for use in advanced biofuel production. J. Biotechnol. 2014, 187, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Neto, D.C. Process to Produce Biodiesel and/or Fuel Oil. U.S. Patent US20100330615, 30 December 2010. [Google Scholar]
- Rangaswamy, V.; Saran, S.; Kannabiran, M.; Thiru, M.; Sankh, S. Process for Biodiesel Production from a Yeast Strain. U.S. Patent US20120317877, 20 December 2012. [Google Scholar]
- Moon, N.J.; Hammond, G. Process for Converting Whey Permeate to Oil-Containing Yeast. U.S. Patent US4235933, 25 November 1980. [Google Scholar]
- Franklin, S.; Decker, S.M.; Wee, J. Fuel and Chemical Production from Oleaginous Yeast. U.S. Patent US20110252696, 20 October 2011. [Google Scholar]
- Thiru, M.; Sankh, S.; Rangaswamy, V. Process for biodiesel production from Cryptococcus curvatus. Bioresour. Technol. 2011, 102, 10436–10440. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Streekstra, H.; Cohen, Z.; Fichtali, J. Downstream processing, extraction, and purification of single cell oils. In Single Cell Oils: Microbial and Algal Oils; AOCS (American Oil Chemists’ Society) Press: Urbana, IL, USA, 2010; pp. 179–197. [Google Scholar]
- Anschau, A.; Xavier, M.C.A.; Hernalsteens, S.; Franco, T.T. Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour. Technol. 2014, 157, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Bonturi, N.; Miranda, E.A.; Rova, U.; Christakopoulos, P. High concentrations of dried sorghum stalks as a biomass feedstock for single cell oil production by Rhodosporidium toruloides. Biotechnol. Biofuels 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Hu, C.; Wu, S.; Wang, Q.; Jin, G.; Shen, H.; Zhao, Z.K. Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum. Biotechnol. Biofuels 2011, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Vannela, R.; Rittrnann, B.E. Evaluation of methods to extract and quantify lipids from Synechocystis PCC 6803. Bioresour. Technol. 2011, 102, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Bai, F.; Zhao, Z.K. Recycling microbial lipid production wastes to cultivate oleaginous yeasts. Bioresour. Technol. 2015, 175, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, A.; Takashima, M.; Sugita, T.; Endoh, R.; Kikukawa, M.; Yamaguchi, S.; Sakuradani, E.; Ogawa, J.; Ohkuma, M.; Shima, J. Cryptococcus terricola is a promising oleaginous yeast for biodiesel production from starch through consolidated bioprocessing. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Sitepu, I.R.; Sestric, R.; Ignatia, L.; Levin, D.; German, J.B.; Gillies, L.A.; Almada, L.A.G.; Boundy-Mills, K.L. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 2013, 144, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.J.; Fernandez, C.M.; Casas, A.; Rodriguez, L.; Perez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Automotive Fuels. Fatty Acid Methyl Esters [FAME] for Diesel Engines. Requirements and Test Methods; European Standard UNE-EN 14214; CEN—European Committee for Standardization: Brussels, Belgium, 2000.
- Li, Y.; Naghdi, F.G.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A comparative study: The impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Factories 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Meesters, P.; Huijberts, G.N.M.; Eggink, G. High cell density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Varavallo, M.A.; de Queiroz, M.V.; Pereira, J.F.; de Araújo, E.F. Isolation and regeneration of Penicillium brevicompactum protoplasts. Acta Sci. 2004, 26, 475–479. [Google Scholar]
- Nelson, G.J. Isolation and purification of lipids from animal tissues. In Analysis of Lipids & Lipoproteins; Perkins, E.G., Ed.; AOCS (American Oil Chemists’ Society): Champaign, IL, USA, 1975. [Google Scholar]
- Appelqvist, L.-A. Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing the accumulation of lipid contaminants. Arkiv Kemi 1968, 28, 551–570. [Google Scholar]
- Krisnangkura, K. A simple method for estimation of cetane index of vegetable oil methyl-esters. J. Am. Oil Chem. Soc. 1986, 63, 552–553. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonturi, N.; Matsakas, L.; Nilsson, R.; Christakopoulos, P.; Miranda, E.A.; Berglund, K.A.; Rova, U. Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction. Energies 2015, 8, 5040-5052. https://doi.org/10.3390/en8065040
Bonturi N, Matsakas L, Nilsson R, Christakopoulos P, Miranda EA, Berglund KA, Rova U. Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction. Energies. 2015; 8(6):5040-5052. https://doi.org/10.3390/en8065040
Chicago/Turabian StyleBonturi, Nemailla, Leonidas Matsakas, Robert Nilsson, Paul Christakopoulos, Everson Alves Miranda, Kris Arvid Berglund, and Ulrika Rova. 2015. "Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction" Energies 8, no. 6: 5040-5052. https://doi.org/10.3390/en8065040