Dehydriding Process and Hydrogen–Deuterium Exchange of LiBH4–Mg2FeD6 Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dehydriding Property of 0.25LiBH4 + 0.75Mg2FeD6
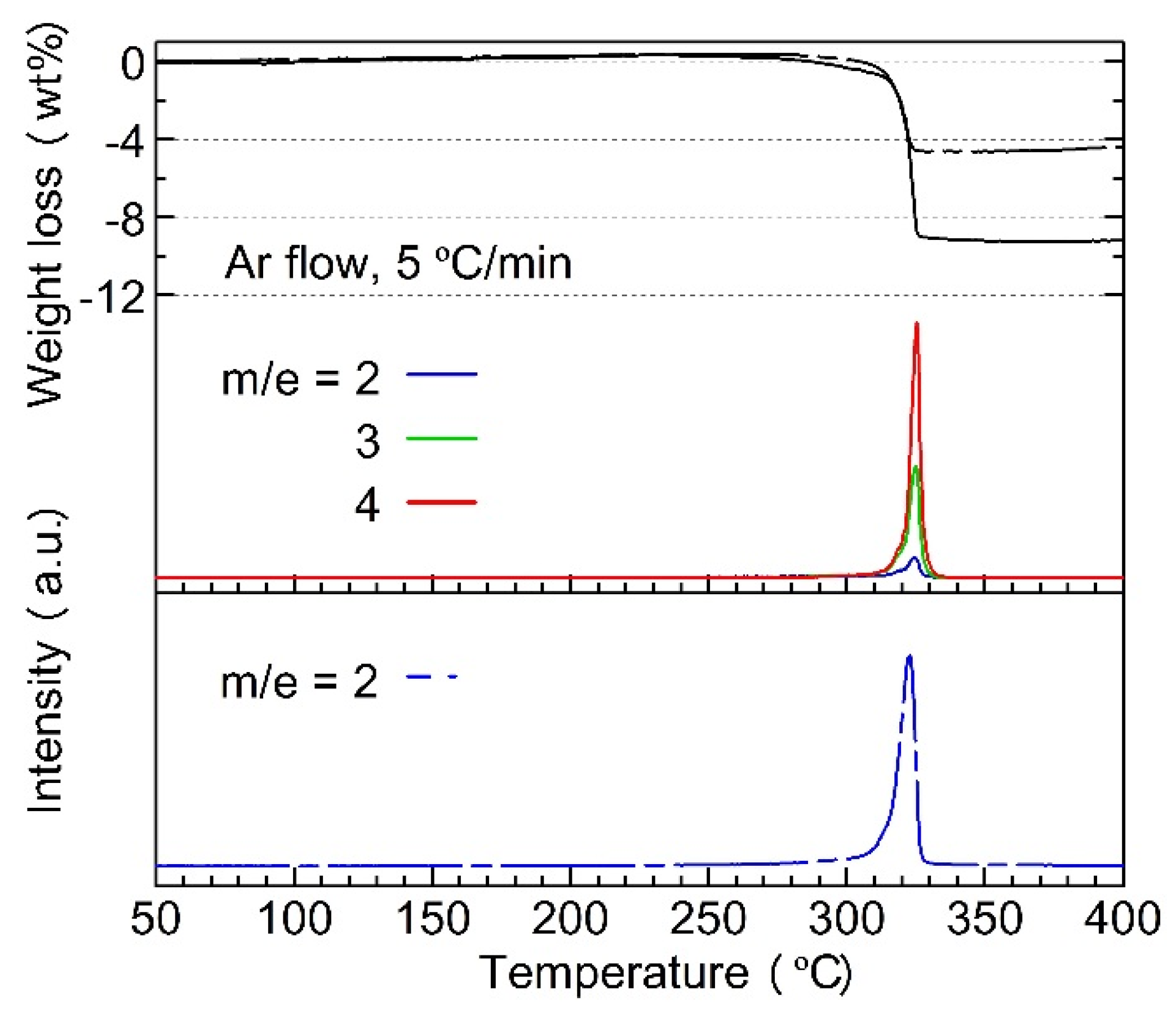

- (a)
- H–D exchange occurred during ball-milling and the heating process but stopped as soon as the dehydriding started;
- (b)
- H2, HD, or D2 molecules were directly released from either Mg2FeHy/3D6 − y/3 or LiBH4 − yDy; H or D atoms derived from the two different complex hydrides cannot combine to form gas molecules.
2.2. Dehydriding Property of 0.75LiBH4 + 0.25Mg2FeD6

3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Züttel, A.; Borgschulte, A.; Orimo, S. Tetrahydroborates as new hydrogen storage materials. Scr. Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloy Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Gross, A.F.; Vajo, J.J.; Van Atta, S.L.; Olson, G.L. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- Liu, X.F.; Peaslee, D.; Jost, C.Z.; Majzoub, E.H. Controlling the decomposition pathway of LiBH4 via confinement in highly ordered nanoporous carbon. J. Phys. Chem. C 2010, 114, 14036–14041. [Google Scholar] [CrossRef]
- Ngene, P.; Adelhelm, P.; Beale, A.M.; de Jong, K.P.; de Jongh, P.E. LiBH4/SBA-15 nanocomposites prepared by melt infiltration under hydrogen pressure: Synthesis and hydrogen sorption properties. J. Phys. Chem. C 2010, 114, 6163–6168. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P.; Vajo, J.J. Phase boundaries and reversibility of LiBH4/MgH2 hydrogen storage material. J. Phys. Chem. C 2007, 111, 12881–12885. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ichikawa, T.; Hanada, N.; Kojima, Y.; Fujii, H. Thermal analysis on the Li-Mg-B-H systems. J. Alloy Compd. 2007, 446, 306–309. [Google Scholar] [CrossRef]
- Jin, S.A.; Lee, Y.S.; Shim, J.H.; Cho, Y.W. Reversible hydrogen storage in LiBH4-MH2 (M = Ce, Ca) composites. J. Phys. Chem. C 2008, 112, 9520–9524. [Google Scholar] [CrossRef]
- Fu, H.; Yang, J.Z.; Wang, X.J.; Xin, G.B.; Zheng, J.; Li, X.G. Preparation and dehydrogenation properties of lithium hydrazidobis(borane) (LiNH(BH3)NH2BH3). Inorg. Chem. 2014, 53, 7334–7339. [Google Scholar] [CrossRef] [PubMed]
- Au, M.; Jurgensen, A.; Zeigler, K. Modified lithium borohydrides for reversible hydrogen storage. J. Phys. Chem. B 2006, 110, 26482–26487. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Matsuo, M.; Deledda, S.; Sato, R.; Hauback, B.C.; Orimo, S. Dehydriding property of LiBH4 combined with Mg2FeH6. Mater. Trans. 2013, 54, 1532–1534. [Google Scholar] [CrossRef]
- Langmi, H.W.; McGrady, G.S.; Newhouse, R.; Rönnebro, E. Mg2FeH6–LiBH4 and Mg2FeH6–LiNH2 composite materials for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 6694–6699. [Google Scholar] [CrossRef]
- Deng, S.S.; Xiao, X.Z.; Han, L.Y.; Li, Y.; Li, S.Q.; Ge, H.W.; Wang, Q.D.; Chen, L.X. Hydrogen storage performance of 5LiBH4+Mg2FeH6 composite system. Int. J. Hydrog. Energ. 2012, 37, 6733–6740. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Catti, M.; Nale, A. Thermodynamics of dehydrogenation of the 2LiBH4–Mg2FeH6 composite. J. Phys. Chem. C 2012, 116, 26694–26699. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Corno, M.; Damin, A.; Spoto, G.; Ugliengo, P.; Baricco, M. Vibrational properties of MBH4 and MBF4 crystals (M = Li, Na, K): A combined DFT, infrared, and Raman Study. J. Phys. Chem. C 2011, 115, 18890–18900. [Google Scholar] [CrossRef]
- Parker, S.F.; Williams, K.P.J.; Bortz, M.; Yvon, K. Inelastic neutron scattering, infrared, and Raman spectroscopic studies of Mg2FeH6 and Mg2FeD6. Inorg. Chem. 1997, 36, 5218–5221. [Google Scholar] [CrossRef]
- Borgschulte, A.; Jain, A.; Ramirez-Cuesta, A.J.; Martelli, P.; Remhof, A.; Friedrichs, O.; Gremaud, R.; Züttel, A. Mobility and dynamics in the complex hydrides LiAlH4 and LiBH4. Faraday Discuss. 2011, 151, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.R.; Gremaud, R.; Borgschulte, A.; Ramirez-Cuesta, A.J.; Züttel, A.; Hamm, P. Vibrational dynamics of LiBH4 by infrared pump-probe and 2D spectroscopy. J. Phys. Chem. A 2009, 113, 12838–12846. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, H.; D’Anna, V.; Rapin, J.P.; Yvon, K. Deuterium-hydrogen exchange in solid Mg(BH4)2. J. Phys. Chem. C 2010, 114, 10045–10047. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Matsuo, M.; Aoki, K.; Ikeshoji, T.; Orimo, S.-i. Dehydriding Process and Hydrogen–Deuterium Exchange of LiBH4–Mg2FeD6 Composites. Energies 2015, 8, 5459-5466. https://doi.org/10.3390/en8065459
Li G, Matsuo M, Aoki K, Ikeshoji T, Orimo S-i. Dehydriding Process and Hydrogen–Deuterium Exchange of LiBH4–Mg2FeD6 Composites. Energies. 2015; 8(6):5459-5466. https://doi.org/10.3390/en8065459
Chicago/Turabian StyleLi, Guanqiao, Motoaki Matsuo, Katsutoshi Aoki, Tamio Ikeshoji, and Shin-ichi Orimo. 2015. "Dehydriding Process and Hydrogen–Deuterium Exchange of LiBH4–Mg2FeD6 Composites" Energies 8, no. 6: 5459-5466. https://doi.org/10.3390/en8065459




