Lignin-Furfural Based Adhesives
Abstract
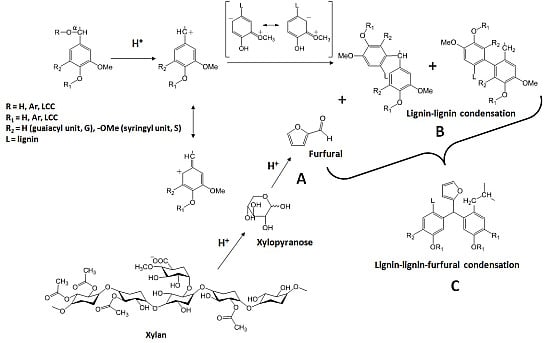
:1. Introduction


2. Results and Discussion
2.1. Characterization of Lignin
| Lignin fraction | Acid insoluble lignin (%) | Acid soluble lignin (%) | Total lignin content (%) |
|---|---|---|---|
| L | 86.39 | 1.85 | 88.24 ± 1.48 |
| LAH | 85.75 | 4.89 | 90.57 ± 0.35 |


2.2. Molecular Weight Distribution of Adhesive Blends

2.3. Effect of Curing Pressure and Temperature on Mechanical Properties of Adhesive Reinforced Glass Fibers

2.4. Effect of pH on Mechanical Properties of Adhesive Reinforced Glass Fibers

3. Experimental Section
3.1. Materials
3.2. Lignin Isolation and Characterization
3.3. Preparation of Adhesives
| Sample ID | Furfural content (%) | Reaction pH condition |
|---|---|---|
| F16_1 | 16 | 1 |
| F8_1 | 8 | 1 |
| F5_1 | 5 | 1 |
| F0_1 | 0 | 1 |
| F5_065 | 5 | 0.65 |
| F0_065 | 0 | 0.65 |
| F5_03 | 5 | 0.3 |
| F0_03 | 0 | 0.3 |
3.4. Mechanical Testing
| Curing conditions | Pressure (MPa) | ||
|---|---|---|---|
| 1.9 | 3.8 | ||
| Temperature (°C) | 180 | pH = 1 | pH = 1 |
| pH = 0.3 | pH = 0.3 | ||
| pH = 0.65 | - | ||
| 150 | pH = 1 | - | |
| pH = 0.3 | |||
3.5. Molecular Weight Distribution
3.6. Nuclear Magnetic Spectroscopy (NMR)
3.7. Acetylation
4. Conclusions and Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pizzi, A. Wood Adhesives; Mittal, K.L., Ed.; CRC Press: Leiden, The Netherlands; Boston, MA, USA, 2011. [Google Scholar]
- Kent, J.A. Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology, 11th ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Pizzi, A. Advanced Wood Adhesives Technology; CRC Press: New York, NY, USA, 1994. [Google Scholar]
- Jin, Y.; Cheng, X.; Zheng, Z. Preparation and characterization of phenol-formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and properties of lignin-phenol-formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind. Crops Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Ҫetin, N.S.; Ӧzmen, N. Studies on Lignin-Based Adhesives for Particleboard Panels. Turk. J. Agric. For. 2003, 27, 183–189. [Google Scholar]
- Wolfgang, H.; Jürgen, L. Phenolic Resins. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Inc.: Somerset, NJ, USA, 2011. [Google Scholar]
- Gardziella, A.; Pilato, L.; Knop, A. Phenolic Resins: Chemistry, Applications, Standardization, Safety and Ecology, 2nd ed.; Springer: New York, NY, USA, 2000. [Google Scholar]
- Chung, H.; Washburn, N.R. Chemistry of lignin-based materials. Green Mater. 2012, 1, 137–160. [Google Scholar] [CrossRef]
- Ye, P.; Cheng, L.; Ma, H.; Bujanovic, B.; Goundalkar, M.J.; Amidon, T.E. Biorefinery with Water. In The Role of Green Chemistry in Biomass Processing and Conversion; Xie, H., Gathergood, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 135–180. [Google Scholar]
- Thomas, E.; Amidon, C.D.W. Biorefinery: Conversion of Woody Biomass to Chemicals, Energy and Materials. J. Biobased Mater. Bioenergy 2008, 2, 100–120. [Google Scholar]
- Hu, F.; Ragauskas, A. Pretreatment and Lignocellulosic Chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Sjöström, E.; Alen, R. Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Kubo, S.; Kadla, J.F. Poly(ethylene oxide)/organosolv lignin blends: Relationship between thermal properties, chemical structure, and blend behavior. Macromolecules 2004, 37, 6904–6911. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Lignin-Based Carbon Fibers: Effect of Synthetic Polymer Blending on Fiber Properties. J. Polym. Environ. 2005, 13, 97–105. [Google Scholar] [CrossRef]
- Mansouri, N.E.; Pizzi, A.; Salvadó, J. Lignin-based wood panel adhesives without formaldehyde. Holz Roh Werkst. 2006, 65, 65–70. [Google Scholar] [CrossRef]
- Liou, T.H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Montané, D.; Torné-Fernández, V.; Fierro, V. Activated carbons from lignin: Kinetic modeling of the pyrolysis of kraft lignin activated with phosphoric acid. Chem. Eng. J. 2005, 106, 1–12. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P. Production of furfural from pentosan-rich biomass: Analysis of process parameters during simultaneous furfural stripping. Bioresour. Technol. 2013, 143, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. J. Catal. 2005, 229, 414–423. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Acidic cesium salts of 12-tungstophosphoric acid as catalysts for the dehydration of xylose into furfural. Carbohydr. Res. 2006, 341, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.B.; Chagnault, V.; Postel, D. Reactivity of d-fructose and d-xylose in acidic media in homogeneous phases. Carbohydr. Res. 2015, 409, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, G.; Hao, W.; Tang, X.; Sun, Y.; Lin, L.; Liu, S. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184–11206. [Google Scholar] [CrossRef]
- Binder, J.B.; Blank, J.J.; Cefali, A.V.; Raines, R.T. Synthesis of Furfural from Xylose and Xylan. ChemSusChem 2010, 3, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Sakostschikoff, A.P.; Iwanowa, W.T.; Kurennowa, A.M. Determination of Pentosans in Vegetable Materials Containing Tannins. Ind. Eng. Chem. Anal. Ed. 1934, 6, 205–208. [Google Scholar] [CrossRef]
- Samuel, R.; Cao, S.; Das, B.K.; Hu, F.; Pu, Y.; Ragauskas, A.J. Investigation of the fate of poplar lignin during autohydrolysis pretreatment to understand the biomass recalcitrance. RSC Adv. 2013, 3, 5305–5309. [Google Scholar] [CrossRef]
- Bu, L.; Tang, Y.; Gao, Y.; Jian, H.; Jiang, J. Comparative characterization of milled wood lignin from furfural residues and corncob. Chem. Eng. J. 2011, 175, 176–184. [Google Scholar] [CrossRef]
- Binder, J.B.; Gray, M.J.; White, J.F.; Zhang, Z.C.; Holladay, J.E. Reactions of lignin model compounds in ionic liquids. Biomass Bioenergy 2009, 33, 1122–1130. [Google Scholar] [CrossRef]
- Gerrit, H.; Klashorst, V.D. Modification of Lignin at the 2- and 6-Positions of the Phenylpropanoid Nuclei. ACS Symp. Ser. 1989, 397, 346–360. [Google Scholar]
- Li, J.; Gellerstedt, G. Improved lignin properties and reactivity by modifications in the autohydrolysis process of aspen wood. Ind. Crops Prod. 2008, 27, 175–181. [Google Scholar] [CrossRef]
- Lundquist, K. Low-molecular weight lignin hydrolysis products. Appl. Polym. Symp. 1976, 28, 1393–1407. [Google Scholar]
- Lora, J.; Wayman, M. Delignification of hardwoods by autohydrolysis and extraction. Tappi J. 1979, 61, 47–50. [Google Scholar]
- Robert, D.; Gellerstedt, G.; Bardet, M. Carbon-13 NMR analysis of lignin obtained after sulfonation of stream exploded aspen wood. Nord. Pulp Pap. Res. 1986, 1, 18–25. [Google Scholar] [CrossRef]
- Roberts, R.; Muzzy, J.D.; Faasa, G.S. Process of extracting lignin from lignocellulosic material using an aqueous organic solvent and an acid neutralizing agent. U.S. Patent 4,746,401, 24 May 1988. [Google Scholar]
- Wayman, M.; Lora, J.H. Aspen autohydrolysis. The effects of 2-naphthol and other aromatic compounds. Tappi J. 1978, 61, 55–57. [Google Scholar]
- Chua, M.G.S.; Wayman, M. Characterization of autohydrolysis aspen (P. tremuloides) lignins. Part 3. Infrared and ultraviolet studies of extracted autohydrolysis lignin. Can. J. Chem. 1979, 57, 2603–2611. [Google Scholar] [CrossRef]
- Martínez, C.; Sedano, M.; Munro, A.; López, P.; Pizzi, A. Evaluation of Some Synthetic Oligolignols as Adhesives: A Molecular Docking Study. J. Adhes. Sci. Technol. 2010, 24, 1739–1751. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; van Dam, J.E.G.; de Jong, E.; Gellerstedt, G.; Scott, E. Effect of periodate on lignin for wood adhesive application. Holzforschung 2011, 65, 155–162. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2012, 20, 829–846. [Google Scholar] [CrossRef]
- Pizzi, A. Natural Phenolic Adhesives I: Tannins. In Handbook of Adhesive Technology; Marcel-Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Vázquez, G.; González, J.; Freire, S.; Antorrena, G. Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour. Technol. 1997, 60, 191–198. [Google Scholar] [CrossRef]
- Gonçalves, A.R.; Benar, P. Hydroxymethylation and oxidation of organosolv lignins and utilization of the products. Bioresour. Technol. 2001, 79, 103–111. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, X.; Chen, X.; Wang, Z. An approach to improve the application of acid-insoluble lignin from rice hull in phenol–formaldehyde resin. Colloids Surf. A. Physicochem. Eng. Asp. 2011, 377, 284–289. [Google Scholar] [CrossRef]
- Lee, W.J.; Chang, K.C.; Tseng, I.M. Properties of phenol-formaldehyde resins prepared from phenol-liquefied lignin. J. Appl. Polym. Sci. 2012, 124, 4782–4788. [Google Scholar] [CrossRef]
- Olivares, M.; Guzmán, J.A.; Natho, A.; Saavedra, A. Kraft lignin utilization in adhesives. Wood Sci. Technol. 1988, 22, 157–165. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Cavdar, A.D.; Kalaycioglu, H.; Hiziroglu, S. Some of the properties of oriented strandboard manufactured using kraft lignin phenolic resin. J. Mater. Process. Technol. 2008, 202, 559–563. [Google Scholar] [CrossRef]
- Goundalkar, M. Investigating the Effect of Hot-Water Extraction on Phenolic Constituents of Selected Hardwoods; State University of New York, College of Environmental Science and Forestry: Syracuse, NY, USA, 2014. [Google Scholar]
- Zuckerstätter, G.; Weber, H.; Patt, R.; Sixta, S. Effect of autohydrolysis of Eucalyptus globulus wood on lignin structure. Part 1: Comparison of different lignin fractions formed during water prehydrolysis. Holzforschung 2008, 62, 645–652. [Google Scholar]
- Sjostrom, E. Wood Chemistry: Fundamentals and Applications, 2nd ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Dongre, P.; Driscoll, M.; Smith, J.; Amidon, T.; Bujanovic, B. Characterization of hot-water extracted lignin and its potential use in adhesive production. In Abstracts of Papers of the American Chemical Society; American Chemical Soc.: Washington, DC, USA, 2013; Volume 245. [Google Scholar]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. A Comprehensive Approach for Quantitative Lignin Characterization by NMR Spectroscopy. J. Agric. Food Chem. 2004, 52, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. Quantitative Characterization of a Hardwood Milled Wood Lignin by Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food Chem. 2005, 53, 9639–9649. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org. Biomol. Chem. 2010, 8, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Schoening, A.G.; Johansson, G. Absorptiometric Determination of Acid-Soluble Lignin in Semichemical Pulps and in Some Woods and Plants. Sven. Papp. 1965, 68, 607–613. [Google Scholar]
- Johansson, I.K.G. Method of producing synthetic resin from waste products. U.S. Patent 4,289,663, 15 September 1981. [Google Scholar]
- Chen, C.L.; Danielle, R. Characterization of lignin by 1H and 13C NMR spectroscopy. In Methods in Enzymology; Academic Press: New York, NY, USA, 1988; Volume 161, pp. 137–174. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dongre, P.; Driscoll, M.; Amidon, T.; Bujanovic, B. Lignin-Furfural Based Adhesives. Energies 2015, 8, 7897-7914. https://doi.org/10.3390/en8087897
Dongre P, Driscoll M, Amidon T, Bujanovic B. Lignin-Furfural Based Adhesives. Energies. 2015; 8(8):7897-7914. https://doi.org/10.3390/en8087897
Chicago/Turabian StyleDongre, Prajakta, Mark Driscoll, Thomas Amidon, and Biljana Bujanovic. 2015. "Lignin-Furfural Based Adhesives" Energies 8, no. 8: 7897-7914. https://doi.org/10.3390/en8087897