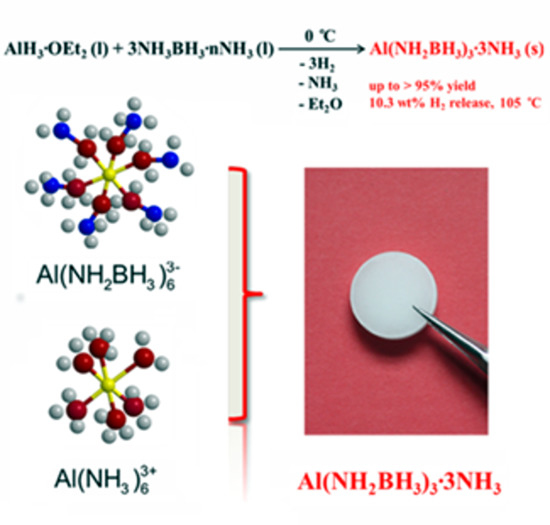
Efficient Synthesis of an Aluminum Amidoborane Ammoniate
Abstract
:1. Introduction
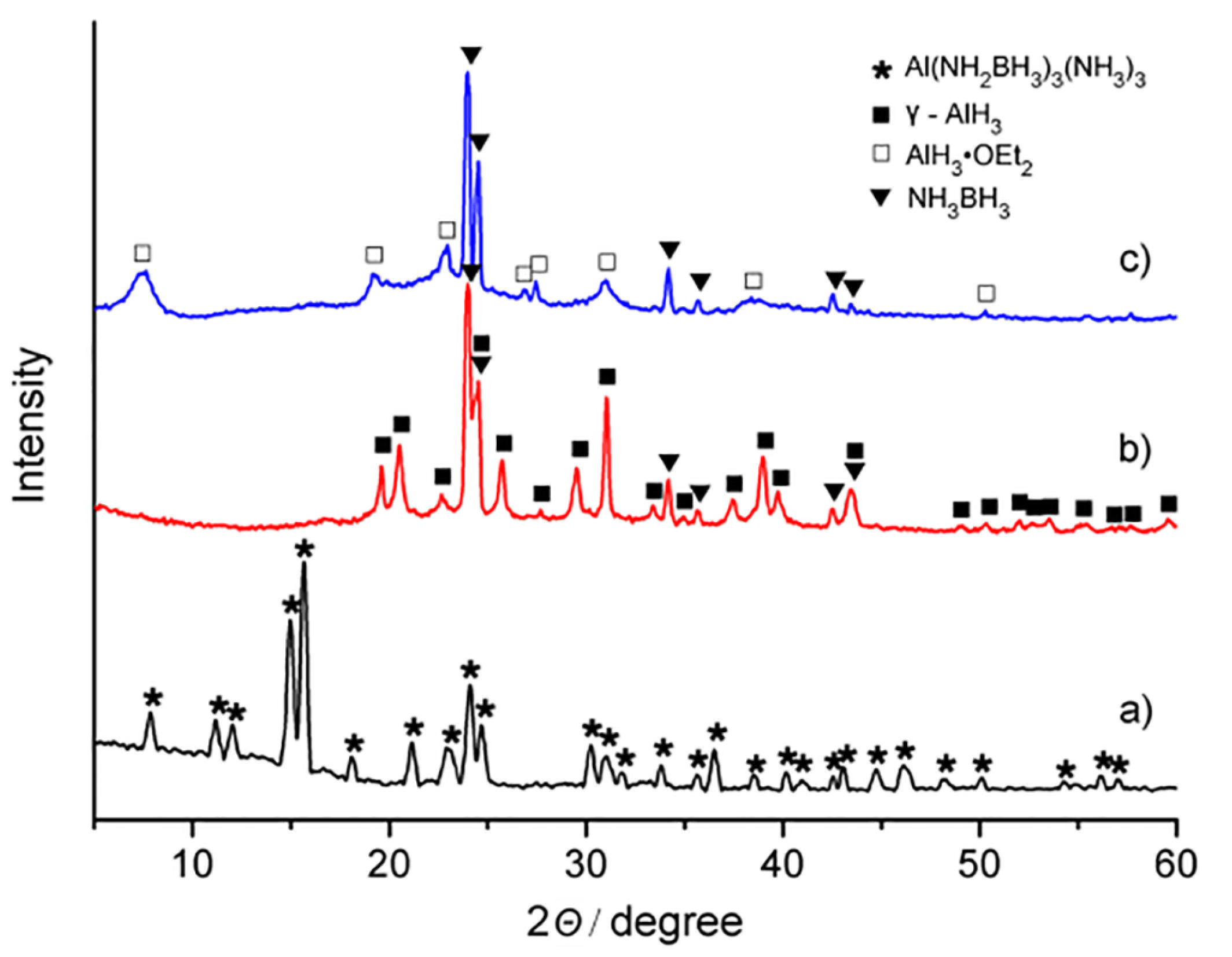
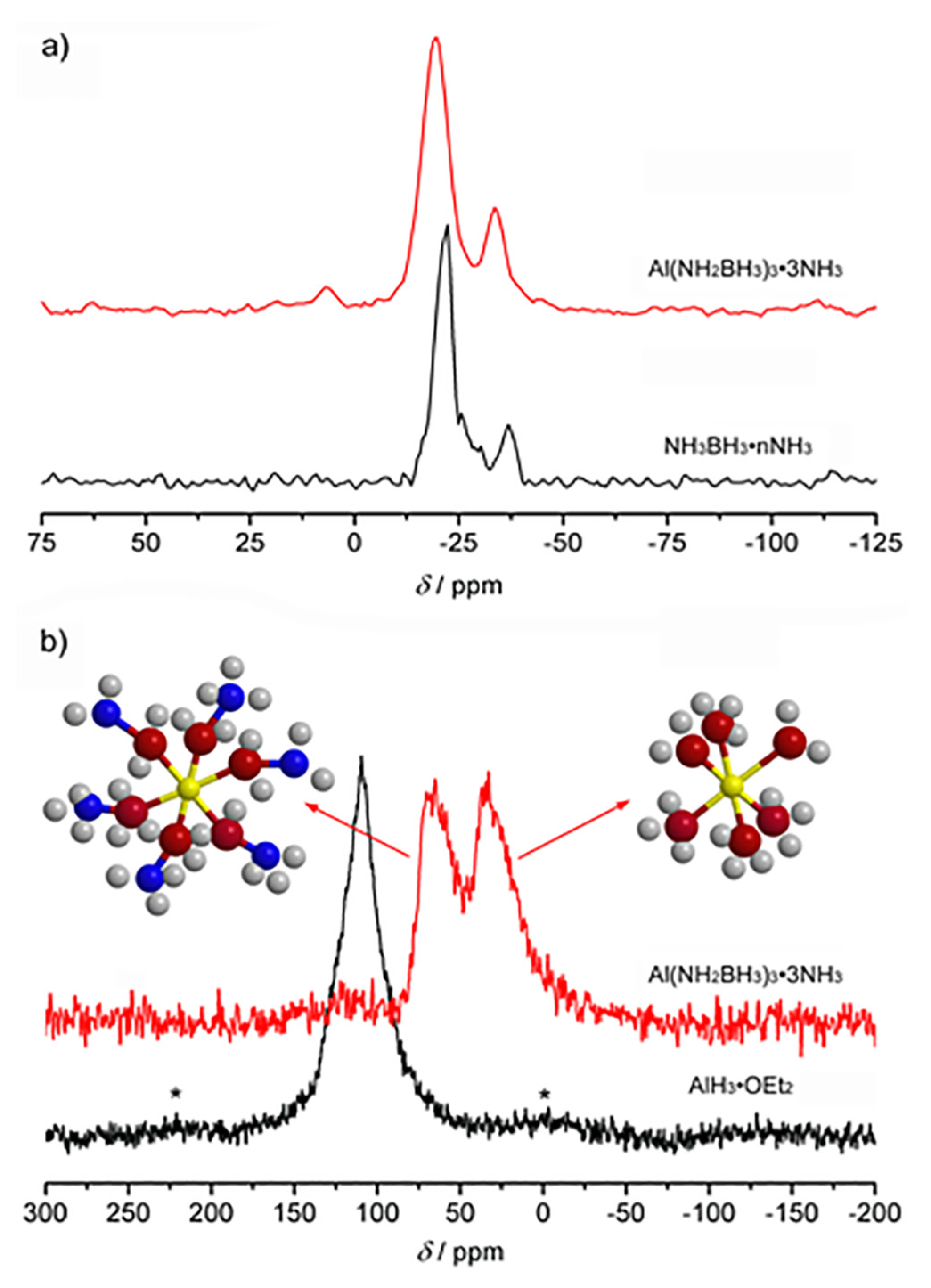
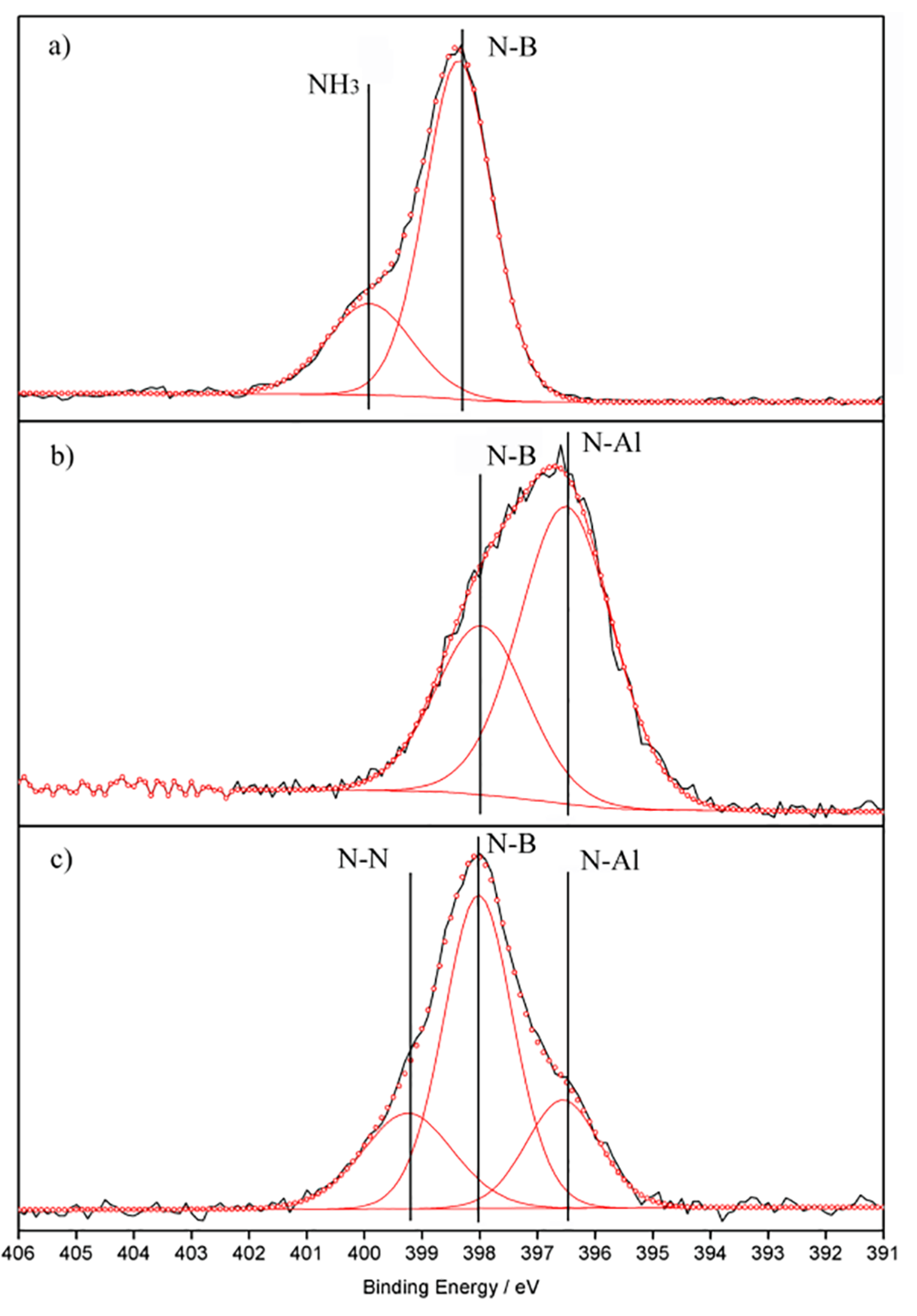
2. Results and Discussion

3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.S.; Chen, P.; Wu, G.; Xiong, Z. Development of amidoboranes for hydrogen storage. Chem. Commun. 2011, 47, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Wolverton, C. Crystal structures, phase stabilities, and hydrogen storage properties of metal amidoboranes. J. Phys. Chem. C 2012, 116, 14224–14231. [Google Scholar] [CrossRef]
- Zheng, X.L.; Wu, G.T.; Li, W.; Xiong, Z.T.; He, T.; Guo, J.P.; Chen, H.; Chen, P. Releasing 17.8 wt% H2 from lithium borohydride ammoniate. Energy Environ. Sci. 2011, 4, 3593–3600. [Google Scholar] [CrossRef]
- Guo, Y.H.; Wu, H.; Zhou, W.; Yu, X.B. Dehydrogenation tuning of ammine borohydrides using double-metal cations. J. Am. Chem. Soc. 2011, 133, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ley, M.B.; Lee, Y.-S.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jørgensen, J.E.; Besenbacher, F.; Jensen, T.R.; et al. Boron-nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef]
- Sutton, A.D.; Burrell, A.K.; Dixon, D.A.; Garner, E.B.; Gordon, J.C.; Nakagawa, T.; Ott, K.C.; Robinson, P.; Vasiliu, M. Regeneration of ammonia borane spent fuel by direct reaction with hydrazine and liquid ammonia. Science 2011, 331, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.W.; Tan, Y.B.; Chen, X.W.; Yu, X.B. Regenerable hydrogen storage in lithium amidoborane. Chem. Commun. 2012, 48, 9296–9298. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, Z.; Gong, Q.; Yu, X.; Beaumont, P.R.; Jensen, C.M. Phenyl introduced ammonium borohydride: Synthesis and reversible dehydrogenation properties. J. Mater. Chem. 2012, 22, 21017–21023. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Jalisatgi, S.S.; Safronov, A.V.; Lee, H.B.; Wu, J. Chemical Hydrogen Storage Using Polyhedral Borane Anions and Aluminum-Ammonia-Borane Complexes. Available online: http://www.osti.gov/scitech//servlets/purl/990217-xUxbgx/ (accessed on 28 June 2015).
- Harder, S.; Spielmann, J. Unprecedented reactivity of an aluminium hydride complex with ArNH2BH3: Nucleophilic substitution versus deprotonation. Chem. Commun. 2011, 47, 11945–11947. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Ketchum, D.R.; Hamilton, E.J.M.; Florian, P.A.; Vermillion, K.E.; Grandinetti, P.J.; Shore, S.G. Reactions of aluminum hydride derivatives with ammonia-borane: A new approach toward AlN/BN materials. Chem. Mater. 1996, 8, 2839–2842. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, X.; Sun, W.; Sun, D.; Yang, W. The hydrogen-enriched Al-B-N system as an advanced solid hydrogen-storage candidate. Angew. Chem. Int. Ed. 2011, 50, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Jiang, Y.X.; Xia, G.L.; Yu, X.B. Ammine aluminium borohydrides: An appealing system releasing over 12 wt% pure H2 under moderate temperature. Chem. Commun. 2012, 48, 4408–4410. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Tan, Y.; Chen, X.; Guo, Z.; Liu, H.; Yu, X. Mixed-metal (Li, Al) amidoborane: Synthesis and enhanced hydrogen storage properties. J. Mater. Chem. A 2013, 1, 1810–1820. [Google Scholar] [CrossRef]
- Xia, G.L.; Yu, X.B.; Guo, Y.H.; Wu, Z.; Yang, C.Z.; Liu, H.K.; Dou, S.X. Ammine lithium amidoborane Li(NH3)NH2BH3: A new coordination compound with favorable dehydrogenation characteristics. Chem. Eur. J. 2010, 16, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.D.; Wu, H.; Luo, J.H.; Zhou, W.; Wang, P. A simple and efficient approach to synthesize amidoborane ammoniates: Case study for Mg(NH2BH3)2(NH3)3 with unusual coordination structure. J. Mater. Chem. 2012, 22, 13174–13179. [Google Scholar] [CrossRef]
- Chua, Y.S.; Wu, H.; Zhou, W.; Udovic, T.J.; Wu, G.T.; Xiong, Z.T.; Wong, M.W.; Chen, P. Monoammoniate of calcium amidoborane: Synthesis, structure, and hydrogen-storage properties. Inorg. Chem. 2012, 51, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fang, H.C.; Li, Z.H.; Yu, X.B.; Fan, K.N. Liquefaction of solid-state BH3NH3 by gaseous NH3. Inorg. Chem. 2011, 50, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Graetz, J.; Chaudhuri, S.; Wegrzyn, J.; Celebi, Y.; Johnson, J.R.; Zhou, W.; Reilly, J.J. Direct and reversible synthesis of AlH3-triethylenediamine from Al and H2. J. Phys. Chem. C 2007, 111, 19148–19152. [Google Scholar] [CrossRef]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and properties of aluminum-hydride. J. Am. Chem. Soc. 1976, 98, 2450–2453. [Google Scholar] [CrossRef]
- Giannasi, A.; Colognesi, D.; Fichtner, M.; Rohm, E.; Ulivi, L.; Ziparo, C.; Zoppi, M. Temperature behavior of the AlH3 polymorph by in situ investigation using high resolution raman scattering. J. Phys. Chem. A 2011, 115, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Humphries, T.D.; Munroe, K.T.; Decken, A.; McGrady, G.S. Lewis base complexes of AlH3: Prediction of preferred structure and stoichiometry. Dalton Trans. 2013, 42, 6965–6978. [Google Scholar] [CrossRef] [PubMed]
- Stowe, A.C.; Shaw, W.J.; Linehan, J.C.; Schmid, B.; Autrey, T. In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: Mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material. Phys. Chem. Chem. Phys. 2007, 9, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Zhang, Y.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Solid state NMR study on the thermal decomposition pathway of sodium amidoborane NaNH2BH3. J. Mater. Chem. 2011, 21, 2609–2615. [Google Scholar] [CrossRef]
- Shimoda, K.; Doi, K.; Nakagawa, T.; Zhan, Y.; Miyaoka, H.; Ichikawa, T.; Tansho, M.; Shimizu, T.; Burrell, A.K.; Kojima, Y. Comparative study of structural changes in NH3BH3, LiNH2BH3, and KNH2BH3 during dehydrogenation process. J. Phys. Chem. C 2012, 116, 5957–5964. [Google Scholar] [CrossRef]
- Semenenko, K.N.; Shilkin, S.P.; Polyakova, V.B. Vibrational spectra and structure of the di- and tetraammoniate of aluminum borohydride. Bull. Acad. Sci. USSR Div. Chem. Sc. 1978, 27, 859–864. [Google Scholar] [CrossRef]
- Chua, Y.S.; Wu, G.T.; Xiong, Z.T.; Karkamkar, A.; Guo, J.P.; Jian, M.X.; Wong, M.W.; Autrey, T.; Chen, P. Synthesis, structure and dehydrogenation of magnesium amidoborane monoammoniate. Chem. Commun. 2010, 46, 5752–5754. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.S.; Wu, G.T.; Xiong, Z.T.; He, T.; Chen, P. Calcium amidoborane ammoniate-synthesis, structure, and hydrogen storage properties. Chem. Mater. 2009, 21, 4899–4904. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.; Rupprecht, G. Normal modes in hexagonal boron nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Marchetti, P.S.; Kwon, D.K.; Schmidt, W.R.; Interrante, L.V.; Maciel, G.E. High-field B11 magic angle spinning NMR characterization of boron nitrides. Chem. Mater. 1991, 3, 482–486. [Google Scholar] [CrossRef]
- Jeschke, G.; Hoffbauer, W.; Jansen, M. A comprehensive NMR study of cubic and hexagonal boron nitride. Solid State Nucl. Magn. Reson. 1998, 12, 1–7. [Google Scholar] [CrossRef]
- Humphries, T.D.; Munroe, K.T.; DeWinter, T.M.; Jensen, C.M.; McGrady, G.S. NMR spectroscopic and thermodynamic studies of the etherate and the α, α’, and γ phases of AlH3. Int. J. Hydrog. Energy 2013, 38, 4577–4586. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Beaumont, P.R.; Humphries, T.D.; Jensen, C.M.; Li, X. Efficient Synthesis of an Aluminum Amidoborane Ammoniate. Energies 2015, 8, 9107-9116. https://doi.org/10.3390/en8099107
Yang J, Beaumont PR, Humphries TD, Jensen CM, Li X. Efficient Synthesis of an Aluminum Amidoborane Ammoniate. Energies. 2015; 8(9):9107-9116. https://doi.org/10.3390/en8099107
Chicago/Turabian StyleYang, Junzhi, Paul R. Beaumont, Terry D. Humphries, Craig M. Jensen, and Xingguo Li. 2015. "Efficient Synthesis of an Aluminum Amidoborane Ammoniate" Energies 8, no. 9: 9107-9116. https://doi.org/10.3390/en8099107