Modeling of a Pouch Lithium Ion Battery Using a Distributed Parameter Equivalent Circuit for Internal Non-Uniformity Analysis
Abstract
:1. Introduction
2. Experiments
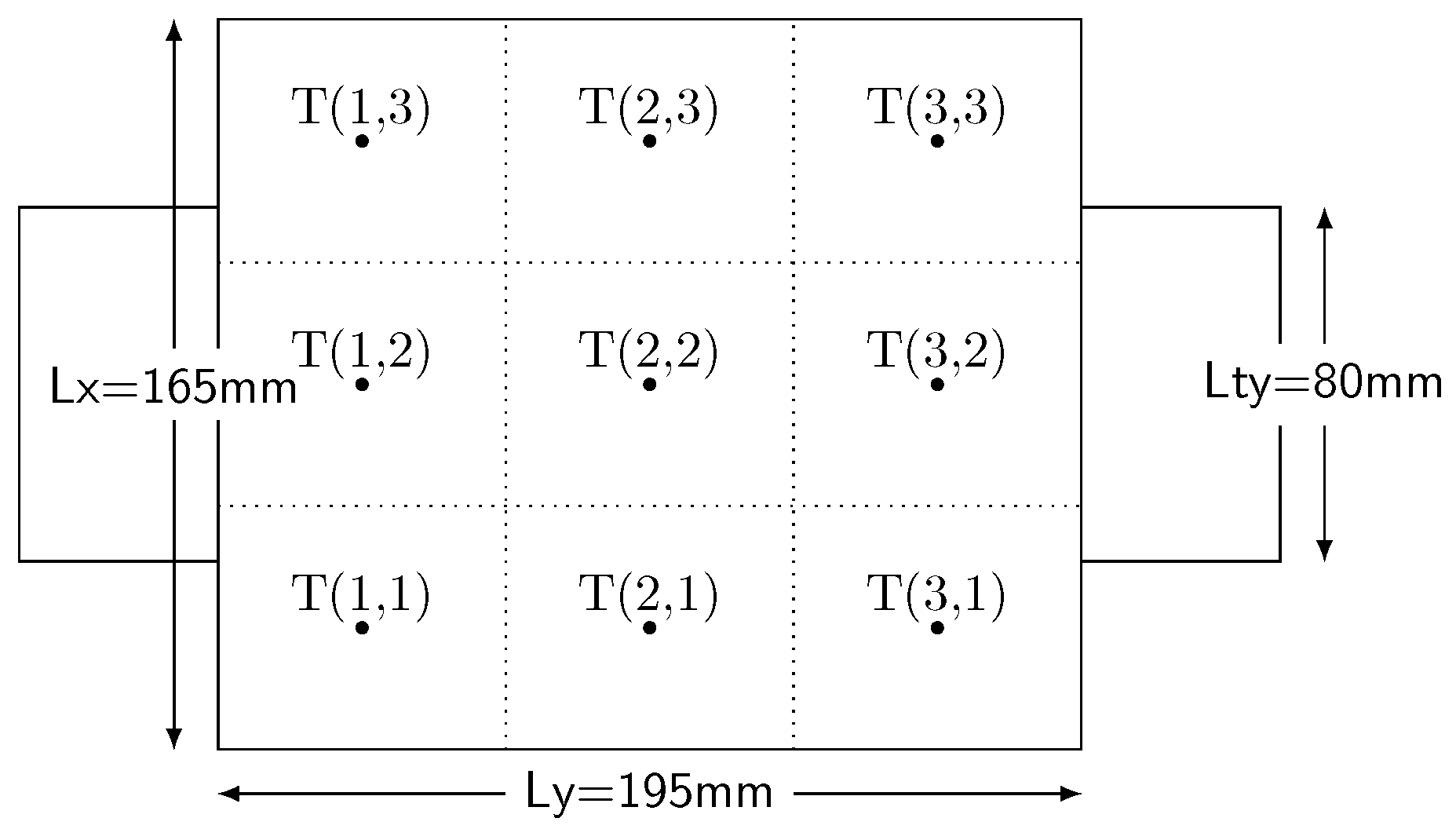
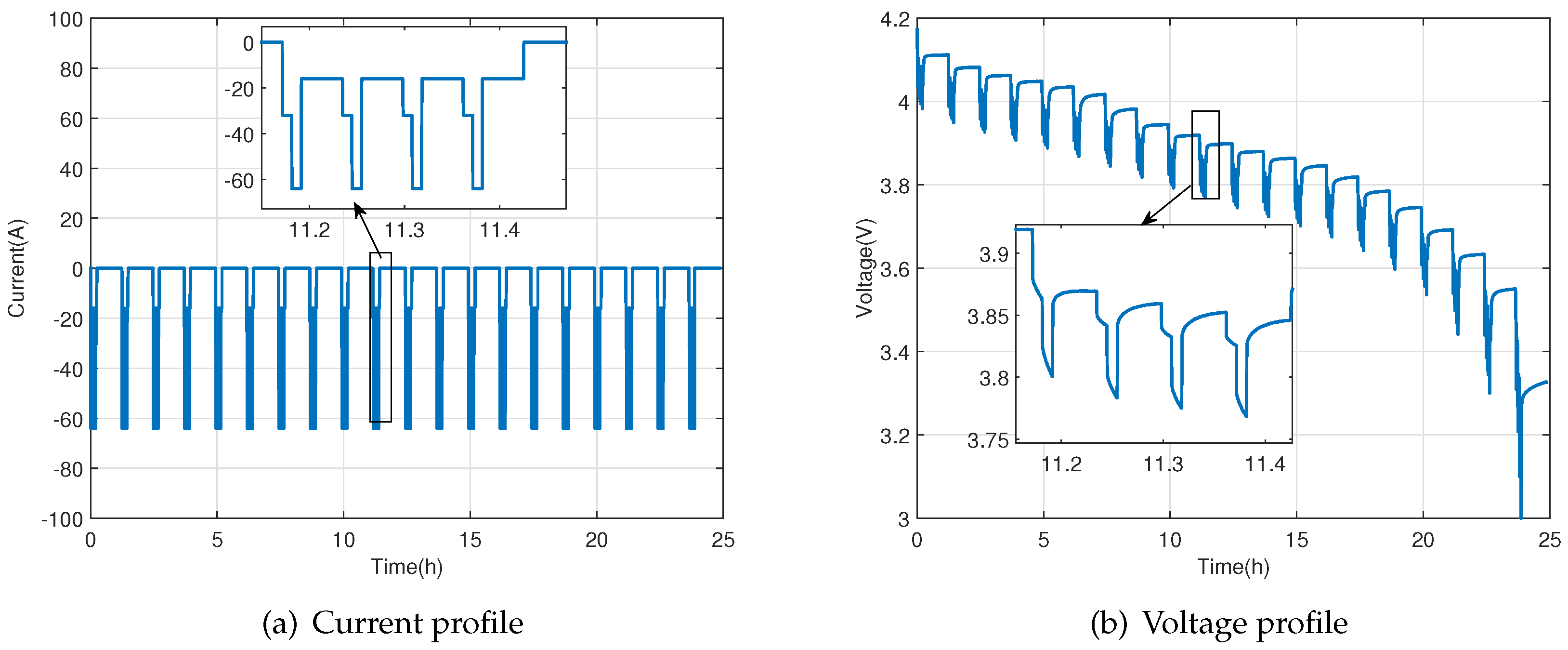
2.1. Parameters Identification Experiments
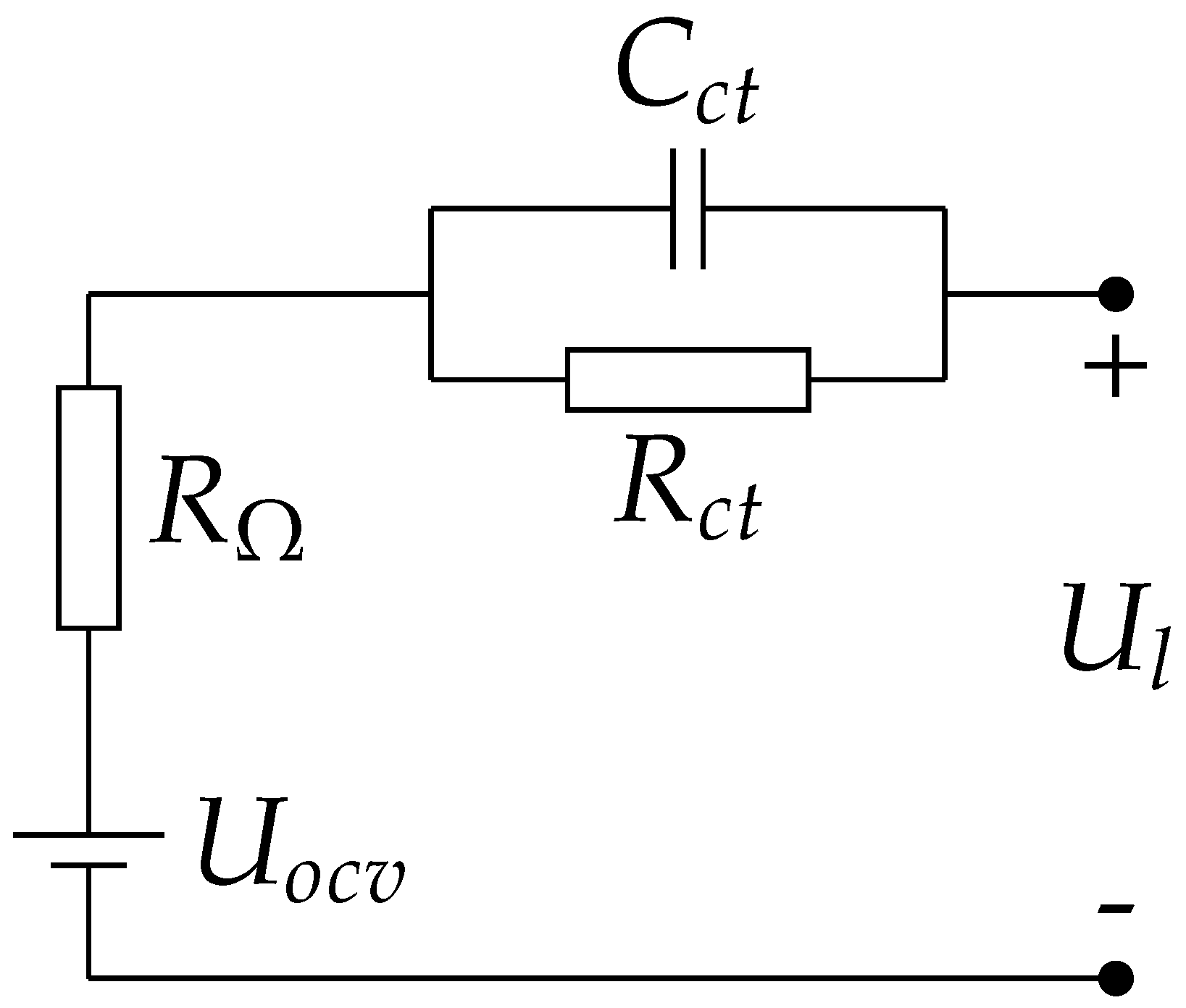
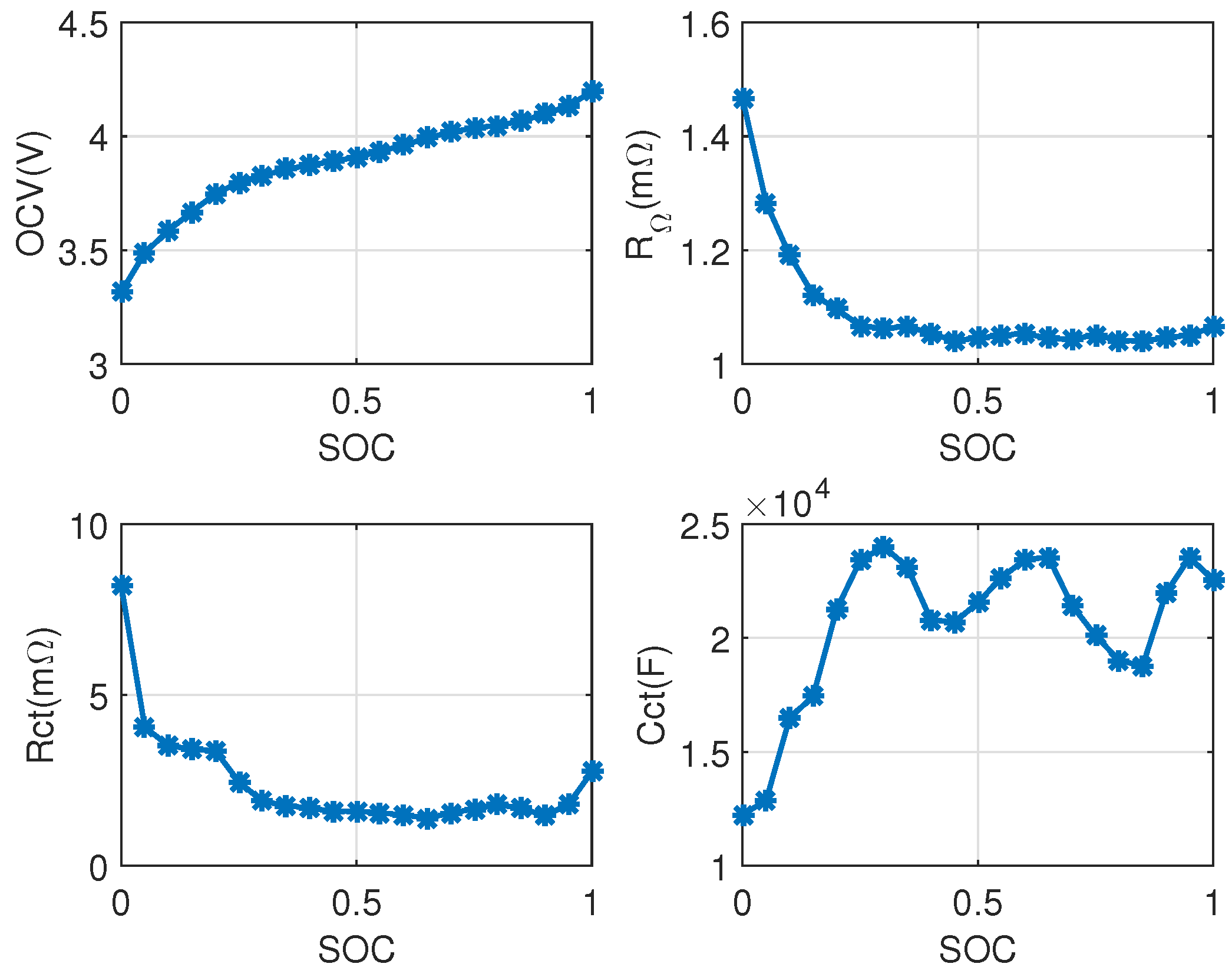
2.1.1. Parameters Identification of the Lumped First-Order Resistor-Capacitor (RC) Model
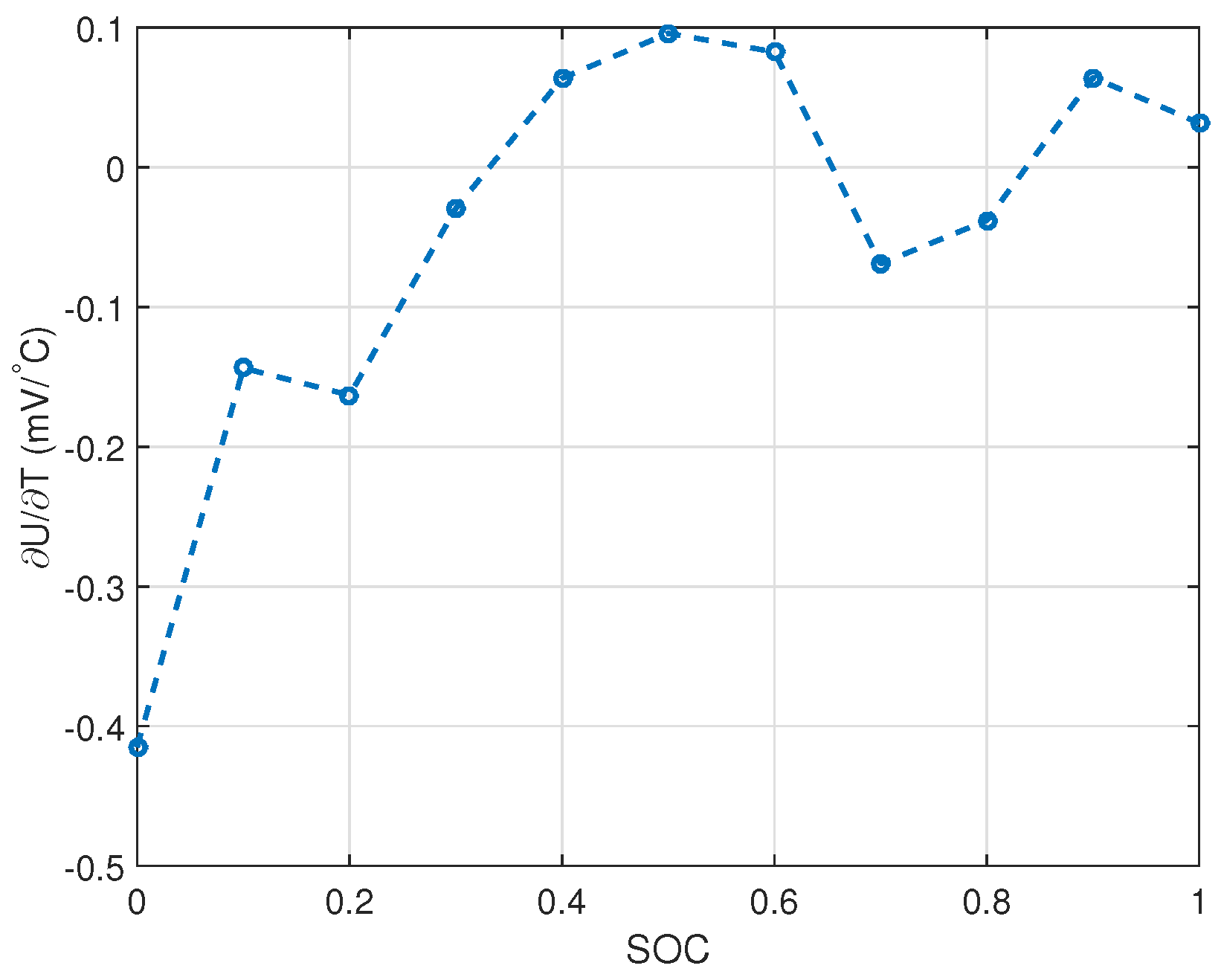
2.1.2. Entropy Change Measurement
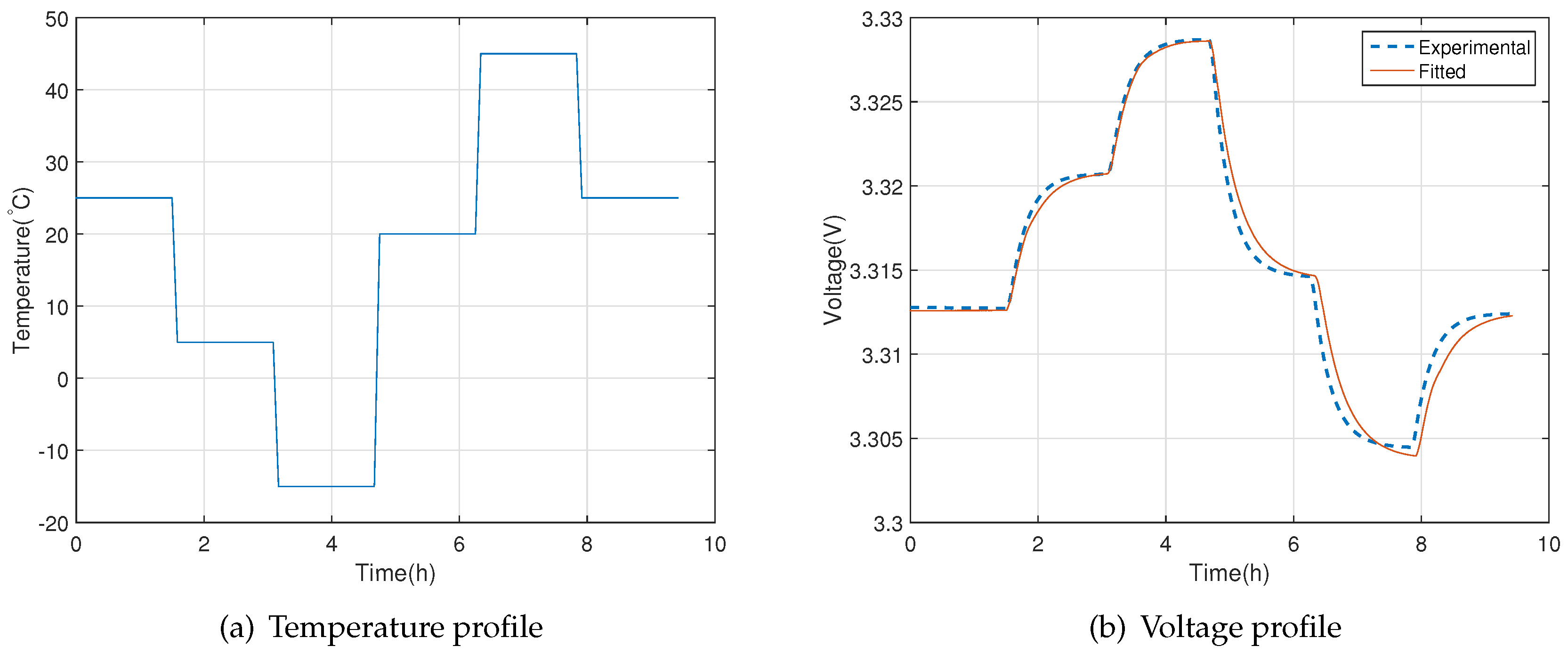
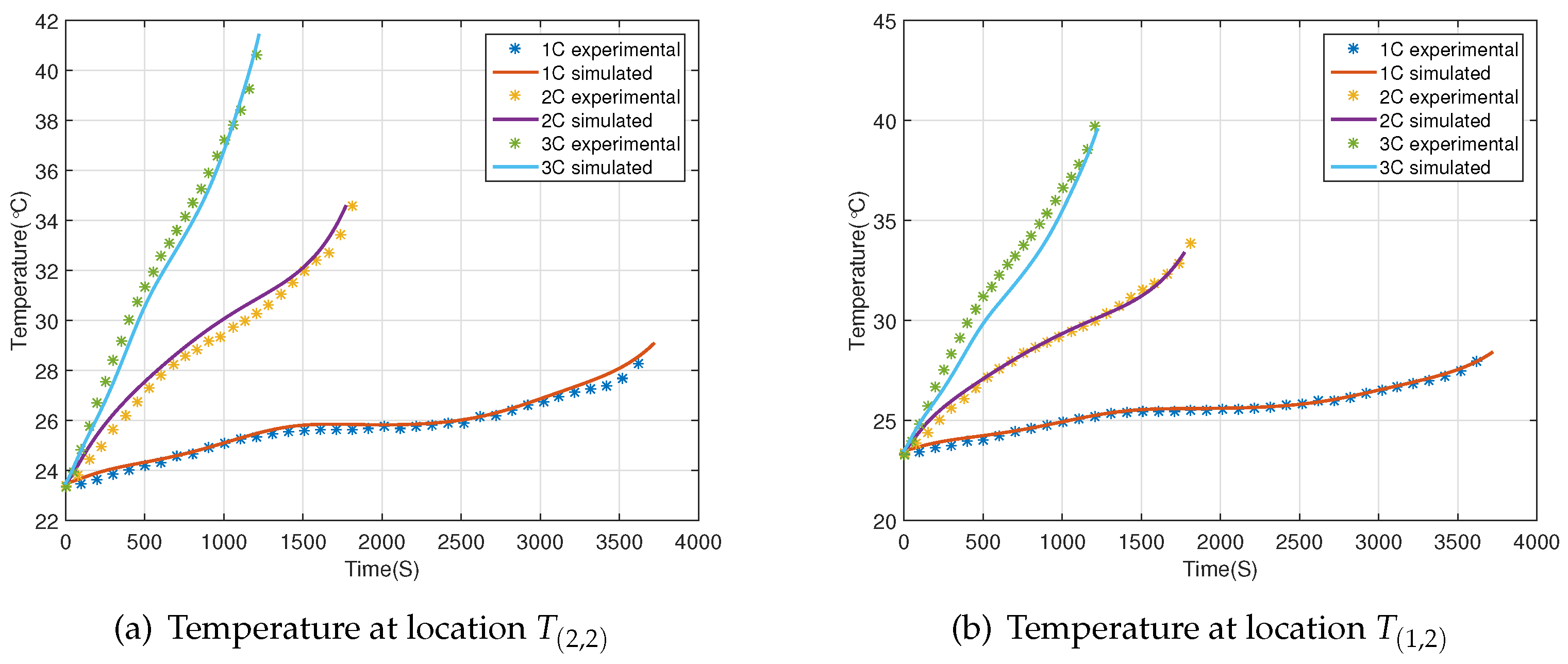
2.2. Validation Experiments
3. Modeling and Validation
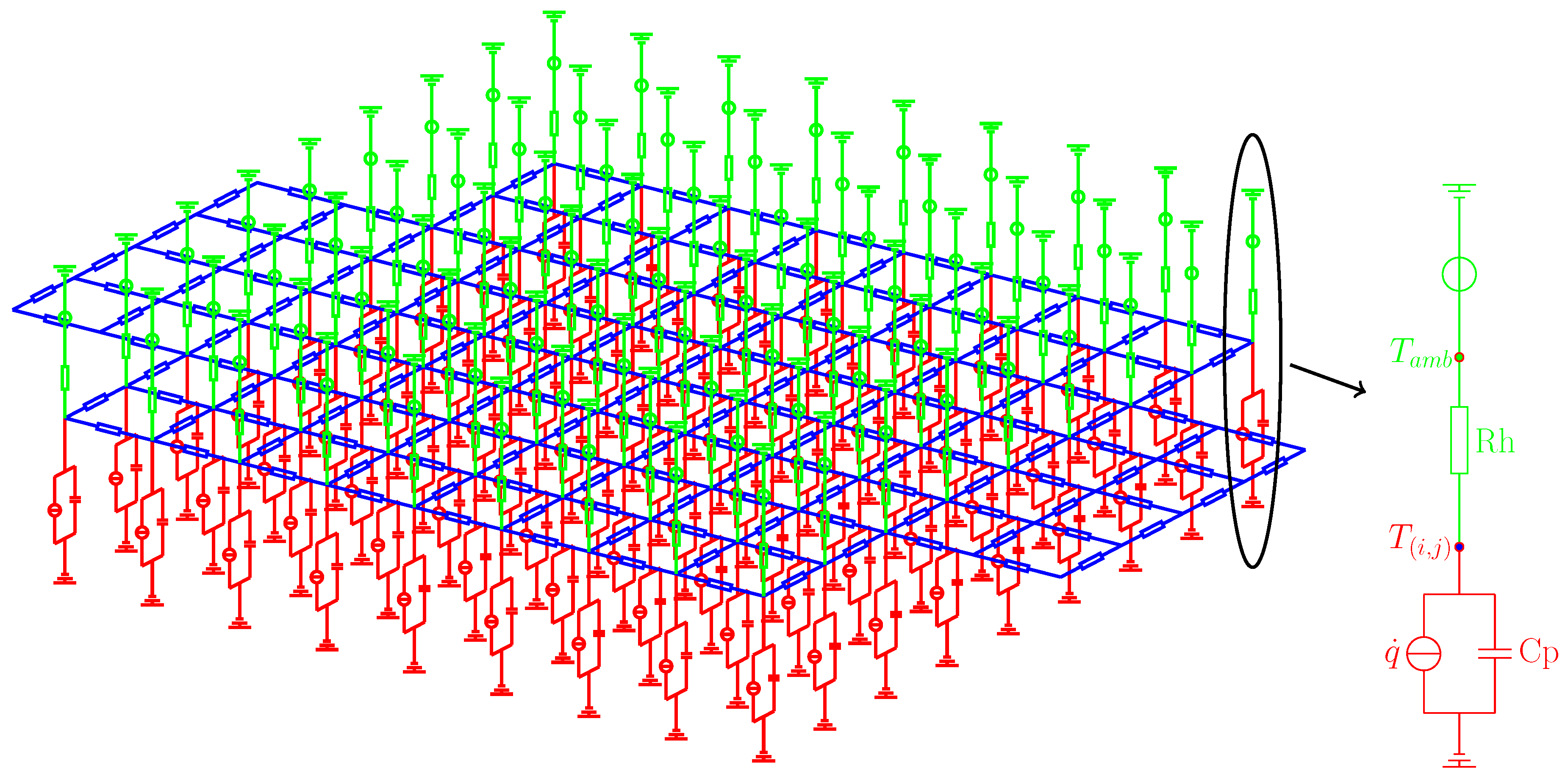
3.1. Electrical Model
3.2. Thermal Model
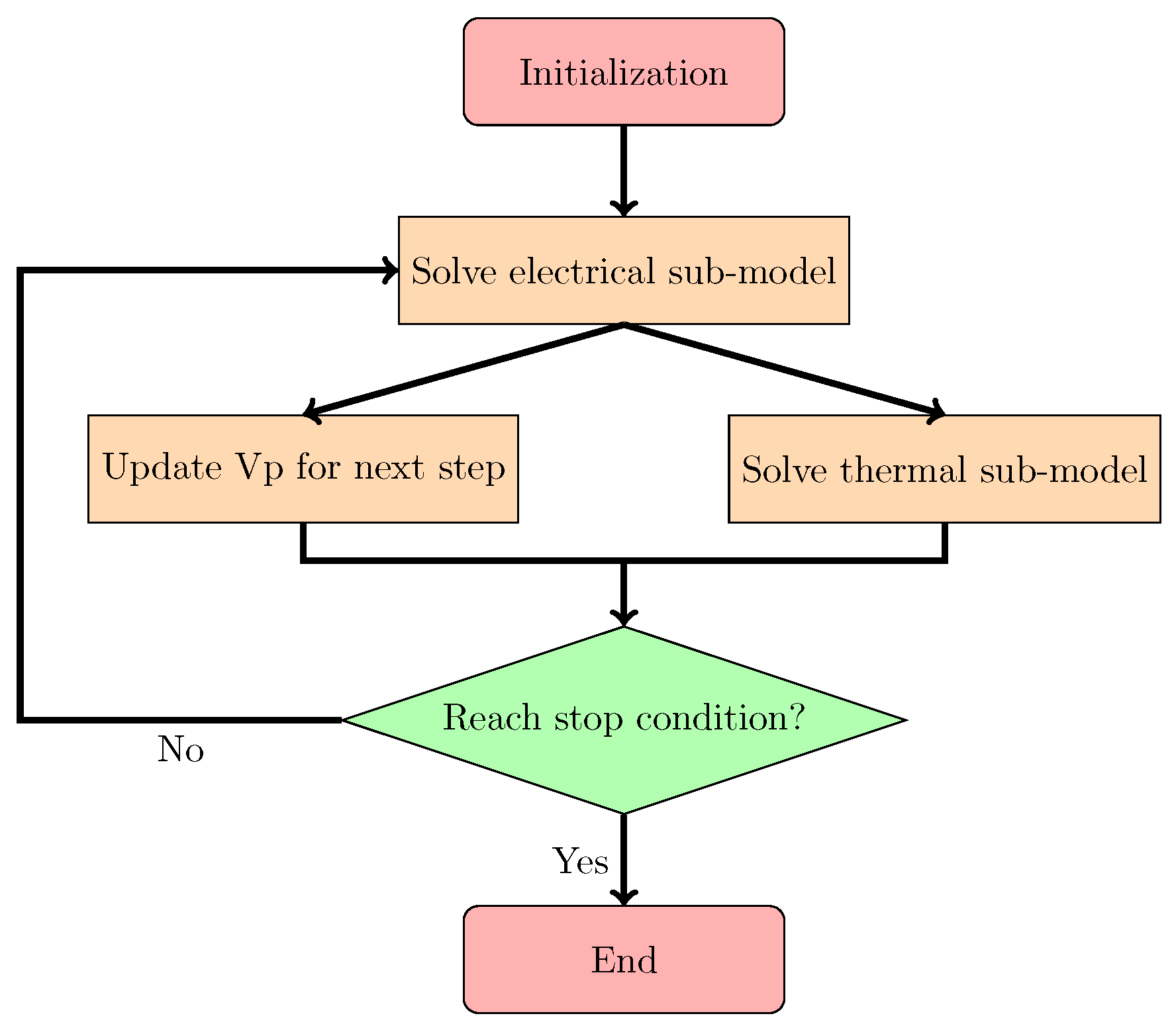
3.3. Solution
4. Results and Discussion
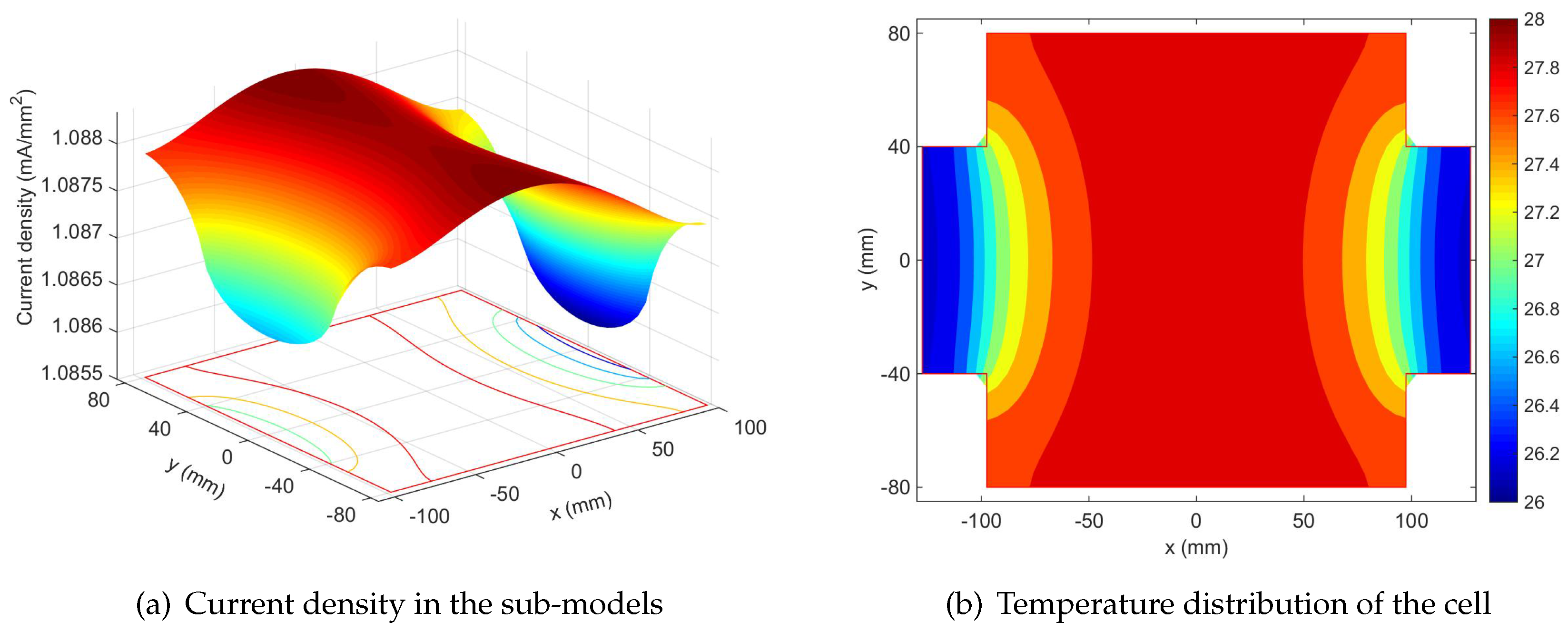
4.1. Non-Uniformity Distribution at the Initial State
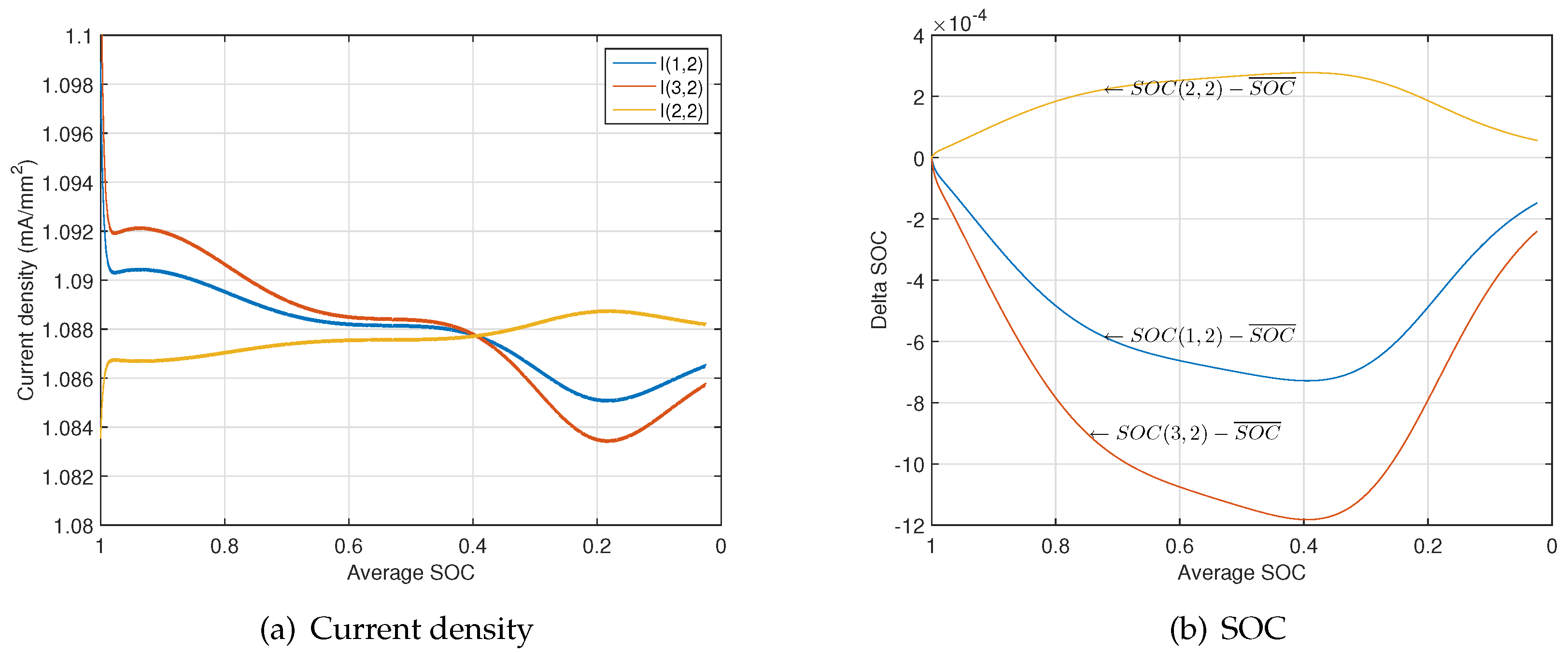
4.2. Non-Uniformity Evolution During Discharge
4.3. Limitation of Technique
5. Conclusions
- The initial non-uniformity of current density inside a 35-Ah NCM pouch cell is caused by the electrode foil resistance and relatively narrow tab.
- The current flow through the sub-models close to tabs is higher than that at the center at the beginning of discharge, because the potential drops in positive and negative foils.
- A current cross point exists during the constant discharge process. The local SOC difference increases with the reduction of the average SOC until the average SOC arrives at that point and then decreases until the average SOC arrives at zero.
- The current flow through the sub-models close to tabs becomes lower than that at the center at the end of discharge due to the accumulation of the local SOC difference, rapid OCV drop and rapid resistance increase.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Luo, W.; Lyu, C.; Wang, L.; Zhang, L. A new extension of physics-based single particle model for higher charge–discharge rates. J. Power Sources 2013, 241, 295–310. [Google Scholar] [CrossRef]
- Kim, G.H.; Smith, K.; Lee, K.J.; Santhanagopalan, S.; Pesaran, A. Multi-domain modeling of lithium-ion batteries encompassing multi-physics in varied length scales. J. Electrochem. Soc. 2011, 158, A955–A969. [Google Scholar] [CrossRef]
- Lee, K.J.; Smith, K.; Pesaran, A.; Kim, G.H. Three dimensional thermal-, electrical-, and electrochemical-coupled model for cylindrical wound large format lithium-ion batteries. J. Power Sources 2013, 241, 20–32. [Google Scholar] [CrossRef]
- Huo, W.; He, H.; Sun, F. Electrochemical–thermal modeling for a ternary lithium ion battery during discharging and driving cycle testing. RSC Adv. 2015, 5, 57599–57607. [Google Scholar] [CrossRef]
- Mazumder, S.; Lu, J. Faster-Than-Real-Time Simulation of Lithium Ion Batteries with Full Spatial and Temporal Resolution. Int. J. Electrochem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Pals, C.R.; Newman, J. Thermal modeling of the lithium/polymer battery I. Discharge behavior of a single cell. J. Electrochem. Soc. 1995, 142, 3274–3281. [Google Scholar] [CrossRef]
- Anwar, S.; Zou, C.; Manzie, C. Distributed thermal-electrochemical modeling of a lithium-ion battery to study the effect of high charging rates. IFAC Proc. Vol. 2014, 47, 6258–6263. [Google Scholar] [CrossRef]
- Kwon, K.H.; Shin, C.B.; Kang, T.H.; Kim, C.S. A two-dimensional modeling of a lithium-polymer battery. J. Power Sources 2006, 163, 151–157. [Google Scholar] [CrossRef]
- Kim, U.S.; Shin, C.B.; Kim, C.S. Effect of electrode configuration on the thermal behavior of a lithium-polymer battery. J. Power Sources 2008, 180, 909–916. [Google Scholar] [CrossRef]
- Kim, U.S.; Shin, C.B.; Kim, C.S. Modeling for the scale-up of a lithium-ion polymer battery. J. Power Sources 2009, 189, 841–846. [Google Scholar] [CrossRef]
- Kohneh, M.M.M.; Samadani, E.; Farhad, S.; Fraser, R.; Fowler, M. Three-Dimensional Electrochemical Analysis of a Graphite/LiFePO4 Li-Ion Cell to Improve Its Durability; SAE International: Warrendale, PA, USA, April 2015. [Google Scholar]
- Liu, B.; Yin, S.; Xu, J. Integrated computation model of lithium-ion battery subject to nail penetration. Appl. Energy 2016, 183, 278–289. [Google Scholar] [CrossRef]
- Samba, A. Battery Electrical Vehicles-Analysis of Thermal Modelling and Thermal Management. Ph.D. Thesis, LUSAC (Laboratoire Universitaire des Sciences Appliquées de Cherbourg), Université de caen Basse Normandie; MOBI (the Mobility, Logistics and Automotive Technology Research Centre), Vrije Universiteit Brussel, Brussels, Belgium, 2015. [Google Scholar]
- Mastali, M.; Samadani, E.; Farhad, S.; Fraser, R.; Fowler, M. Three-dimensional Multi-Particle Electrochemical Model of LiFePO4 Cells based on a Resistor Network Methodology. Electrochim. Acta 2016, 190, 574–587. [Google Scholar] [CrossRef]
- Tong, S.; Klein, M.P.; Park, J.W. Comprehensive Battery Equivalent Circuit Based Model for Battery Management Application. In Proceedings of the ASME 2013 Dynamic Systems and Control Conference, American Society of Mechanical Engineers, Stanford, CA, USA, 21–23 October 2013. V001T05A005.
- Sun, F.; Xiong, R.; He, H. A systematic state-of-charge estimation framework for multi-cell battery pack in electric vehicles using bias correction technique. Appl. Energy 2016, 162, 1399–1409. [Google Scholar] [CrossRef]
- Yazdanpour, M.; Taheri, P.; Mansouri, A.; Schweitzer, B. A circuit-based approach for electro-thermal modeling of lithium-ion batteries. In Proceedings of the 2016 32nd Thermal Measurement, Modeling & Management Symposium (SEMI-THERM), San Jose, CA, USA, 14–17 March 2016.
- Knap, V.; Stroe, D.I.; Teodorescu, R.; Swierczynski, M.; Stanciu, T. Electrical circuit models for performance modeling of Lithium-Sulfur batteries. In Proceedings of the 2015 IEEE Energy Conversion Congress and Exposition (ECCE), Montreal, QC, Canada, 20–24 September 2015.
- Blanco, C.; Sanchez, L.; Gonzalez, M.; Anton, J.C.; Garcia, V.; Viera, J.C. An Equivalent Circuit Model with Variable Effective Capacity for Batteries. IEEE Trans. Veh. Technol. 2014, 63, 3592–3599. [Google Scholar] [CrossRef]
- Fok, C.W.E. Simulation of Lithium-Ion Batteries Based on Pulsed Current Characterization. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar]
- Chen, X.K.; Sun, D. Modeling and state of charge estimation of lithium-ion battery. Adv. Manuf. 2015, 3, 202–211. [Google Scholar] [CrossRef]
- Xiong, R.; Sun, F.; Gong, X.; Gao, C. A data-driven based adaptive state of charge estimator of lithium-ion polymer battery used in electric vehicles. Appl. Energy 2014, 113, 1421–1433. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, R.; Cao, J. Battery durability and longevity based power management for plug-in hybrid electric vehicle with hybrid energy storage system. Appl. Energy 2016, 179, 316–328. [Google Scholar] [CrossRef]
- Xiong, R.; Sun, F.; Chen, Z.; He, H. A data-driven multi-scale extended Kalman filtering based parameter and state estimation approach of lithium-ion polymer battery in electric vehicles. Appl. Energy 2014, 113, 463–476. [Google Scholar] [CrossRef]
- Sun, F.; Xiong, R. A novel dual-scale cell state-of-charge estimation approach for series-connected battery pack used in electric vehicles. J. Power Sources 2015, 274, 582–594. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Siegel, J.B.; Stefanopoulou, A.G.; Li, Y.; Anderson, R.D.; Ding, Y.; Castanier, M.P. Online parameterization of lumped thermal dynamics in cylindrical lithium ion batteries for core temperature estimation and health monitoring. IEEE Trans. Control Syst. Technol. 2013, 21, 1745–1755. [Google Scholar]
- Forgez, C.; Do, D.V.; Friedrich, G.; Morcrette, M.; Delacourt, C. Thermal modeling of a cylindrical LiFePO4/graphite lithium-ion battery. J. Power Sources 2010, 195, 2961–2968. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Bohlen, O.; Roscher, M.A.; Bäker, B. Current density and state of charge inhomogeneities in Li-ion battery cells with LiFePO4 as cathode material due to temperature gradients. J. Power Sources 2011, 196, 4769–4778. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, J.; Wang, Z.; Duan, Y.; Zhang, Y.; Shi, W. Research on Distribution Parameters Equivalent-Circuit Model of Power Lithium-Ion Batteries. Trans. China Electrotechn. Soc. 2013, 7, 025. [Google Scholar]
- Zhang, G.; Shaffer, C.E.; Wang, C.Y.; Rahn, C.D. In-situ measurement of current distribution in a Li-Ion cell. J. Electrochem. Soc. 2013, 160, A610–A615. [Google Scholar] [CrossRef]
- Zhang, G.; Shaffer, C.E.; Wang, C.Y.; Rahn, C.D. Effects of non-uniform current distribution on energy density of Li-ion cells. J. Electrochem. Soc. 2013, 160, A2299–A2305. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Wu, B.; Huang, J.; Nie, Z.; Sun, Y.; An, F.; Wu, N. Examining temporal and spatial variations of internal temperature in large-format laminated battery with embedded thermocouples. J. Power Sources 2013, 241, 536–553. [Google Scholar] [CrossRef]
- Huria, T.; Ceraolo, M.; Gazzarri, J.; Jackey, R. High fidelity electrical model with thermal dependence for characterization and simulation of high power lithium battery cells. In Proceedings of the 2012 IEEE International Electric Vehicle Conference (IEVC), Greenville, SC, USA, 4–8 March 2012.
- Ceraolo, M.; Lutzemberger, G.; Huria, T. Experimentally-determined Models for High-power Lithium Batteries; SAE International: Warrendale, PA, USA, April 2011. [Google Scholar]
- Xiao, M.; Choe, S.Y. Dynamic modeling and analysis of a pouch type LiMn2O4/Carbon high power Li-polymer battery based on electrochemical-thermal principles. J. Power Sources 2012, 218, 357–367. [Google Scholar] [CrossRef]
- Samba, A.; Omar, N.; Gualous, H.; Capron, O.; Van den Bossche, P.; Van Mierlo, J. Impact of Tab Location on Large Format Lithium-Ion Pouch Cell Based on Fully Coupled Tree-Dimensional Electrochemical-Thermal Modeling. Electrochim. Acta 2014, 147, 319–329. [Google Scholar] [CrossRef]
- Fu, R.; Xiao, M.; Choe, S.Y. Modeling, validation and analysis of mechanical stress generation and dimension changes of a pouch type high power Li-ion battery. J. Power Sources 2013, 224, 211–224. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Peng, H. A comparative study of equivalent circuit models for Li-ion batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Chen, S.; Wan, C.; Wang, Y. Thermal analysis of lithium-ion batteries. J. Power Sources 2005, 140, 111–124. [Google Scholar] [CrossRef]





| Component | Thickness | Heat Conductivity Coefficient | Specific Heat Capacity | Electrical Resistivity |
|---|---|---|---|---|
| (μm) | (w·m·k) | (J·kg·k) | (Ω·m) | |
| Positive foil | 20 | 238 | 903 | |
| Positive material | 160 | 3.9 | 839 | — |
| Separator | 40 | 0.33 | 1978 | — |
| Negative material | 110 | 3.3 | 1064 | — |
| Negative foil | 10 | 398 | 385 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Jiang, J.; Li, X.; Wang, Z.; Zhang, W. Modeling of a Pouch Lithium Ion Battery Using a Distributed Parameter Equivalent Circuit for Internal Non-Uniformity Analysis. Energies 2016, 9, 865. https://doi.org/10.3390/en9110865
Chen D, Jiang J, Li X, Wang Z, Zhang W. Modeling of a Pouch Lithium Ion Battery Using a Distributed Parameter Equivalent Circuit for Internal Non-Uniformity Analysis. Energies. 2016; 9(11):865. https://doi.org/10.3390/en9110865
Chicago/Turabian StyleChen, Dafen, Jiuchun Jiang, Xue Li, Zhanguo Wang, and Weige Zhang. 2016. "Modeling of a Pouch Lithium Ion Battery Using a Distributed Parameter Equivalent Circuit for Internal Non-Uniformity Analysis" Energies 9, no. 11: 865. https://doi.org/10.3390/en9110865





