Effects of Biochar Amendment on Chloropicrin Adsorption and Degradation in Soil
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Composition and Structure of Biochar
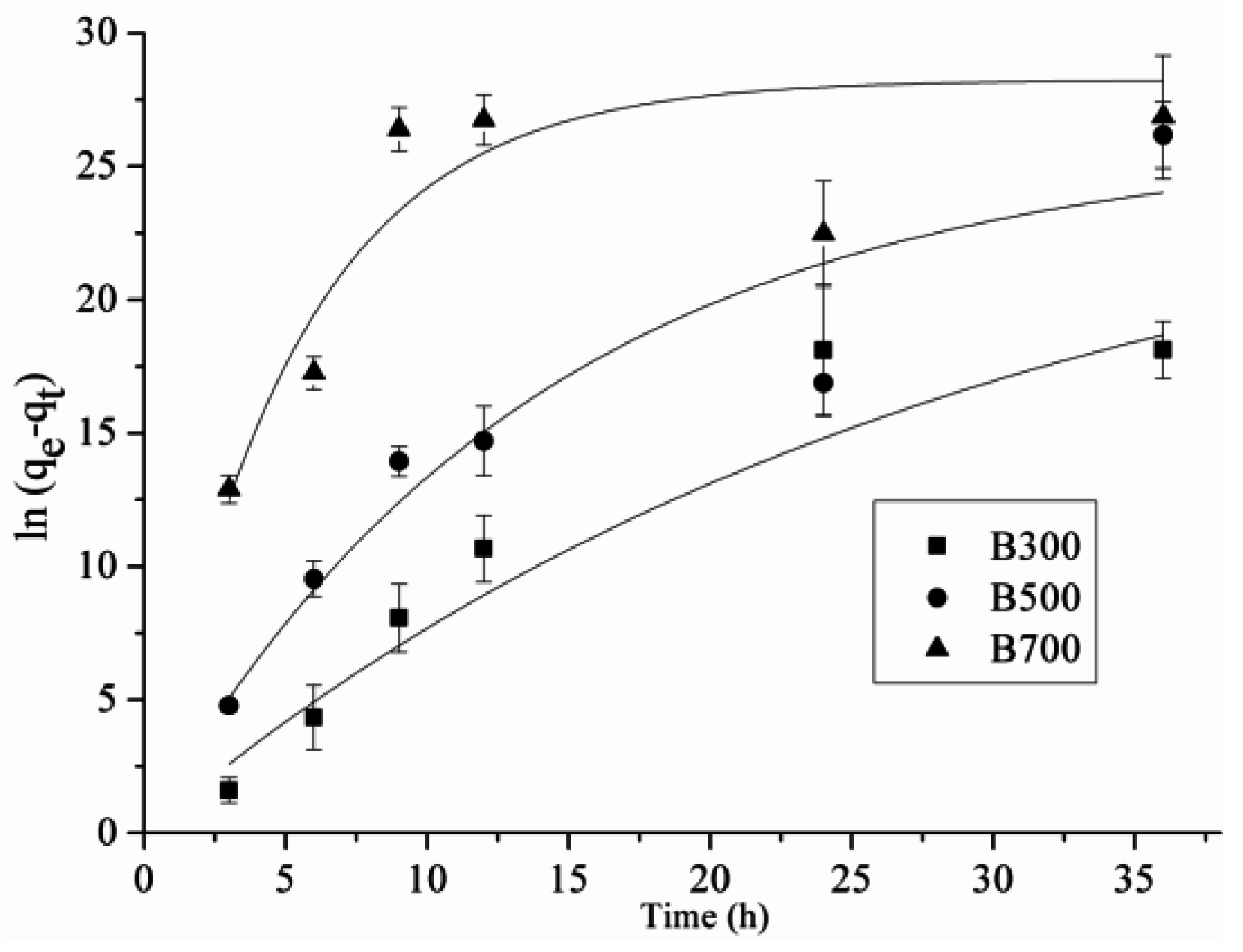
2.2. Adsorption Kinetics
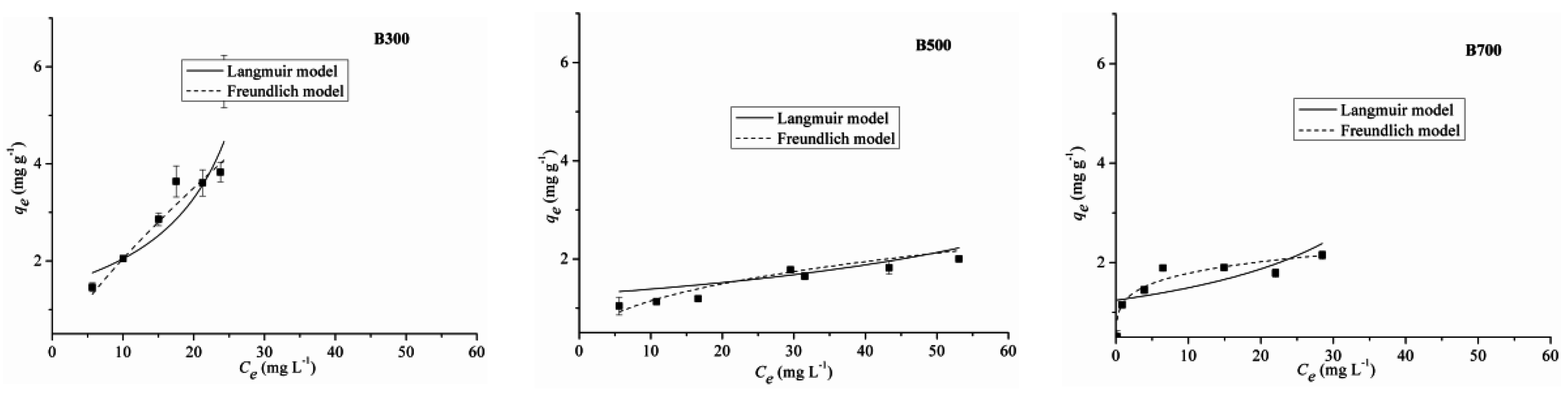
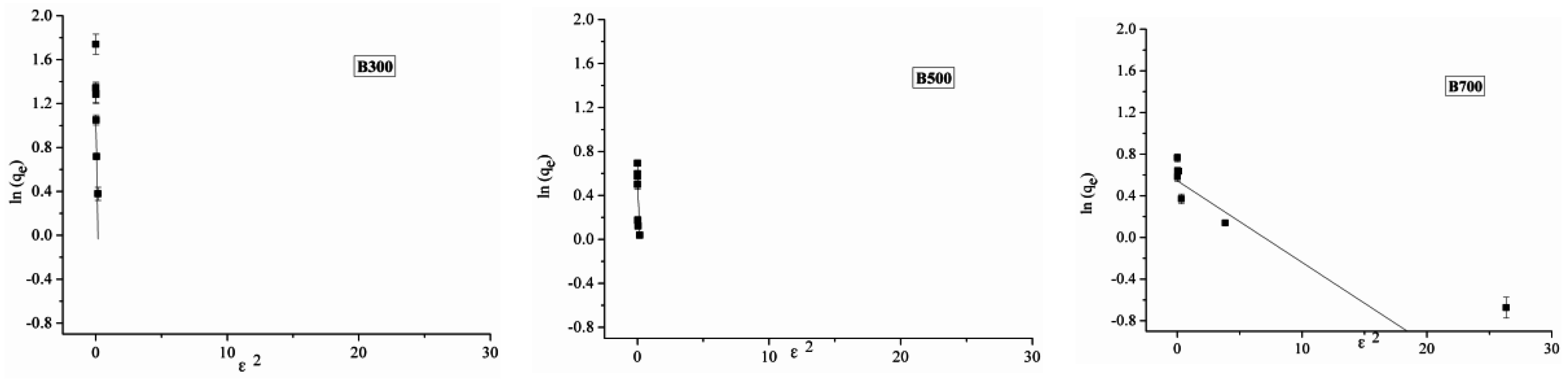
2.3. Adsorption Isotherm
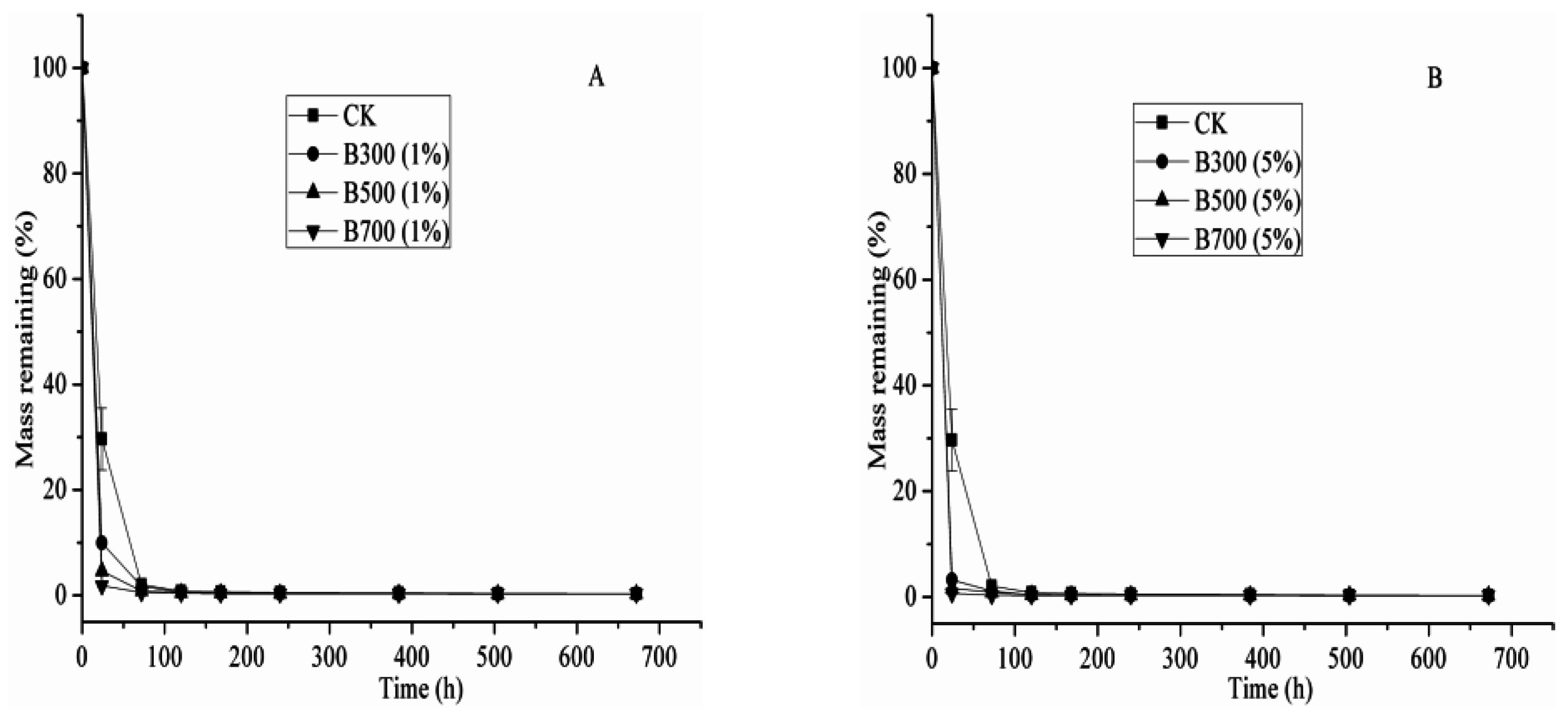
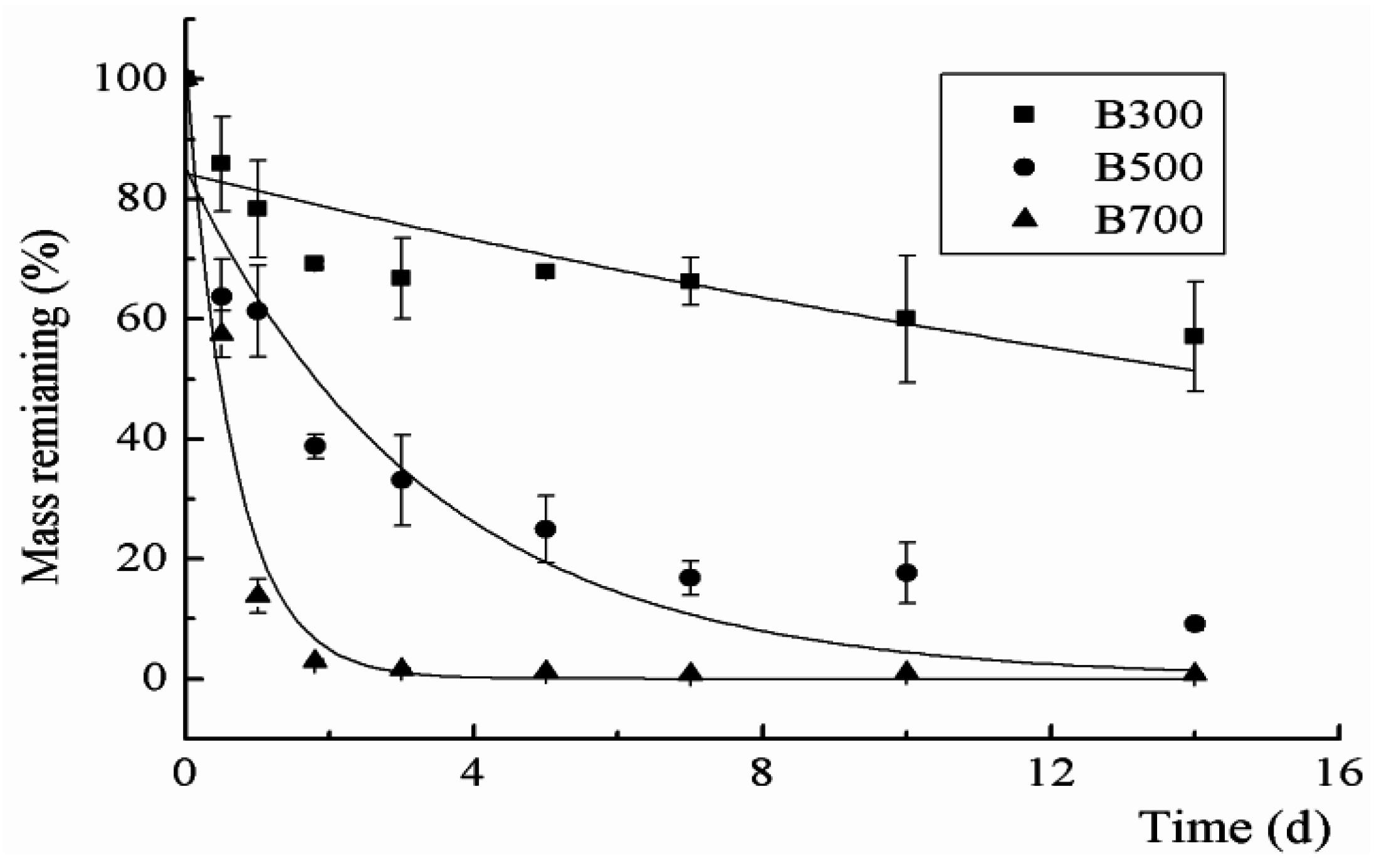
2.4. The Effects of Different Biochar Types and Rates on CP Degradation Kinetics
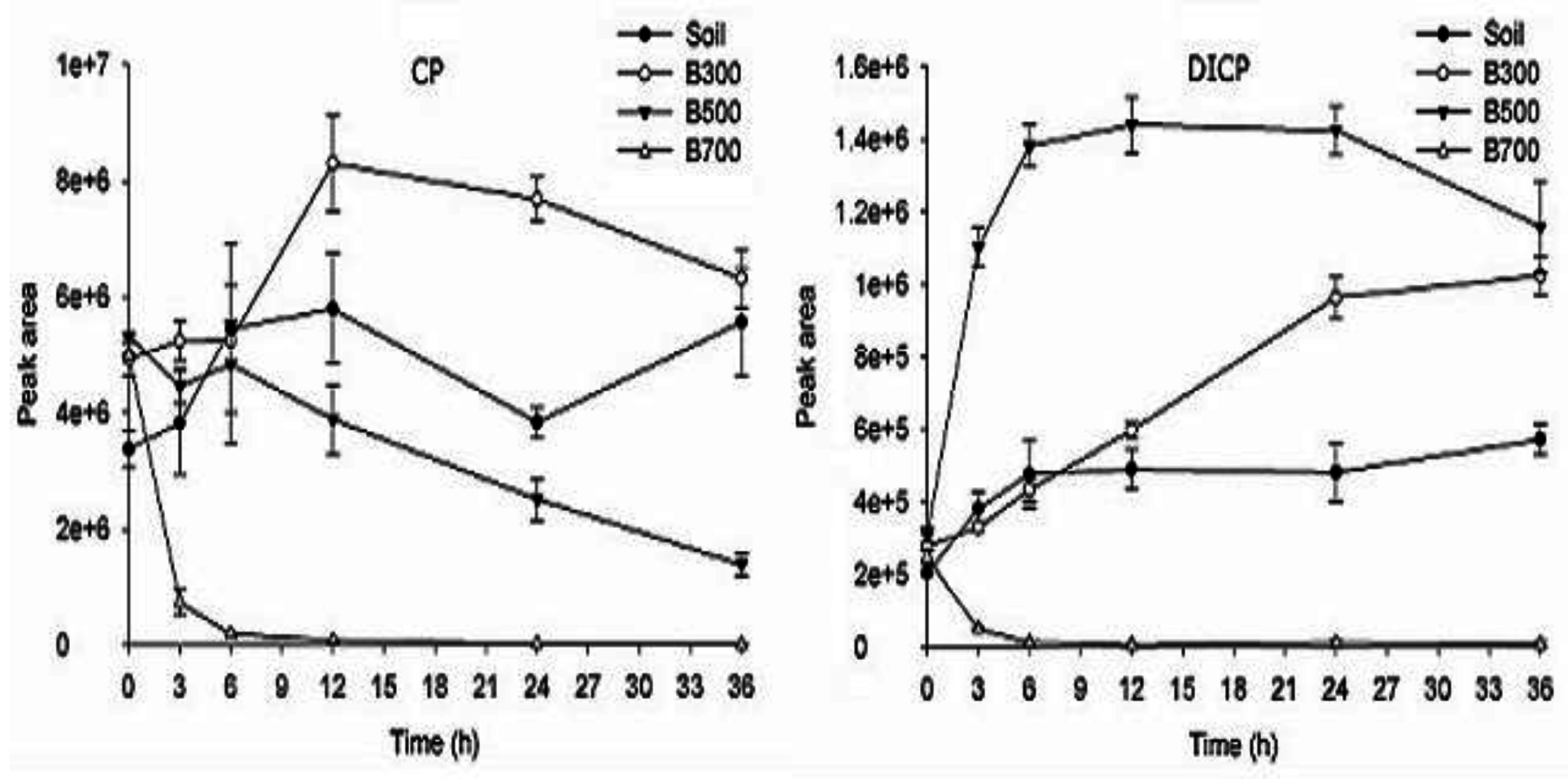
2.5. The Degradation Products of CP in Soil and Biochar
3. Materials and Methods
3.1. Biochar Characteristics
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm Experiment
3.4. Soil and Incubation Experiments
3.5. The Degradation Products of CP in Biochar and Soil
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science & Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; Chapter 2; pp. 13–32. [Google Scholar]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a southeastern Coastal Plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.; Klasson, K.; Wartelle, L. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil—The role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, G.; Huang, M. Bioavailability of diuron in soil containing wheat-straw-derived char. Sci. Total Environ. 2006, 354, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sheng, G.; Wolf, D.C.; Feng, Y. Reduced biodegradation of benzonitrile in soil containing wheat-residue-derived ash. J. Environ. Qual. 2004, 33, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Edwards-Jones, G.; Murphy, D.V. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Krutz, J.L.; Martinazzo, R.; Zajkoska, P.; Hamacher, G.; Borchard, N.; Burauel, P. Transfer of atrazine degradation capability to mineralize aged 14C-labeled atrazine residues in soils. J. Agric. Food Chem. 2013, 61, 6161–6166. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Control of Verticillium wilt and other soil-borne diseases of strawberry in Britain by chemical soil disinfestation. J. Hortic. Sci. Biotechnol. 1990, 65, 401–408. [Google Scholar] [CrossRef]
- Johnson, M.O.; Godfrey, G.H. Chloropicrin for nematode control. Ind. Eng. Chem. 1932, 24, 311–313. [Google Scholar] [CrossRef]
- Cole, S.K.; Cooper, W.J.; Fox, R.V.; Gardinali, P.R.; Mezyk, S.P.; Mincher, B.J.; O’Shea, K.E. Free radical chemistry of disinfection byproducts. 2. Rate constants and degradation mechanisms of trichloronitromethane (chloropicrin). Environ. Sci. Technol. 2007, 41, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Bourke, J.; Manley-Harris, M.; Fushim, C.; Dowaki, K.; Nunoura, T.; Antal, M.J. Do all carbonized charcoals have the same chemical structure? 2. A model of the chemical structure of carbonized charcoal. Ind. Eng. Chem. Res. 2007, 46, 5954–5967. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolytic characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolytic. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.; Xing, B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals. Int. J. Environ. Sci. Technol. 2014, 11, 967–976. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Gan, J.; Yates, S.R.; Ernst, F.F.; Jury, W.A. Degradation and volatilization of the fumigant chloropicrin after soil treatment. J. Environ. Qual. 2000, 29, 1391–1397. [Google Scholar] [CrossRef]
- Castro, C.E.; Wade, R.S.; Belser, N.O. Biodehalogenation. The metabolism of chloropicrin by Pseudomonas sp. J. Agric. Food Chem. 1983, 31, 1184–1187. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Joseph, S.; Husson, O.; Graber, E.R.; Donne, S.W. The electrochemical properties of biochars and how they affect soil redox properties and processes. Agronomy 2015, 5, 322–340. [Google Scholar] [CrossRef] [Green Version]
- Graber, E.R.; Tsechansky, L.; Lew, B.; Cohen, E. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur. J. Soil Sci. 2014, 65, 162–172. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Kilcoyne, A.L.D.; Parikh, S.J. Alteration of biochar carbon chemistry during soil incubations: SR-FTIR and NEXAFS investigation. Soil Sci. Soc. Am. J. 2014, 78, 1632–1640. [Google Scholar] [CrossRef]
- Swancutt, K.L.; Dail, M.K.; Mezyk, S.P.; Ishida, K.P. Absolute kinetics and reaction efficiencies of hydroxyl-radical-induced degradation of methyl isothiocyanate (MITC) in different quality waters. Chemosphere 2010, 81, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Mezyk, S.P.; Helgeson, T.; Cole, S.K.; Cooper, W.J.; Fox, R.V.; Gardinali, P.R.; Mincher, B.J. Free radical chemistry of disinfection-byproducts. 1. Kinetics of hydrated electron and hydroxyl radical reactions with halonitromethanes in water. J. Phys. Chem. A 2006, 110, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.K.; Bandosz, T.J.; Thomson, K.T.; Gubbins, K.E. Water in porous carbons. Colloid Surf. A 2001, 187, 539–568. [Google Scholar] [CrossRef]
- Feng, J.W.; Zheng, S.; Maciel, G.E. EPR investigations of the effects of inorganic additives on the charring and char/air interactions of cellulose. Energy Fuels 2004, 18, 1049–1065. [Google Scholar] [CrossRef]
- Emmerich, F.G.; Rettori, C.; Luengo, C.A. ESR in heat treated carbons from the endocarp of babassu coconut. Carbon 1991, 29, 305–311. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Wu, J.; Strömqvist, M.E.; Claesson, O.; Fängmark, I.E.; Hammarström, L.G. A systematic approach for modelling the affinity coefficient in the Dubinin–Radushkevich equation. Carbon 2002, 40, 2587–2596. [Google Scholar] [CrossRef]
- Wang, Q.X.; Wang, D.; Tang, J.T.; Yan, D.D.; Zhang, H.J.; Wang, F.Y.; Guo, M.X.; Cao, A.C. Gas-phase distribution and emission of chloropicrin applied in gelatin capsules to soil columns. J. Environ. Qual. 2010, 39, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Gan, J.; Papiernik, S.K.; Becker, J.O.; Yates, S.R. Degradation of soil fumigants as affected by initial concentration and temperature. J. Environ. Qual. 2001, 30, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.R.; Mengle, D.; Epstein, D.M.; Fredson, D.; Kelly, K.; Jackson, R.J. Acute symptoms in persons residing near a field treated with the soil fumigants methyl bromide and chloropicrin. West. J. Med. 1987, 147, 95–98. [Google Scholar] [PubMed]
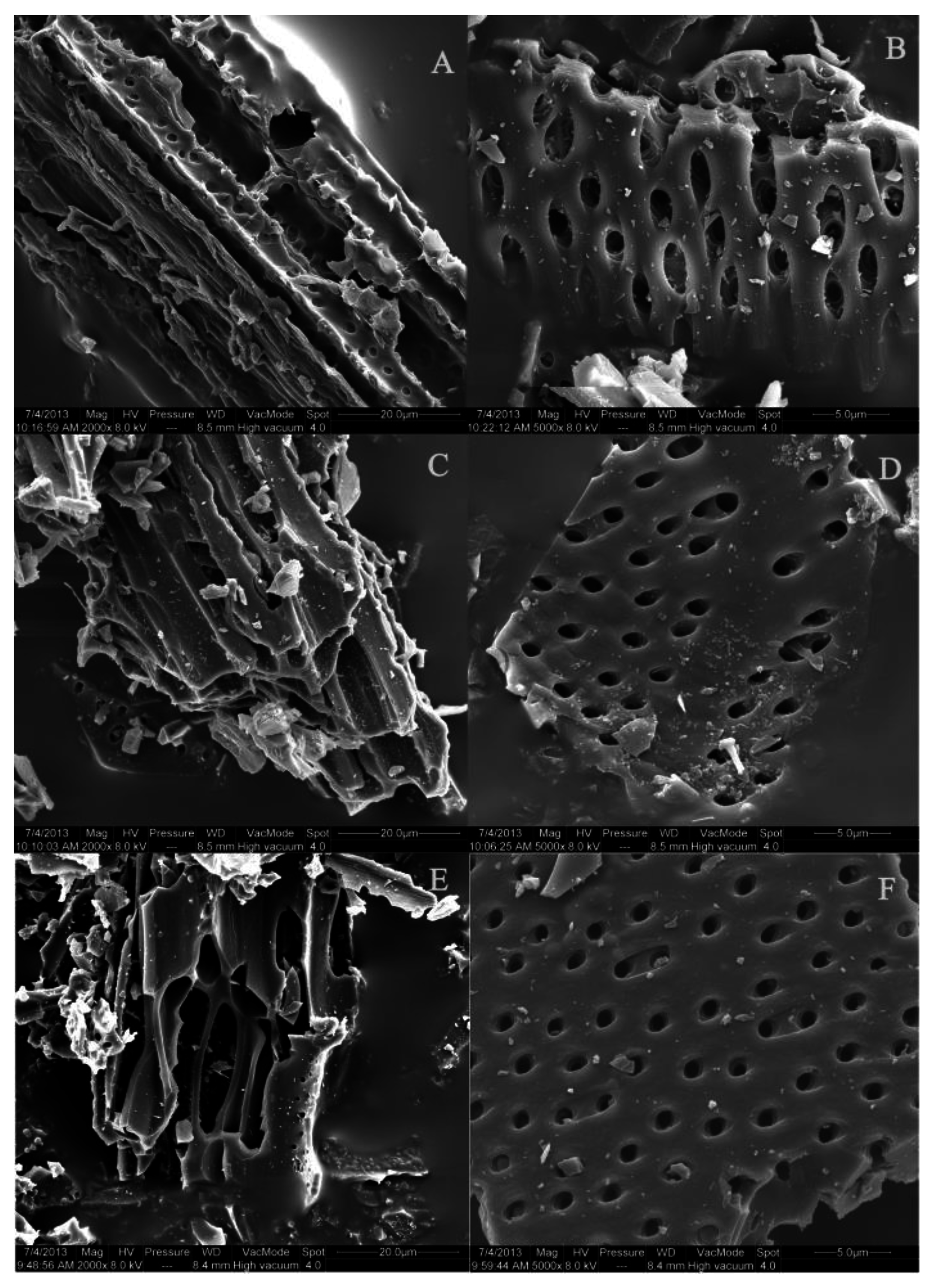
| Types | Oxygen-Containing Functional Groups | |||||||||||
| Carboxyl (mmoL/g) | Phenol Hydroxyl (mmoL/g) | Lactones (mmoL/g) | ||||||||||
| B300 | 0.37 ± 0.03 1 a | 0.01 ± 0.001 a 2 | 0.56 ± 0.01 a | |||||||||
| B500 | 0.29 ± 0.01 b | 0.32 ± 0.01 b | 0.44 ± 0.01 b | |||||||||
| B700 | 0.26 ± 0.01 b | 0.39 ± 0.01 c | 0.47 ± 0.03 b | |||||||||
| Elemental composition | ||||||||||||
| Carbon (mol %) | Hydrogen (mol %) | Nitrogen (mol %) | Oxygen (mol %) | (O + N)/C 3 | O/C | H/C | ||||||
| B300 | 67.97 a | 2.68 a (B500) | 0.94 a | 24.28 a | 0.37 | 0.36 | 0.04 | |||||
| B500 | 76.17 b | 2.36 b (B300) | 0.99 b | 14.35 b | 0.20 | 0.19 | 0.04 | |||||
| B700 | 83.55 c | 1.62 c | 0.97 c | 6.58 c | 0.09 | 0.08 | 0.01 | |||||
| SSA, pore size and ash content | ||||||||||||
| SSA (m2/g) 4 | BJH pore size (nm) | Pore volume (cm3/g) | Ash content (%) | pH (1:2.5) | ||||||||
| B300 | 1.81 | 15.59 | 0.00034 | 4.62 ± 0.01 a | 7.42 ± 0.05 a | |||||||
| B500 | 68.36 | 5.34 | 0.02 | 5.86 ± 0.14 b | 9.39 ± 0.08 b | |||||||
| B700 | 364.63 | 4.35 | 0.13 | 7.74 ± 0.01 c | 10.14 ± 0.11 c | |||||||
| Biochar Type | qe (mg/g) | k (min−1) | R2 |
|---|---|---|---|
| B300 | 23.81 | 0.01 | 0.98 1 |
| B500 | 25.72 | 0.07 | 0.87 |
| B700 | 26.03 | 0.24 | 0.74 |
| AC | 176.86 | 0.27 | 0.88 |
| Biochar Type | Langmuir | Freundlich | D-R | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax | kL | R2 | kf | n | R2 | qmax | E | R2 | |
| B300 | 0.51 | 0.004 | 0.82 | 0.26 | 1.1 | 0.82 | 4.79 | 0.29 | 0.67 |
| B500 | 2.24 | 0.1 | 0.86 | 0.53 | 3 | 0.93 | 1.73 | 0.49 | 0.58 |
| B700 | 1.97 | 1.64 | 0.92 | 1.1 | 5.04 | 0.94 | 1.81 | 3.78 | 0.82 |
| Biochar Types | Applied Amount (mg) | Amount in Gas Phase (mg) | Amount in Biochar (mg) | Degradation Amount (mg) | Degradation Ratio (%) | Average Degradation Ratio (%) |
|---|---|---|---|---|---|---|
| B300 | 0.4 | 0.11 ± 0.003 1 | 0.22 ± 0.01 | 0.07 ± 0.01 | 16.92 | 17.46 a 2 |
| 0.6 | 0.2 ± 0.01 | 0.31 ± 0.002 | 0.09 ± 0.01 | 15.36 | ||
| 0.8 | 0.3 ± 0.05 | 0.43 ± 0.02 | 0.07 ± 0.01 | 8.72 | ||
| 1 | 0.25 ± 0.18 | 0.55 ± 0.05 | 0.2 ± 0.02 | 20.46 | ||
| 1.2 | 0.43 ± 0.03 | 0.54 ± 0.04 | 0.23 ± 0.05 | 19.52 | ||
| 1.4 | 0.48 ± 0.02 | 0.57 ± 0.03 | 0.35 ± 0.01 | 25.01 | ||
| 1.6 | 0.49 ± 0.04 | 0.85 ± 0.08 | 0.26 ± 0.04 | 16.26 | ||
| B500 | 0.4 | 0.11 ± 0.01 | 0.16 ± 0.03 | 0.13 ± 0.04 | 33.24 | 25.65 b |
| 0.6 | 0.22 ± 0.005 | 0.17 ± 0.003 | 0.21 ± 0.005 | 35.58 | ||
| 0.8 | 0.33 ± 0.05 | 0.18 ± 0.006 | 0.29 ± 0.05 | 36.13 | ||
| 1 | 0.59 ± 0.06 | 0.27 ± 0.002 | 0.14 ± 0.06 | 14.23 | ||
| 1.2 | 0.63 ± 0.15 | 0.25 ± 0.01 | 0.32 ± 0.14 | 26.84 | ||
| 1.4 | 0.87 ± 0.06 | 0.27 ± 0.02 | 0.26 ± 0.07 | 18.66 | ||
| 1.6 | 1.06 ± 0.09 | 0.3 ± 0.005 | 0.24 ± 0.08 | 14.86 | ||
| B700 | 0.4 | 0.003 ± 0.0004 | 0.08 ± 0.02 | 0.32 ± 0.02 | 80.13 | 59.35 c |
| 0.6 | 0.02 ± 0.004 | 0.17 ± 0.002 | 0.41 ± 0.006 | 68.53 | ||
| 0.8 | 0.08 ± 0.005 | 0.22 ± 0.01 | 0.5 ± 0.01 | 63.10 | ||
| 1 | 0.13 ± 0.01 | 0.28 ± 0.01 | 0.59 ± 0.02 | 58.68 | ||
| 1.2 | 0.30 ± 0.10 | 0.28 ± 0.006 | 0.62 ± 0.10 | 51.38 | ||
| 1.4 | 0.44 ± 0.06 | 0.27 ± 0.01 | 0.69 ± 0.07 | 49.36 | ||
| 1.6 | 0.57 ± 0.08 | 0.32 ± 0.01 | 0.71 ± 0.08 | 44.29 |
| Treatment | k (d−1) | t1/2 (d) | R2 |
|---|---|---|---|
| Soil (CK) | 1.22 ± 0.02 1 | 0.57 | >0.99 |
| Soil + 1% B300 | 2.30 ± 0.07 | 0.30 | >0.99 |
| Soil + 1% B500 | 2.44 ± 0.05 | 0.28 | >0.99 |
| Soil + 1% B700 | 4.05 ± 0.21 | 0.17 | >0.99 |
| Soil + 5% B300 | 3.42 ± 0.15 | 0.20 | >0.99 |
| Soil + 5% B500 | 4.17 ± 0.32 | 0.17 | >0.99 |
| Soil + 5% B700 | 5.10 ± 0.49 | 0.14 | >0.99 |
| Sterilized soil | 0.19 ± 0.01 | 3.65 | 0.98 |
| Sterilized soil + 1% B300 | 1.23 ± 0.02 | 0.56 | >0.99 |
| Sterilized soil + 1% B500 | 0.91 ± 0.03 | 0.76 | >0.99 |
| Sterilized soil + 1% B700 | 0.93 ± 0.03 | 0.75 | >0.99 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Wang, Q.; Yan, D.; Fang, W.; Mao, L.; Wang, D.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Effects of Biochar Amendment on Chloropicrin Adsorption and Degradation in Soil. Energies 2016, 9, 869. https://doi.org/10.3390/en9110869
Liu P, Wang Q, Yan D, Fang W, Mao L, Wang D, Li Y, Ouyang C, Guo M, Cao A. Effects of Biochar Amendment on Chloropicrin Adsorption and Degradation in Soil. Energies. 2016; 9(11):869. https://doi.org/10.3390/en9110869
Chicago/Turabian StyleLiu, Pengfei, Qiuxia Wang, Dongdong Yan, Wensheng Fang, Liangang Mao, Dong Wang, Yuan Li, Canbin Ouyang, Meixia Guo, and Aocheng Cao. 2016. "Effects of Biochar Amendment on Chloropicrin Adsorption and Degradation in Soil" Energies 9, no. 11: 869. https://doi.org/10.3390/en9110869





