2.2. Electrochemical Analysis of OCV
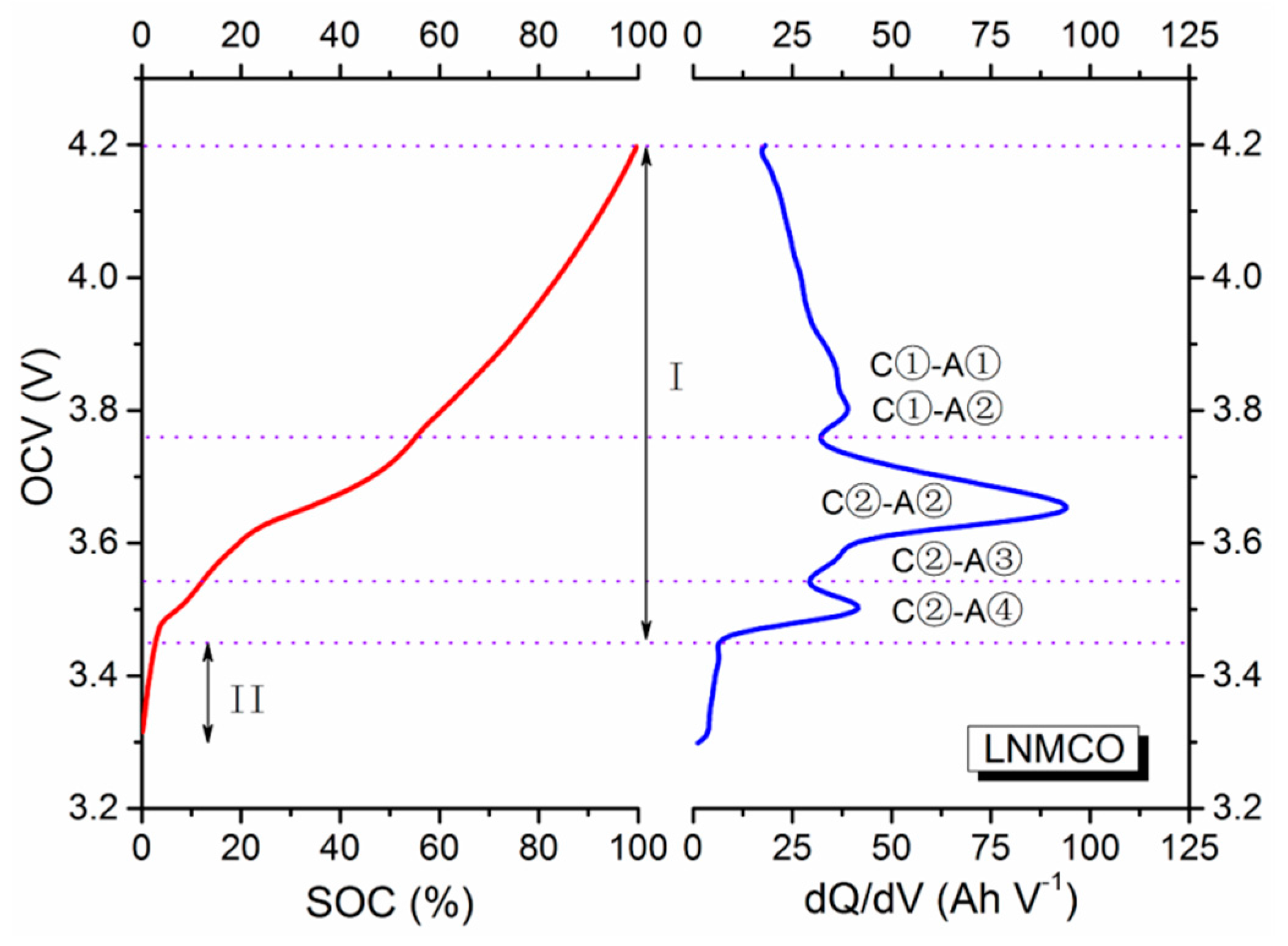
Consider a commercial LIB with an LNMCO cathode and a graphite anode, its experimental OCV curve as a function of SOC and the corresponding dQ/dV profile is displayed in
Figure 1, and its schematic presentation of the electrochemical redox reactions is illustrated in
Table 1 [
23]. The shown OCV behavior is caused by electrode redox reactions experienced by the cathode and anode materials. Particularly, in stage I, where the voltage gradually drops to a certain level, the main electrochemical reactions of the active materials can proceed, resulting in tardy voltage variations. This dynamic voltage decrease can appropriately be represented by a linear function that is associated with the continuous electrochemical redox reactions. In stage II, only small traces of electrochemical reactions occur due to the relatively low cell voltage. The cell voltage then suddenly drops, and can be described by a specific logarithmic function with a real (not complex) power. To better clarify this function form, the dQ/dV profile (differentiates the battery charged capacity (Q) to the terminal voltage (V)) derived from the SOC-OCV curve in
Figure 1 is evaluated, beginning with the layered LNMCO cathode.
At the cathode, its presented voltage gradually increases/decreases throughout the whole electrochemical charging/discharging processes. When it is charged to above 4.5 V, all lithium ions deintercalated from the bulk of the material, along with the oxidation from Ni
2+ to Ni
4+ and from Co
3+ to Co
4+. The layered LNMCO structure is thus damaged, attributed to the degraded electrochemical performance caused by the high charging voltage [
24,
25]. Consequently, the layered LNMCO cathode with homogenous phase reactions only works over the range of 0 ≤ x ≤ 2/3 in Li
1−xCo
1/3Ni
1/3Mn
1/3O
2 [
26]. Moreover, according to the first principle calculations reported, there are two solid state redox reactions occurring when the voltage is below 4.5 V. They are ascribed to Li
1/3Co
1/3Ni
1/3Mn
1/3O
2 and Li
2/3Co
1/3Ni
1/3Mn
1/3O
2 ranging from 4.2 V to 3.9 V (Ni
4+/Ni
3+, labeled as C①), and Li
2/3Co
1/3Ni
1/3Mn
1/3O
2 and LiCo
1/3Ni
1/3Mn
1/3O
2 at 3.9 V–3.7 V (Ni
3+/Ni
2+, labeled as C②), respectively.
In theory, the dQ/dV plot of the LNMCO electrode should therefore show two isolated peaks near these voltage plateaus. The two peaks are not separated, and hence appear as a broad peak in the dQ/dV plot [
27]. On the other hand, for the graphite anode, there are five phase transformation stages during the charging/discharging processes over the voltage range from 0.8 to 0.01 V [
28]. They include three main plateaus corresponding to the three pairs of redox reaction peaks in the dQ/dV plot of the graphite electrode. They are LiC
6↔LiC
12 at 0.10/0.08 V (labeled as A①), LiC
12↔LiC
36 at 0.14/0.11 V (labeled as A②), and LiC
36↔LiC
72 at 0.22/0.20 V (labeled as A③), respectively. In addition, an unobvious plateau (or peak), LiC
72↔C
6 above 0.3 V (labeled as A④), usually appears in the SOC-OCV curve and dQ/dV plot.
The overall SOC-OCV curve of the cell in
Figure 1 can be obtained using the approach reported in [
29]. In other words, when the cathode is on a steady electrochemical reaction plateau, an additional peak will emerge at each distinct anode phase transformation plateau. Therefore, each peak in the dQ/dV plot of the cell can be distinctively identified. For example, C①-A② represents the state in which the cathode is on its first plateau and the anode is on its second plateau. The peak voltage in the dQ/dV plot is the difference in plateau voltage between the cathode and the anode [
23]. It is also informative to point out that the number, location and shape of the peaks in the dQ/dV plot usually vary with operating conditions and degradation of the LIBs. It is therefore possible to identify performance decline origins of many cells based only on their dQ/dV variations.
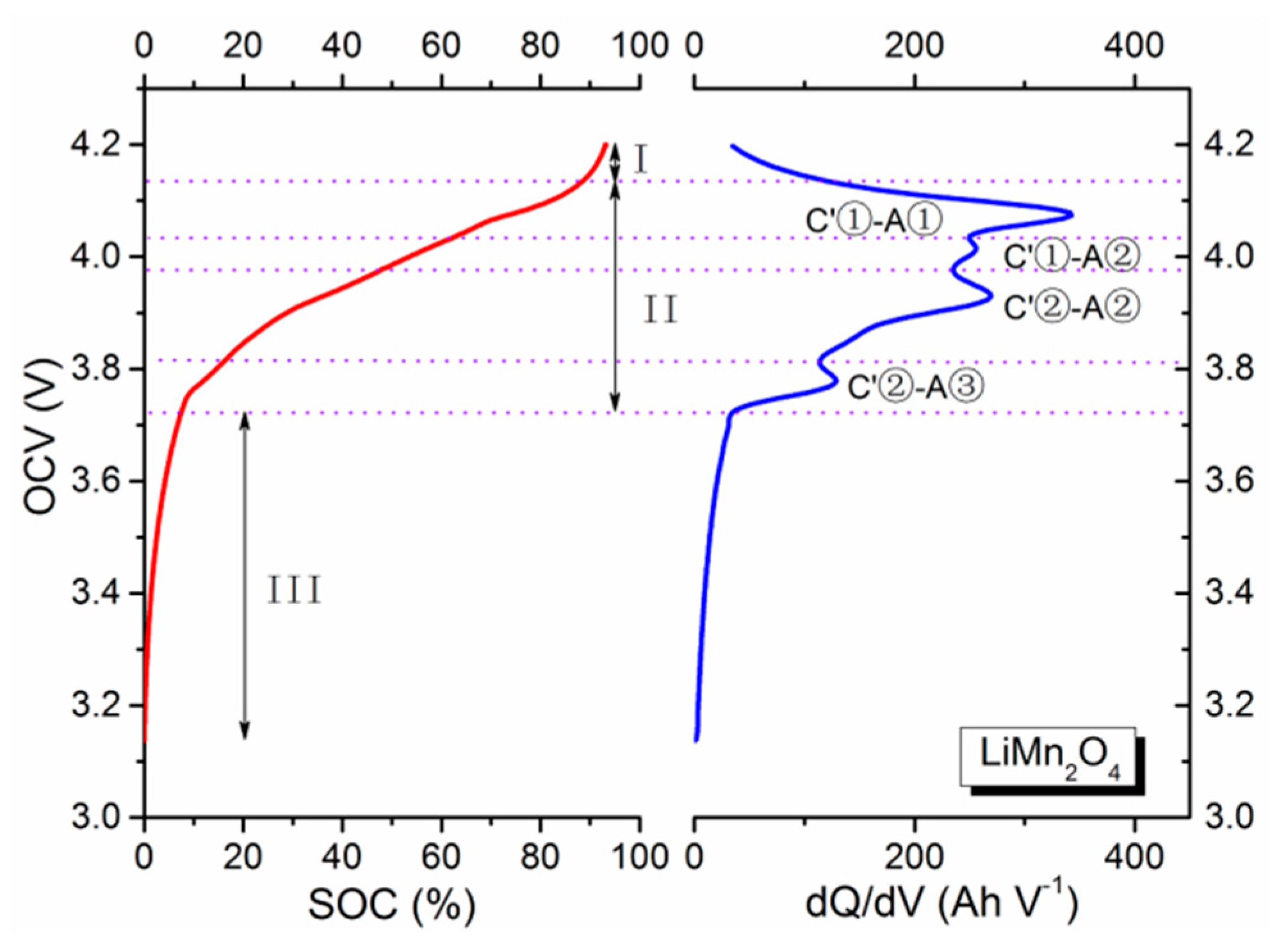
Similarly, in the case of the spinel LMO cathode shown in
Figure 2 and
Table 2, it exhibits two electrochemical plateaus at 4.1 V (labeled as C′①) and 3.95 V (labeled as C′②). They correspond to the two phase transformations notated as Mn
2O
4/Li
0.5Mn
2O
4, and Li
0.5Mn
2O
4/LiMn
2O
4 [
30,
31]. Likewise, for the olive LFP cathode shown in
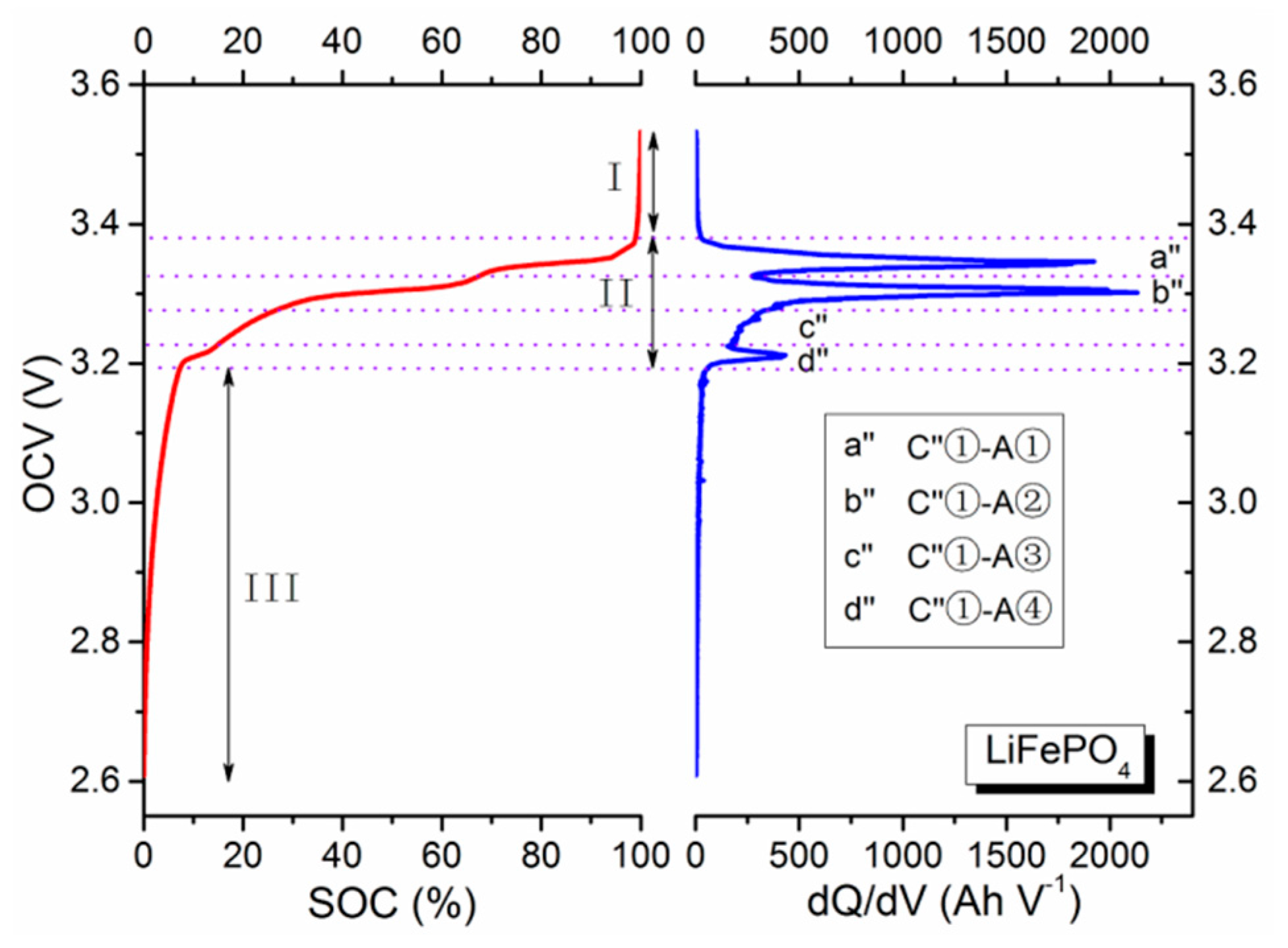
Figure 3 and
Table 3 [
29], it manifests a unique voltage plateau at 3.45 V (labeled as C″①), which has been verified as the phase transformation between FePO
4 and LiFePO
4 [
32]. In addition, unlike the LNMCO/graphite battery, the SOC-OCV curves of the LMO/graphite and LFP/graphite systems can be divided into three stages. In stage I, redox reactions basically completely with only slight traces of them continuing. The main process in this stage is also charge accumulation, whose effect is a rapid decrease of OCV value that can nicely be described by an exponential function. The latter two stages (II and III) are identical to the two stages (I and II) of LNMCO/graphite batteries, and are for representing the main electrochemical reaction and charge accumulation stages.
For example, the voltage of the LFP batteries rapidly decreased in stage I because the main component is basically the FePO
4 without any more phase transformation. Upon reaching stage II, the voltage of the LFP battery remains relatively steady. The low voltage is attributed to the extraction/insertion reactions of the LFP cathode, assigned to the first-order phase transition. A flat voltage plateau is thus produced with two phase regions corresponding to FePO
4 and LiFePO
4. Taking the stage I into consideration, it may therefore introduce some differences when determining parameters for the SOC-OCV model, as compared to the earlier LNMCO battery.
Figure 4 shows the SOC-OCV curve and corresponding dQ/dV profile of the novel lithium-ion batteries with NMC+LiCoO
2 (LCO) cathode and LTO anode, and the phase transformation is relatively uncomplicated.
Table 4 reports its schematic presentation of the electrochemical redox reactions [
33]. For the LTO anode [
34], the phase transformation between Li
7Ti
5O
12 and Li
4Ti
5O
12 occurs near 1.55 V, showing a long plateau. For the LiCoO
2 cathode, it exhibits two very weak peaks at 4.19/4.13 V and 4.06/4.03 V (labeled as C′′′①and C′′′②) and a pair of strong redox peak at 3.97/3.85 V (labeled as C′′′③), corresponding to the reduction/oxidation reactions of Co
4+/Co
3+ [
35], respectively. In view of the flat plateau of the LTO anode, the dQ/dV curve primarily reflects the characteristics of the NMC+LCO cathode and the LTO anode. However, due to the approximate phase transformation voltage of LCO and NMC, overlaps of peaks will appear in the dQ/dV curve of the LTO lithium-ion battery. The three stages of the SOC-OCV curve of the NMC+LCO/LTO batteries are similar to those of LMO/graphite and LFP/graphite batteries.
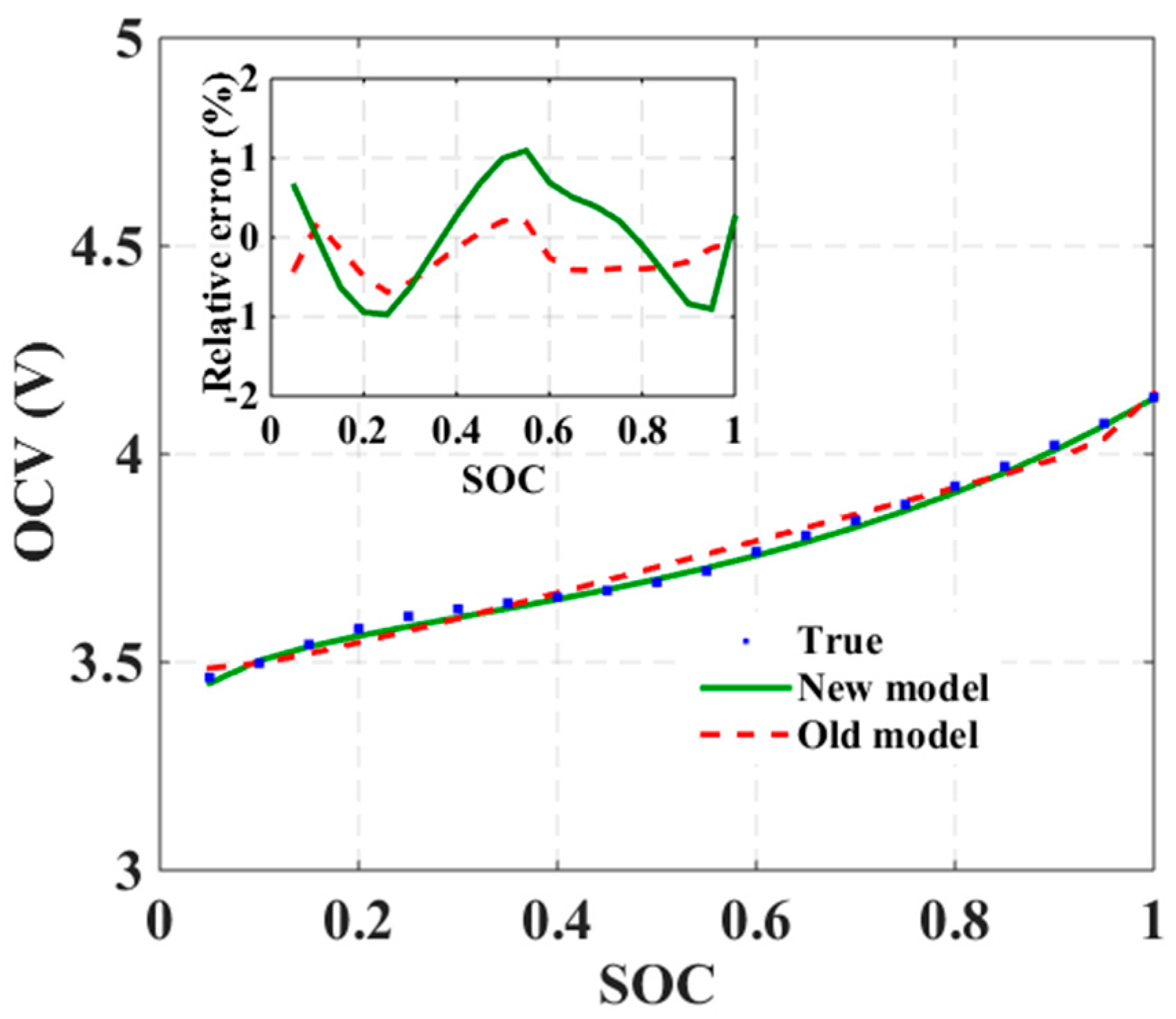
It is confirmed from electrochemical analysis of different types of LIBs that a three-segment SOC-OCV model is more potentially suitable for capturing all characteristic features of the described electrochemical processes. This model structure and its suitability for real-time implementation are described next.
2.3. Generalized SOC-OCV Model
The proposed generalized SOC-OCV model is shown in Equation (1), where a logarithmic function with real (not complex) power, a linear function, and an exponential function with a shifted exponent can clearly be seen:
where
and
s represent the OCV and SOC of the battery, respectively, and
.
To match the processes described in
Section 2.2, the logarithmic function must be tuned to play a predominant role at low SOC, where charge accumulations on the surfaces of the active materials happen within the LIB. The linear function, in turn, dominates the middle SOC range, where primary phase transformation of the active materials occurs. The last exponential function then contributes to the high SOC behavior, where both partial redox reaction and charge accumulation occur. The three functions in Equation (1) are therefore essential, and will interact with each other to form the generalized OCV model over the whole SOC range.
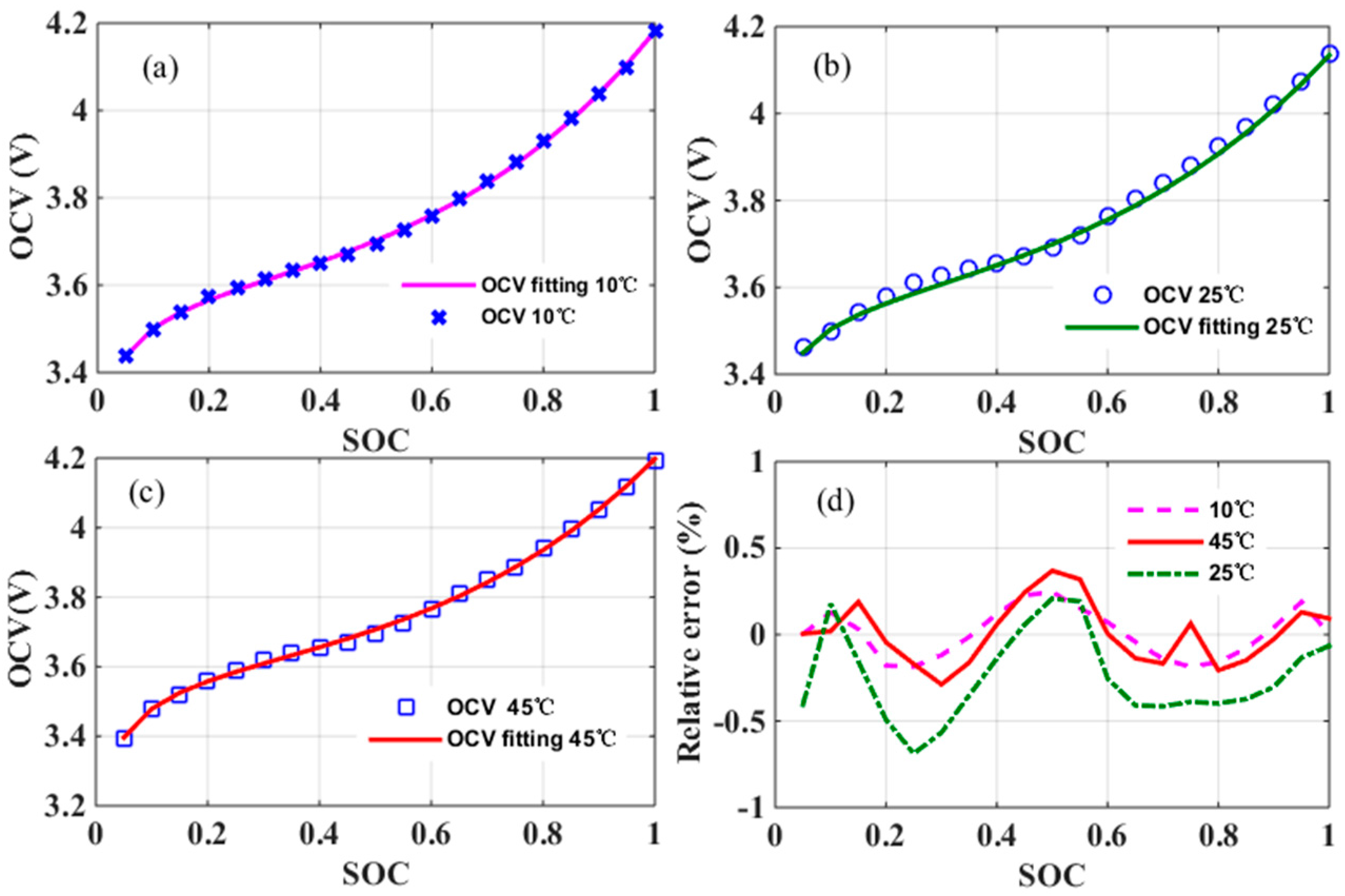
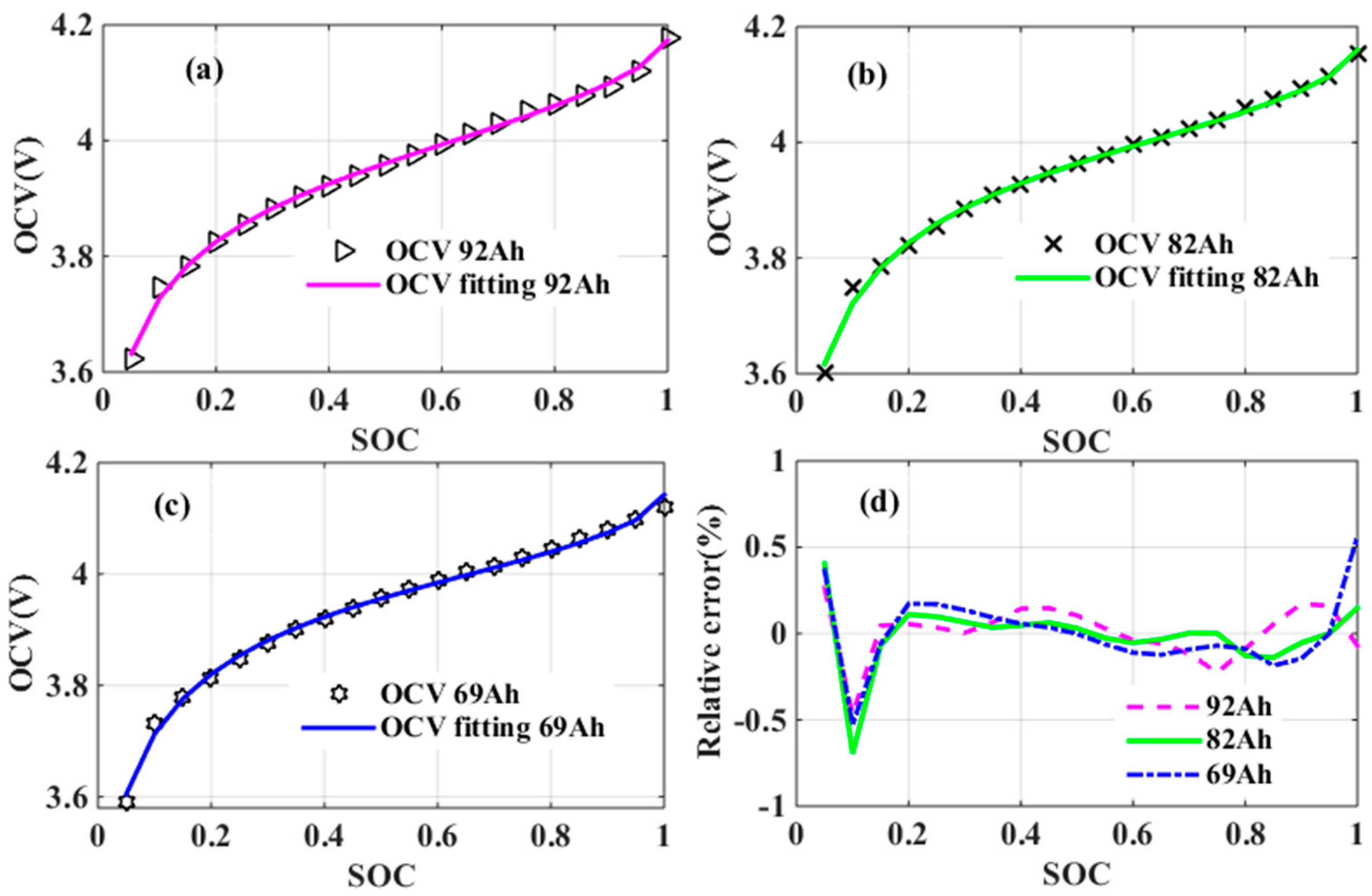
Compared with [
10], coefficients
m and
n included in Equation (1) are for adapting the model to match with different types of LIBs, since they depend on active materials of electrodes used in the LIBs. Their specific values and properties will be discussed in
Section 3, where the hypothesis of
m and
n being invariant for a specific type of LIBs will also be proved. In other words, it will be proved that both
m and
n will not change with temperature and aging. Consequently, the SOC-OCV model is reduced to a sum of only four proportionally scaled terms, whose coefficients are
a,
b,
c and
d in Equation (1). Only these four coefficients require tuning in real-time to arrive at the desired SOC-OCV mapping. The complexity of realizing Equation (1) has therefore been considerably reduced, which is certainly encouraged for real-time state estimation.
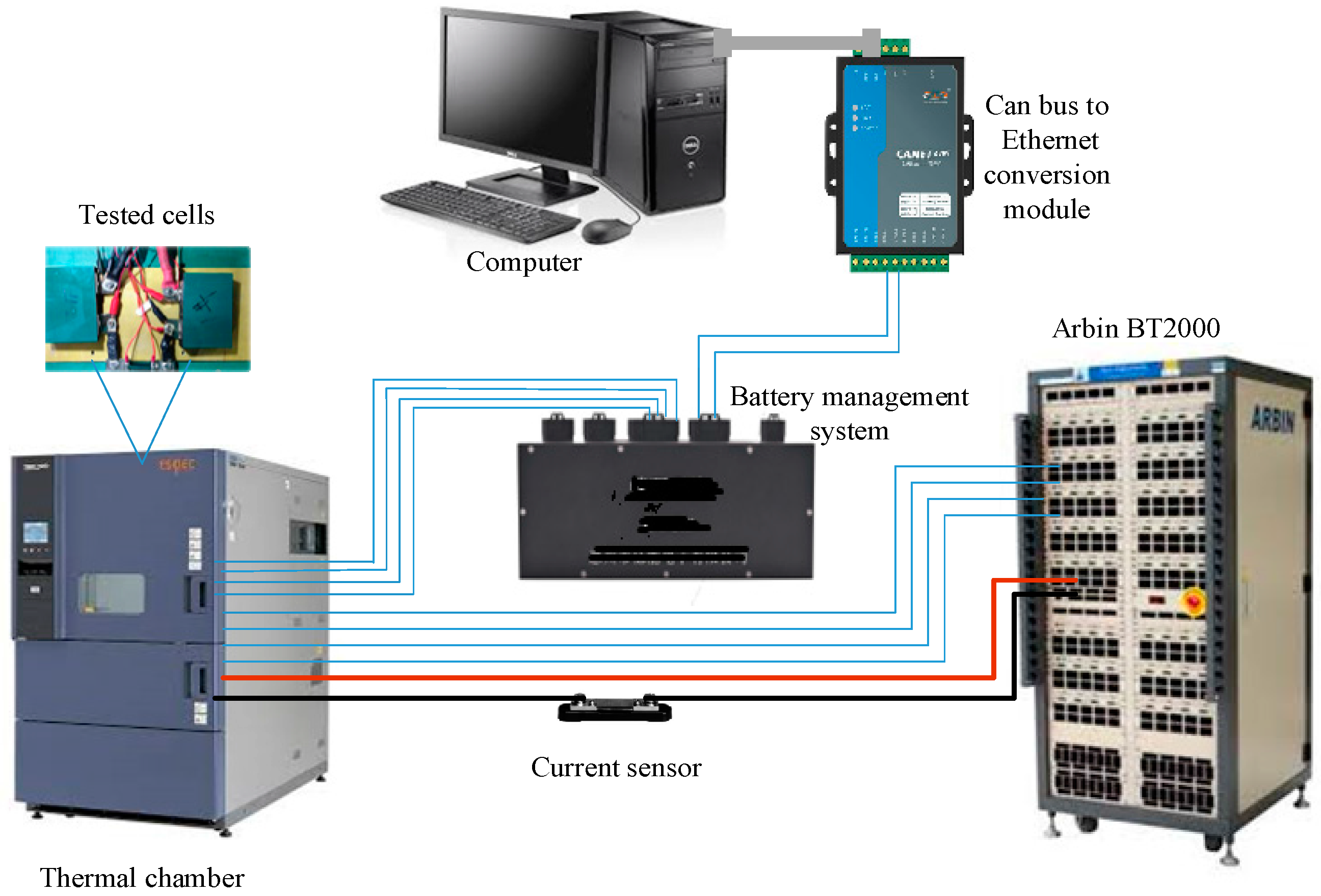
2.4. Recursive Parameter Identification
For a given class of LIBs, its six model parameters in Equation (1) must collectively be determined from offline experimental data. This determination is nonlinear because of the logarithmic and exponential functions included in Equation (1). Some amount of complexity may therefore be involved, but will subsequently be proven to be otherwise since
m and
n are fixed for a given class of LIBs, and hence do not need real-time updating. They must, however, be determined for once at the beginning of real-time execution. Typical non-linear algorithm for optimization is thus needed, and is usually run iteratively. One possibility is the gradient based iterative search method, which mathematically, relies on the following equation, expressed in terms of the unknown parameter vector
:
The obtained
N sets of experimental data (
s(
k),
y(
k)),
k = 1,…,
N, when substituted in Equation (3), further lead to the following equations:
A nonlinear least square estimation problem has thus been formed. The purpose is to find the parameter vector that can minimize the following expression:
The process must usually be executed iteratively, using the gradient based algorithm provided as follows:
where the step size
must be selected to ensure algorithm convergence.
The algorithm is stopped only when is smaller than a pre-defined small threshold.