Results obtained in the experimental campaign, both in outdoor testing and accelerated aging testing, are included in this section.
3.1. Outdoor Testing
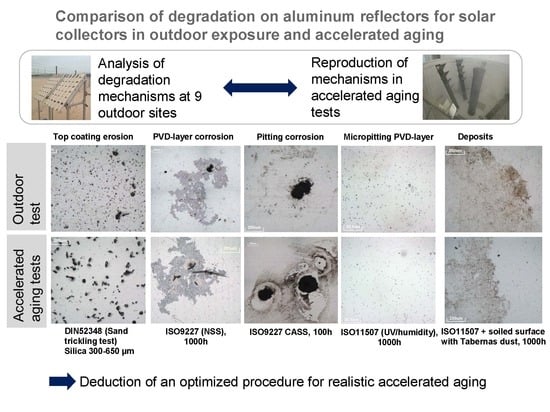
The five main degradation mechanisms that were detected during the outdoor testing campaign are the following:
Corrosion of the aluminum PVD layer
Micropitting in the PVD layer
Non-removable chemical deposition on the surface
Pitting corrosion
Sand abrasion
Table 3 summarizes the degradation mechanisms suffered for each material at every exposure site.
The first mechanism consists of a corrosion produced in the pure Al layer, which is typical for PVD-coated aluminum reflectors (materials A, B, C, D and F). A detailed description of this degradation mechanism can be found in [
24]. Examples of this degradation type can be seen in
Figure 3. According to results presented in
Table 3, in general these corrosion defects mainly appeared in materials A, B, D and F (and also in samples C exposed in coastal sites after longer exposures, although with a lower intensity). For these four material types, PVD layer corrosion was detected in many sites already after 6 months. Typical spot sizes range between 100 μm and a few millimeters. The number of defects detected was higher for D and F samples and for aggressive climates (close to the coast), such as Tan Tan, and increased with the exposure time. Also on sites with severe abrasion, the extent of this defect type is elevated. Here the protective function of the top layers is decreased and the abrasive defects act as starting points for the corrosion.
The second mechanism (micropitting) consists of small perforations of the aluminum PVD layer. Under the light microscope, these kinds of defects are visible as small black spots of a size below one micrometer. Micropitting is only observed on materials A, B and C, as indicated in
Table 3. As all these samples are from one manufacturer it is likely that a characteristic in the production process causes this susceptibility. Typical spots in material C (see
Figure 4 right) are smaller and denser than spots detected in material A and B (see
Figure 4 left). Micropitting degradation appeared in all exposure sites after 6 months. SEM images of the actual holes in the Al layer are presented in
Figure 5. The damaged PVD layer system can be observed in the central part of the image presented in
Figure 5 left, without the delaminated protective top coating.
Figure 5 right shows a picture of the same area but with higher magnification. In this case, small holes can be easily observed in the Al layer, corresponding to the micropitting defects.
The third degradation mechanism detected consists of different kinds of deposits on the surface, which cannot be removed by the usual cleaning process (demineralized water and a soft tissue when necessary). The settled environmental particles seem to chemically interact with the protective coating (see
Figure 6). As can be seen in
Table 3, this is the most common defect observed in the outdoor exposure study because it was detected in all materials and all exposure sites.
The fourth mechanism, called pitting corrosion, is a punctual corrosion that penetrates into the aluminum substrate (see
Figure 7), presenting irregular shape and sizes around 100–200 μm. As can be noted in
Table 3, this degradation mechanism almost exclusively happens on coastal sites (Almería, Canarias, Abu Dhabi and Tan Tan). A remarkable behavior was detected in Tan Tan, where most materials suffer this degradation already after 6 months of outdoor exposure (except C and E). For a deeper understanding, the pitting holes were observed with the 3D microscope, as seen in the profile of a cross section in
Figure 7, right. As can be observed, the depth of a typical pitting hole is around 30–50 μm. In addition the areas surrounding pitting holes are often covered with corrosion products reaching considerable fractions of the samples. The formation of pitting holes in aluminum is associated with the presence of chloride ions [
25], which explains the strong sensitivity to this degradation mechanism at the coastal sites.
The fifth mechanism is an abrasive damage of the materials top coating caused by airborne dust and sand particles. The size of the defects ranges from few micrometers to around 0.5 mm. In
Figure 8 an overview can be seen on the left and a higher magnification picture shows the different defects in the top coating on the right. So far this mechanism has only been detected at the desertic Moroccan sites.
The site of Zagora is especially aggressive concerning this mechanism as it appears after only 6 months and spreads over the entire surface of all samples with a high density and maximum defect size. In Erfoud and Missour the defects only appear after longer exposition durations and with less dense distribution as well as a smaller size.
Additionally, reflectance was measured in all materials and all sites, after the testing period (see
Figure 9.)
As can be seen in
Figure 9, hemispherical reflectance losses are significantly lower than specular reflectance losses, because the main process causing the losses is scattering. Concerning the reflectance decay for the different materials, it can be noticed that in general materials A, B and C show higher losess (mainly in hemispherical reflectance due to the micropitting). And with respect to the sites, the most significant result is that Zagora (and also Erfoud, but in a lower importancy) suffered a considerably higher reflectance decrease. In this site, materials F, G and I present the highest reflectance losses. These results are useful for a better understanding of the performance behaviour of the different materials in the studied sites. However, reflectance measurements are not able to distinguish among the different degradation mechanisms and, consequently, they do not show a clear patterns because all possible effects are mixed. Therefore, reflectance loss was not selected as a representative parameter to derive proper accelerated aging tests that reproduce the degradation suffered in the real outdoor conditions.
3.2. Accelerated Testing
Table 4 summarizes the results of the accelerated aging tests performed with the different materials. The columns show the typical degradation mechanisms that were already discussed in the outdoor testing (see
Section 3.1) and the rows represent the accelerated aging tests conducted. There is an additional column, titled “Side Effects,” where degradation mechanisms that do not happen during outdoor exposure are included. Images of typical appearances of the side effects are linked in the table. This way it can be directly seen which test has which effect on a material. The effects of the two abrasion tests (soil pipe and sandstorm) are not included in this table because they are specifically designed as short-term mechanical tests and thus only reproduce abrasive defects.
From the results of the conventional test program, the following conclusions can be drawn:
Additionally, the following side effects not detected outdoors were also found in the conventional accelerated tests:
A particular defect was detected in materials A, B and C after the NSS test, consisting of cracks in the top layers together with their delamination (see
Figure 10e,f). This effect was also found in material B after the immersion test.
Some unrealistic deposits were detected in all materials after the Damp Heat test, covering large parts of the sample surfaces (see
Figure 10g).
In the UV + Condensation and the Damp Heat tests, the elevated temperature is likely to cause degradation of the polymer top coating of material C. Pictures of these unrealistic changes in the polymer layer are presented in
Figure 10h (Damp Heat test) and
Figure 10i (UV + Condensation test).
Additionally, another side effect appeared in the UV + Condensation test (in materials A, B, D, E and F), consisting of a significant amount of cracks in the top layers (see
Figure 10j).
Finally, some scratches were detected in materials A and F after the Humidity Freeze test (see
Figure 10l).
In the first innovative test performed, a combination of the NSS and the UV + Condensation test was chosen to benefit from the advantages of both tests. This test was only applied to a small set of materials (A, E and I). As can be seen in
Table 4, material A suffered from both PVD- layer corrosion and micropitting corrosion in this test. In addition, it is worth to remark that the typical cracks detected in the UV + Condensation test (see
Figure 10j) also appeared in this combined test, in materials A and E.
In the outdoor results, indications were found that the soiling has an influence on the degradation behavior. In general, samples with stronger soiling also showed more other defects. Therefore, a series of tests with artificially soiled samples has been carried out. The soiled samples have been tested in conventional tests. The first important result is that the Tabernas dust indeed seems to enhance corrosion mechanisms. In particular, the following events were detected:
In the UV + Condensation + Sand test (material B and F) as well as in the Damp Heat + Sand test (material D), PVD-layer corrosion was detected on soiled samples, although this was not the case for clean samples. This degradation mechanism was also found in the Humidity Freeze + Sand test (materials B and F) and the NSS + Sand test (material B), but in this case the results are not as significant because it was also noticed in the same tests without sand. It is important to highlight that the corrosion that appeared in the two tests was stronger than those detected in the same test without sand.
Micropitting corrosion was only detected in the conventional tests when Damp Heat or UV + Condensation conditions were applied. However, when sand is added on the sample surfaces, this degradation pattern was detected not only in these two tests, but also in the NSS test (for material B).
The effect of incompletely removable deposits on the samples, observed after the outdoor tests, is reproduced by all the innovative accelerated aging tests with sand (see
Figure 10l).
Finally, pitting corrosion was not noticed on the innovative tests performed with sand.
There are two main factors explaining the higher susceptibility to degradation of the soiled samples. First, soiling changes the wetting phenomena on the sample surface. While clean samples are very hydrophobic, soiled surfaces tend to retain humidity and thus stay wet for a longer duration during exposure. Second, as natural sand includes many different impurities, aggressive compounds like chlorides can be introduced which act as corrosion activators.
The same side effects observed in the conventional tests were also detected in the tests with sand. For example, cracks in the UV + Condensation+Sand and the Humidity Freeze + Sand tests. In addition, innovative tests presented some side effects caused by the sand application, that is, NSS + Sand test showed noticeable deterioration points (materials B and C, see
Figure 10m) and sand could not be removed from the sample surface of material C both in the Damp Heat +Sand test and in the UV + Condensation + Sand test (see
Figure 10n).