Cathode Assessment for Maximizing Current Generation in Microbial Fuel Cells Utilizing Bioethanol Effluent as Substrate
Abstract
:1. Introduction
2. Results
2.1. Maximum Current Generation Found from the Polarisation Curves
2.2. Resistance in the Anode and Cathode Electrodes
2.3. Current Generation in Relation to Substrate Loading Rate and Switching to Bioethanol Effluent
3. Discussion
3.1. Obtainable Current for the Different Cathode Types
3.2. Potential for Integration of Microbial Fuel Cell in the Bioethanol Biorefinery
4. Materials and Methods
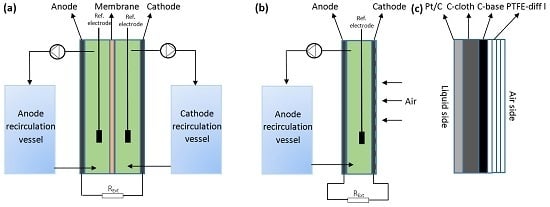
4.1. Reactor Setup
4.2. Operational Condition and Inoculation
4.3. Preparation of Bioethanol Effluent
4.4. Chemical and Electrochemical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AiC | Air cathode |
| CE | Coulombic efficiency |
| COD | Chemical oxygen demand (g) |
| DOC | Dissolved oxygen cathode |
| Ean | Anode electrode potential (V) |
| Eca | Cathode electrode potential (V) |
| EIS | Electrochemical impedance spectroscopy |
| FeC | Ferricyanide cathode |
| Iave | Average current density (A/m2) |
| Lsub | Substrate loading rate g·COD/(L·day) |
| MFC | Microbial fuel cell |
| PTFE | Polytetrafluoroethylene |
| Pt/C | Platinized carbon |
| Rext | External resistance (Ω) |
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Thygesen, A.; Ale, M.T.; Mensah, M.; Poulsen, F.W.; Meyer, A.S. The significance of the initiation process parameters and reactor design for maximizing the efficiency of microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.S.; Criddle, C.S. Three-dimensional carbon nanotube-textile anode for high-performance microbial fuel cells. Nano Lett. 2011, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Rodrigues, D.S.; Thygesen, A.; Fernando, D.; Meyer, A.S. Inocula selection in microbial fuel cells based on anodic biofilm abundance of Geobacter sulfurreducens. Chin. J. Chem. Eng. 2016, 24, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Thygesen, A.; Meyer, A.S. Acetate is a superior substrate for microbial fuel cell initiation preceding bioethanol effluent utilization. Appl. Microbiol. Biotechnol. 2015, 99, 4905–4915. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; de Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Schaetzle, O.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Strik, D.P.B.T.B.; Barriere, F.; Buisman, C.J.N.; Hamelers, H.V.M. Identifying charge and mass transfer resistances of an oxygen reducing biocathode. Energy Environ. Sci. 2011, 4, 5035–5043. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Benetton, X.; Sevda, S.; Vanbroekhoven, K.; Pant, D. The accurate use of impedance analysis for the study of microbial electrochemical systems. Chem. Soc. Rev. 2012, 41, 7228–7246. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Poulsen, F.W.; Min, B.; Angelidaki, I.; Thomsen, A.B. The effect of different substrates and humic acid on power generation in microbial fuel cell operation. Bioresour. Technol. 2009, 100, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Possemiers, S.; Thomsen, A.B.; Verstraete, W. Integration of microbial electrolysis cells (MECs) in the biorefinery for production of ethanol, H2 and phenolics. Waste Biomass Valoriz. 2010, 1, 9–20. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Fan, M.Z.; Cao, X.X.; Wang, C. Composition and distribution of internal resistance in three types of microbial fuel cells. Appl. Microbiol. Biotechnol. 2007, 77, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.C.; Chang, I.S.; Kim, B.H.; Kim, M.; Jang, J.K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- Manohar, A.K.; Bretschger, O.; Nealson, K.H.; Mansfeld, F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.J.A.; Heijne, A.T.; Buisman, C.J.N.; Hamelers, H.V.M. Bioelectrochemical systems: An outlook for practical applications. Chem. Sustain Chem. 2012, 5, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, P.; Verstraete, W. Methanogenesis in membraneless microbial electrolysis cells. Appl. Microbiol. Biotechnol. 2009, 82, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Rabaey, K.; Ossieur, W.; Verhaege, M.; Verstraete, W. Continuous microbial fuel cells convert carbohydrates to electricity. Water Sci. Technol. 2005, 52, 515–523. [Google Scholar] [PubMed]
- Ambye-Jensen, M.; Thomsen, S.T.; Kádár, Z.; Meyer, A.S. Ensiling of wheat straw decreases the required temperature in hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Cathode Type | DOC-MFC | FeC-MFC | AiC-MFC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Part | Anode | Cathode | Cell | Anode | Cathode | Cell | Anode | Cathode | Cell |
| Unit | Ωm2 | Ωm2 | Ωm2 | Ωm2 | Ωm2 | Ωm2 | Ωm2 | Ωm2 | Ωm2 |
| Ohmic resistance | 0.02 | 0.05 | 0.07 | 0.04 | 0.07 | 0.02 | |||
| Internal resistance | 0.08 | 0.43 | 0.46 | 0.07 | 0.11 | 0.18 | 0.22 | 0.10 | 0.32 |
| Parameters | DOC-MFC | FeC-MFC | AiC-MFC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | Time | Lsub | Rext | Ean | Eca | Iave | CE | Ean | Eca | Iave | CE | Ean | Eca | Iave | CE |
| Unit | Day | g·COD/L/d | Ω | mV | mV | mA/m2 | % | mV | mV | mA/m2 | % | mV | mV | mA/m2 | % |
| 1 | 11 | 0.5 | 150 | −470 | −290 | 142 | 18 | −440 | 140 | 444 | 41 | −370 | 150 | 403 | 25 |
| 2 | 3 | 0.5 | 150 | −470 | −290 | 146 | 7 | −420 | 150 | 457 | 22 | −340 | 160 | 397 | 18 |
| 3 | 2 | 1.0 | 150 | −470 | −290 | 145 | 6 | −420 | 160 | 445 | 16 | −440 | 150 | 438 | 13 |
| 4 | 2 | 1.0 | 75 | −470 | −340 | 173 | 15 | −390 | 140 | 691 | 36 | −430 | 100 | 684 | 33 |
| 5 | 2 | 1.0 | 47 | −460 | −390 | 186 | 19 | −360 | 160 | 1114 | 62 | −370 | 50 | 920 | 49 |
| 6 | 4 | 2.0 | 47 | −460 | −390 | 184 | 5 | −390 | 140 | 1279 | 30 | −400 | 50 | 1043 | 23 |
| 7 | 2 | 2.0 | 27 | −420 | −390 | 184 | 4 | −340 | 140 | 1640 | 28 | −50 | 100 | 802 | 12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.; Thygesen, A.; Meyer, A.S. Cathode Assessment for Maximizing Current Generation in Microbial Fuel Cells Utilizing Bioethanol Effluent as Substrate. Energies 2016, 9, 388. https://doi.org/10.3390/en9050388
Sun G, Thygesen A, Meyer AS. Cathode Assessment for Maximizing Current Generation in Microbial Fuel Cells Utilizing Bioethanol Effluent as Substrate. Energies. 2016; 9(5):388. https://doi.org/10.3390/en9050388
Chicago/Turabian StyleSun, Guotao, Anders Thygesen, and Anne S. Meyer. 2016. "Cathode Assessment for Maximizing Current Generation in Microbial Fuel Cells Utilizing Bioethanol Effluent as Substrate" Energies 9, no. 5: 388. https://doi.org/10.3390/en9050388