Modulating the Release Kinetics of Paclitaxel from Membrane-Covered Stents Using Different Loading Strategies
Abstract
:1. Introduction

2. Results and Discussion
2.1. CH-PEO/HA membrane characterization



2.2. Cell viability

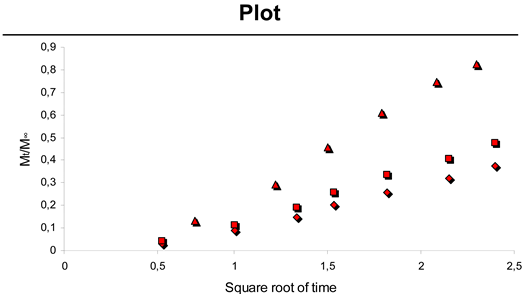
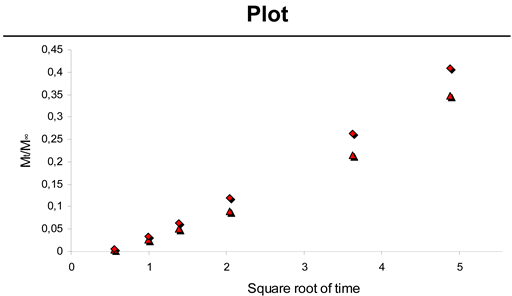
2.3. Paclitaxel Release from CH-PEO/HA membranes


| Sample | K | a | R2 |
|---|---|---|---|
| 0.60 mg Pac | 0.19 | -0.07 | 0.9959 |
| 0.51 mg Pac | 0.25 | -0.09 | 0.9956 |
| 0.24 mg Pac | 0.48 | -0.21 | 0.9959 |
 | |||



| Sample | K | a | R2 |
|---|---|---|---|
| 8.0 mg spheres | 0.08 | -0.056 | 0.9881 |
| 9.5 mg spheres | 0.09 | -0.060 | 0.9941 |
 | |||
2.4. Thrombogenecity

3. Experimental Section
3.1. Preparation of hyaluronan (HA) ester prodrug of paclitaxel (Pac)
3.2. CH-PEO membrane formation
3.3. Membrane-covered stent preparation
3.4. Self-assembled monolayer coatings: membrane-HA and membrane HA-Pac preparation
3.5. Membrane assessment during inflation
3.6. Preparation of microspheres
3.7. SEM analysis
3.8. Determination of drug loading
3.9. Drug activity assessment by cell viability studies
3.10. Drug release studies
3.10.1. Drug release study from CH-PEO/HA/Pac membranes
3.10.2. Drug release study from CH-PEO/HA + Pac membranes
3.10.3. Drug release study from CH-PEO/HA + PLGA membranes
3.11. Isolation and labelling of platelets
3.12. Statistics
4. Conclusions
Acknowledgements
References and Notes
- Dobesh, P.P.; Stacy, Z.A.; Ansara, A.J.; Enders, J.M. Drug-eluting stents: A mechanical and pharmacologic approach to coronary artery disease. Pharmacotherapy 2004, 24, 1554–1577. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Kutryk, M.J.; Ong, A.T. Coronary-artery stents. N. Engl. J. Med. 2006, 354, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Wenaweser, P.; Daemen, J.; Zwahlen, M.; van Domburg, R.; Jüni, P.; Vaina, S.; Hellige, G.; Tsuchida, K.; Morger, C.; Boersma, E.; Kukreja, N.; Meier, B.; Serruys, P.W.; Windecker, S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 2008, 52, 1134–1140. [Google Scholar]
- Plum, G.; Tabrizian, M. Clinical Outcomes of Non Pharmaceutical Stent Coatings. In Fundamentals About Stents; Proceedings of the European Symposium of Vascular Biomaterials; 2005; pp. 83–102. [Google Scholar]
- Menown, I.; Lowe, R.; Penn, I. Passive stent coatings in the drug-eluting era. J. Invasive Cardiol. 2005, 17, 222–228. [Google Scholar] [PubMed]
- Presbitero, P.; Asioli, M. Drug-eluting stents do they make the difference? Minerva Cardioangiol. 2002, 50, 431–442. [Google Scholar] [PubMed]
- Billesfeld, L.; Gerckens, U.; Muller, R.; Grube, E. Long-term evaluation of paclitaxel-coated stents for treatment of native coronary lesions. First results of both the clinical and angiographic 18 month follow-up of TAXUS I. Z. Kardiol. 2003, 92, 825–832. [Google Scholar]
- Aoki, J.; Mintz, G.S.; Weissman, N.J.; Mandinov, L.; Grube, E.; Dawkins, K.D.; Ellis, S.G.; Greenberg, J.; Yu, A.; Mann, J.T.; Cannon, L.; Cambier, P.A.; Stone, G.W. Impact of mild or moderate renal insufficiency on the intravascular ultrasonic analysis of chronic vascular response to paclitaxel-eluting and bare-metal stents (from the TAXUS IV, V, and VI trials). Am. J. Cardiol. 2008, 102, 1009–16. [Google Scholar] [CrossRef] [PubMed]
- Mehvar, R. Recent trends in the use of polysaccharides for improved delivery of therapeutic agents: pharmacokinetic and pharmacodynamic perspectives. Curr. Pharm. Biotechnol. 2003, 4, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Merhi, Y.; Silver, J.; Tabrizian, M. Biodegradable membrane-covered stent from chitosan-based polymers. J. Biomed. Mater. Res. A. 2005, 75, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs. 1990, 18, 1–24. [Google Scholar] [PubMed]
- Budtova, T.; Belnikevich, N.; Kalyuzhnaya, L.; Alexeev, V.; Bronnikov, S.; Vesnebolotskkaya, S.; Zoolshoev, Z. Chitosan modified by poly(ethylene oxide): Film and mixture properties. J. Appl. Polym. Sci. 2002, 84, 1114–1122. [Google Scholar] [CrossRef]
- Neto, C.G.; Dantas, T.N.; Fonseca, J.L.; Pereira, M.R. Permeability studies in chitosan membranes. Effects of crosslinking and poly(ethylene oxide) addition. Carbohydr. Res. 2005, 340, 2630–2636. [Google Scholar]
- Amiji, M. Permeability and blood compatibility properties of chitosan-poly(ethylene oxide) blend membranes for haemodialysis. Biomaterials 1995, 16, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Amiji, M.; Park, K. Surface modification of polymeric biomaterials with poly(ethylene oxide), albumin, and heparin for reduced thrombogenicity. J. Biomater. Sci. Polym. Ed. 1993, 4, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Winnik, F.M.; Merhi, Y.; Silver, J.; Tabrizian, M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules 2003, 4, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Kujawa, P.; Tkaczyk, C.; Winnik, F.M.; Bilodeau, L.; Tabrizian, M. Delivery platform for hydrophobic drugs: prodrug approach combined with self-assembled multilayers. J. Am. Chem. Soc. 2005, 127, 1626–1627. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Moussa, I. Percutaneous coronary intervention and drug-eluting stents. Can. Med. Ass. J. 2005, 172, 323–325. [Google Scholar] [CrossRef]
- Schneider, A.; Vodouhe, C.; Richert, L.; Francius, G.; Le Guen, E.; Schaaf, P.; Voegel, J.C.; Frisch, B.; Picart, C. Multifunctional polyelectrolyte multilayer films: Combining mechanical resistance, biodegradability, and bioactivity. Biomacromolecules 2007, 8, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Markou, C.P.; Salame, M.Y.; Wan, B.; King, S.B.; Robinson, K.A.; Chronos, N.A.; Hanson, S.R. Reduced thrombus formation by hyaluronic acid coating of endovascular devices. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Hoffman, A.S. Preparation and characterization of biocompatible polyelectrolyte complex multilayer of hyaluronic acid and poly-l-lysine. Int. J. Biol. Macromol. 2005, 37, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Heublein, B.; Evagorou, E.G.; Rohde, R.; Ohse, S.; Meliss, R.R.; Barlach, S.; Haverich, A. Polymerized degradable hyaluronan--a platform for stent coating with inherent inhibitory effects on neointimal formation in a porcine coronary model. Int. J. Artif. Organs 2002, 25, 1166–1173. [Google Scholar] [PubMed]
- Lu, H.; Hu, N. Loading behavior of (chitoan/hyaluronic acid)n layer-by-layer assembly films toward myoglobin: an electrochemical study. J. Phys. Chem. B 2006, 110, 23710–23718. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, H.M.; Glinski, J.A.; Hernandez, M.; Haugwitz, R.D.; Narayanan, V.L.; Suffness, M.; Zalkow, M.L. Synthesis of congeners and prodrugs. 3. Water-soluble prodrugs of taxol with potent antitumor activity. J. Med. Chem. 1989, 32, 788–792. [Google Scholar]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, J.G.; Tanguay, J.F.; Chauvet, P.; Merhi, Y. Relationship between platelets and neutrophil adhesion and neointimal growth after repeated arterial wall injury induced by angioplasty in pigs. J. Vasc. Res. 2001, 38, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Mehilli, J.; Dirschinger, J.; Dotzer, F.; Schuhlen, H.; Neumann, F.J.; Fleckenstein, M.; Pfafferott, C.; Seyfarth, M.; Schomig, A. Intracoronary stenting and angiographic results: Strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001, 103, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ziebell, M.R.; Prestwich, G.D. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules 2000, 1, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Plum, G. Freisetzungsverhalten von unterschiedlich hydrophil/hydrophoben Modellsubstanzen aus Poly-(D,L-lactid)- und Poly-(D,L-lactid)-co-PEO-co-poly-(D,L-lactid)-Mikrosphären und pharmazeutische Anwendungen. Thesis, RWTH-Aachen, 2004. [Google Scholar]
- Obara, K.; Ishihara, M.; Ozeki, Y.; Ishizuka, T.; Hayashi, T.; Nakamura, S.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Takase, B.; Ishihara, M.; Kikuchi, M.; Maehara, T. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J. Contr. Release. 2005, 110, 79–89. [Google Scholar] [CrossRef]
- Siepmann, J.; Elkharraz, K.; Siepmann, F.; Klose, D. How autocatalysis accelerates drug release from PLGA-based microparticles: a quantitative treatment. Biomacromolecules 2005, 6, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, L.P.; Srinivas, A.; Ramamurthi, A. In vitro hemocompatibility testing of UV-modified hyaluronan hydrogels. Biomaterials 2006, 27, 1416–1424. [Google Scholar] [CrossRef]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sydow-Plum, G.; Haidar, Z.S.; Merhi, Y.; Tabrizian, M. Modulating the Release Kinetics of Paclitaxel from Membrane-Covered Stents Using Different Loading Strategies. Materials 2008, 1, 25-43. https://doi.org/10.3390/ma1010025
Sydow-Plum G, Haidar ZS, Merhi Y, Tabrizian M. Modulating the Release Kinetics of Paclitaxel from Membrane-Covered Stents Using Different Loading Strategies. Materials. 2008; 1(1):25-43. https://doi.org/10.3390/ma1010025
Chicago/Turabian StyleSydow-Plum, Georg, Ziyad S. Haidar, Yahye Merhi, and Maryam Tabrizian. 2008. "Modulating the Release Kinetics of Paclitaxel from Membrane-Covered Stents Using Different Loading Strategies" Materials 1, no. 1: 25-43. https://doi.org/10.3390/ma1010025





