Microwave-Assisted Polyol Synthesis of Water Dispersible Red-Emitting Eu3+-Modified Carbon Dots
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Analytical Tools
3. Results and Discussion
3.1. Eu-Modified C-Dots via Polyol Synthesis and Conventional Resistance Heating
3.2. Eu3+-Modified C-Dots via Polyol Synthesis and MW-Heating
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots-emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]
- Wang, Q.H.; Bellisario, D.O.; Drahushuk, L.W.; Jain, R.M.; Kruss, S.; Landry, M.P.; Mahajan, S.G.; Shimizu, S.F.E.; Ulissi, Z.W.; Strano, M.S. Low dimensional carbon materials for applications in mass and energy transport. Chem. Mater. 2014, 26, 172–183. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Q.; Zhu, Y.; Tan, B.; Xu, Z.P.; Dou, S.X. Ultra-small fluorescent inorganic nanoparticles for bioimaging. J. Mater. Chem. B 2014, 2, 2793–2918. [Google Scholar]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv. Mater. 2015, 27, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X. Carbon Dots with continuously tunable full-color emission and their application in Ratiometric pH sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, Y.; Kalytchuk, S.; Kershaw, S.V.; Wang, Y.; Wang, P.; Zhang, T.; Zhao, Y.; Zhang, H.; et al. Color-switchable electroluminescence of carbon dot light-emitting diodes. ACS Nano 2013, 7, 11234–11241. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.Q.; Yang, K.; Ma, Z.; Wan, J.M.; Zhang, Y.J.; Kang, Z.H.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.G.; Farkas, D. Biomedical Optical Imaging; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Dong, H.; Kuzmanoski, A.; Gößl, D.M.; Popescu, R.; Gerthsen, D.; Feldmann, C. Polyol-mediated C-dot formation showing efficient Tb3+/Eu3+ emission. Chem. Commun. 2014, 50, 7503–7506. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Roming, M.; Feldmann, C. Unexpected fluorescence of polyols and PEGylated nanoparticles derived from carbon dot formation. Part. Part. Syst. Charact. 2015, 32, 467–475. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.C.; Feldmann, C. Polyol synthesis of nanoparticles: Status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.; Zboril, R. Toxicity of carbon dots—Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Q.; Wang, J.; Zhang, C. Imaging two targets in live cells based on rational design of lanthanide organic structure appended carbon dots. Carbon 2015, 93, 671–680. [Google Scholar] [CrossRef]
- Song, Y.; Chen, J.; Hu, D.; Liu, F.; Li, P.; Li, H.; Chen, S. Ratiometric fluorescent detection of biomakers for biological warfare agents with carbon dots chelated europium-based nanoscale coordination polymers. Sens. Actuators B 2015, 221, 586–592. [Google Scholar] [CrossRef]
- Ye, Z.; Tang, R.; Wu, H.; Wang, B.; Tan, M.; Yuan, J. Preparation of europium complex-conjugated carbon dots for ratiometric fluorescence detection of copper(II) ions. New J. Chem. 2014, 38, 5721–5726. [Google Scholar] [CrossRef]
- Samanta, T.; Hazra, C.; Mahalingam, V. C-dot sensitized Eu3+ luminescence from Eu3+-doped LaF3-C dot nanocomposites. New J. Chem. 2016, 39, 106–109. [Google Scholar] [CrossRef]
- De Mello, J.C.; Wittmann, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 1997, 9, 230–232. [Google Scholar] [CrossRef]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure, Reactivity; Pearson: New York, NY, USA, 2008. [Google Scholar]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Shiral, K.A.; Fern, O.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chatti, M.; Mallick, A.; Purkayastha, P. pH triggered reversible photoinduced electron transfer to and from carbon nanoparticles. Chem. Commun. 2014, 50, 6890–6893. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.; Esteves da Silva, J.C. Fluorescent carbon dots capped with PEG200 and mercaptosuccinic acid. J. Fluoresc. 2010, 20, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zheng, H.; Long, Y.; Du, J.; Hao, J.; Wang, L.; Zhou, D. Study on the fluorescence characteristics of carbon dots. Spectrochim. Acta A 2010, 75, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Blasse, G.; Grabmaier, C. Luminescent Materials; Springer: Berlin, Germany, 1994. [Google Scholar]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Shi, W.; Xu, N.; Cheng, P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg. Chem. 2013, 52, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Thielemann, D.T.; Wagner, A.T.; Birtalan, E.; Kölmel, D.; Heck, J.; Rudat, B.; Neumaier, M.; Feldmann, C.; Schepers, U.; Bräse, S.; et al. A luminescent cell-penetrating pentadecanuclear lanthanide cluster. J. Am. Chem. Soc. 2013, 135, 7454–7457. [Google Scholar] [CrossRef] [PubMed]

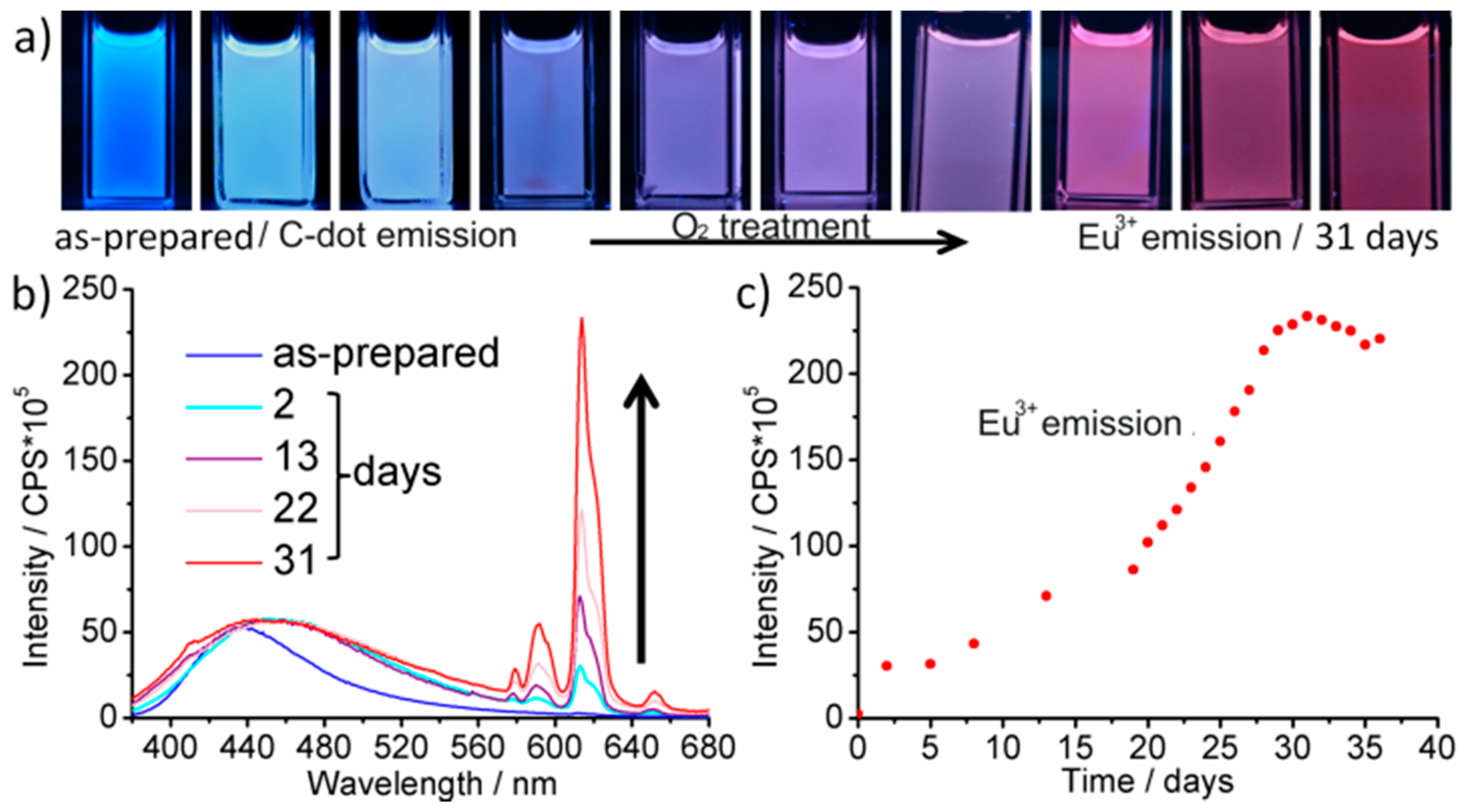
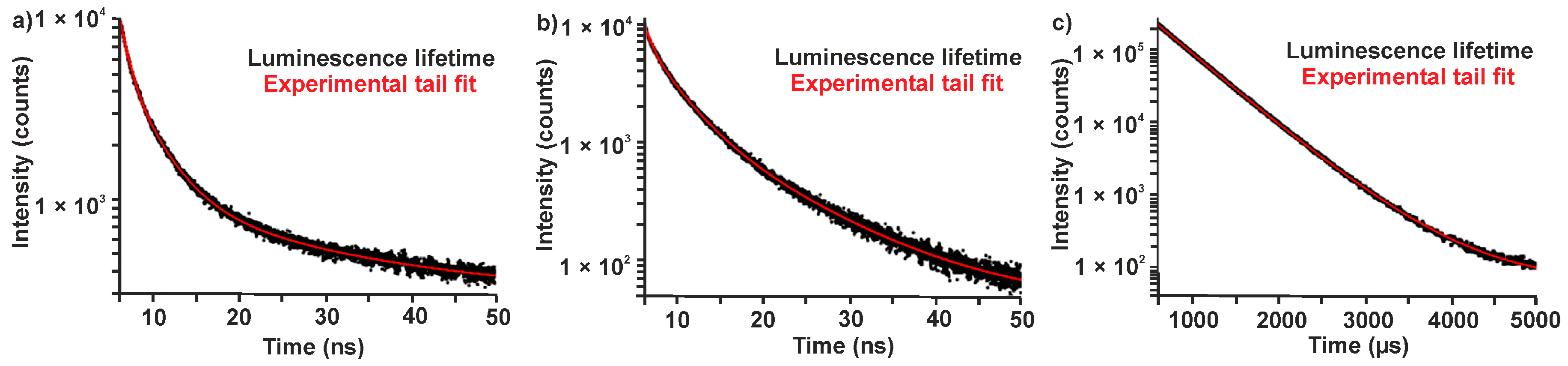

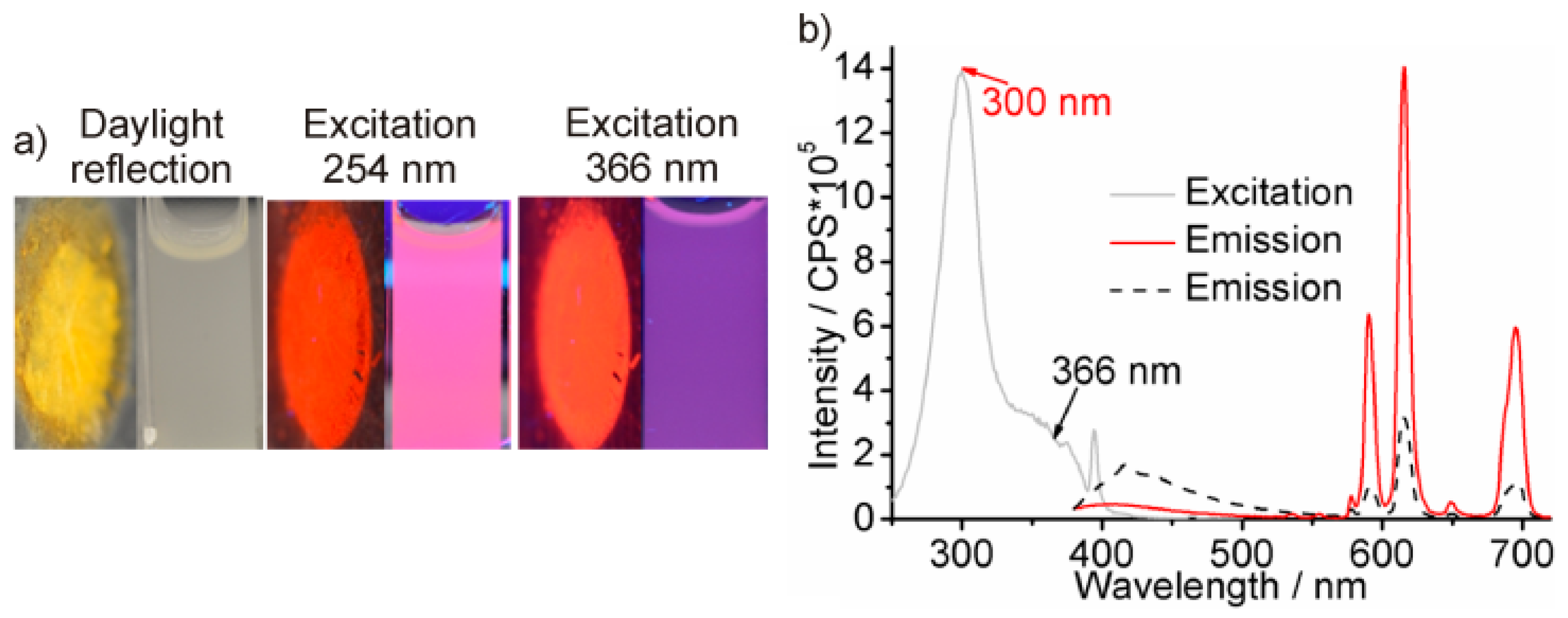

| Sample | λem/nm | B1/% | τ1/ns | B2/% | τ2/ns | B3/% | τ3/ns | χ2 |
|---|---|---|---|---|---|---|---|---|
| As-prepared (Eu2+-modified) | 440 | 14.9 | 0.57 ± 0.01 | 45.3 | 2.56 (±0.02) | 39.7 | 11.31 ± 0.10 | 1.23 |
| Dry-air treatment (Eu3+-modified) | 440 | 11.9 | 0.53 ± 0.01 | 37.0 | 2.52 (±0.03) | 51.1 | 8.43 ± 0.04 | 1.23 |
| Dry-air treatment (Eu3+-modified) | 615 | 93.7 | 474 × 103 ±1.0 | 6.3 | 875 × 103 (±15) | / | / | 1.36 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Kuzmanoski, A.; Wehner, T.; Müller-Buschbaum, K.; Feldmann, C. Microwave-Assisted Polyol Synthesis of Water Dispersible Red-Emitting Eu3+-Modified Carbon Dots. Materials 2017, 10, 25. https://doi.org/10.3390/ma10010025
Dong H, Kuzmanoski A, Wehner T, Müller-Buschbaum K, Feldmann C. Microwave-Assisted Polyol Synthesis of Water Dispersible Red-Emitting Eu3+-Modified Carbon Dots. Materials. 2017; 10(1):25. https://doi.org/10.3390/ma10010025
Chicago/Turabian StyleDong, Hailong, Ana Kuzmanoski, Tobias Wehner, Klaus Müller-Buschbaum, and Claus Feldmann. 2017. "Microwave-Assisted Polyol Synthesis of Water Dispersible Red-Emitting Eu3+-Modified Carbon Dots" Materials 10, no. 1: 25. https://doi.org/10.3390/ma10010025






