1. Introduction
Stable graphene, as a two-dimensional single sheet, was firstly discovered by Andre Geim and Konstantin Novoselov [
1]. Graphene plays a very important role in nano-materials due to its excellent physical and electrical properties [
2,
3], and in polymer fields, many applications of graphene have been reported in that it can significantly improve the properties of polymers [
4]. However, graphene is not suitable to be produced on a large scale due to the limited existing producing technologies [
5]. Graphene oxide (GO) is the precursor of graphene, which is more convenient to be produced at a large scale. It also has a two-dimensional structure and inherits the beneficial properties of graphene.
So many researchers have investigated the possibilities of modifying polymers with GO. Chen used GO in epoxy/silica composites and found that it could greatly enhance the mechanical properties of epoxy resin [
6]. Yongjin found that the conductivity, thermal, mechanical, and rheological properties of poly(methylmethacrylate) were improved by introducing GO [
7]. As reported in Zhenhai’s paper, GO could significantly increase the modulus and tensile strength of butadiene–styrene–vinyl pyridine rubber [
8]. Moreover, various polymers, such as carboxylated acrylonitrile butadiene rubber [
9,
10], polypropylene [
11], and poly(arylene disulfide) [
12], that were modified by GO to enhance their physical properties. In addition to the increasing physical properties, a gas barrier feature is also endowed with GO-modified polymers. GO sheet is impermeable for different gases (He, O
2, CO
2, etc.) due to the high potential energy barrier, which provides many potential applications, such as inhibiting oxidation and aging of polymers [
13].
Asphalt is considered as a kind of organic mixture, which is made by the distillation of crude oil. Just like polymers, asphalt also shows viscous-elastic properties. It has been widely applied in the pavement constructions for its comfortableness and smoothness. However, it is also facing many problems that may shorten the serving time of pavements. The poor elastic modulus and high viscous modulus in summer may lead to rutting on the road surface. On the contrary, a high elastic modulus and poor viscous modulus in winter may lead to surface cracking. Moreover, another of the most serious challenges of asphalt roads is oxidation aging [
14,
15,
16]. Many researchers have investigated traditional anti-aging additives (graphite, montmorillonite) to improve the physical properties and ease the aging process that happens in the serving period [
17,
18,
19]. Moreover, some novel materials (TIO
2, CeO
2, Layered Double Hydroxides) have been studied for their enhancing strength and anti-aging characters [
20,
21,
22]. So far, modifiers of asphalt is one of the most popular research fields, and more novel materials should be investigated to produce the ideal asphalt.
GO, as described before, is a potential material to improve the physical properties and anti-aging ability of asphalt. Thus, in this paper, GO was introduced into asphalt to investigate how it influences the asphalt properties and whether GO could improve the anti-aging properties of asphalt. In this paper, the softening point and penetration were used to evaluate the physical properties. Rheological properties were also carried out to study the effects of GO on asphalt. In the meantime, the anti-aging property was also investigated by these three tests. Finally, the reason why GO does not show significant improvement on the anti-aging property was explained and some interesting applications (warm mixing and flame retardants) were discussed.
2. Experiments
2.1. Materials
GO was bought from Heng Qiu Graphene Technology (Suzhou) Co., Ltd. (Suzhou, China).
Two types of asphalts, with 60/80 pen grade and 80/90 pen grade, were applied in this investigation. These asphalts were supplied by KOCH Asphalt Co., Ltd. (Ezhou, China). The physical properties of two asphalts were listed in
Table 1. 70A and 90A are abbreviations of asphalts with 60/80 pen grade and 80/90 pen grade, respectively.
2.2. Aging Procedure
70A, 90A, and GO-modified asphalts were firstly aged by a thin film oven test (TFOT) at 163 °C. Then the residues from the TFOT were aged by a pressure aging vessel (PAV) test at 95 °C and 2.1 MPa air pressure. TFOT was executed in accordance with ASTM D1754, and the PAV test in accordance with ASTM D6521.
2.3. Preparation Procedure of GO-Modified Asphalt
In general, there are three ways to prepare the GO modified polymer. The first one is the solution-mixing method. However, complete evaporation of the solution is the crucial problem with this method [
23]. The second preparation method is in situ polymerization. However, in situ polymerization also faces the evaporation problem because the solution state also has to be applied when in situ polymerization is conducted [
24]. The last method is the melting method. In the melting method, mechanical mixing is applied to disperse GO into molten polymers. It is a very convenient, economical, and environmentally friendly method to produce GO-modified polymers on a large scale [
25]. In this paper, it is hard to evaporate carbon disulfide, trichloroethylene, or xylene if these solutions were used to prepare the GO-modified asphalt. Thus, the melt method was selected to produce the GO-modified asphalts.
Firstly, the molten asphalt was prepared as the matrix at 135 °C. Then 1% and 3% (mass percentage) of GO were introduced into the two asphalts, respectively. Moreover, in order to produce a good dispersed GO-modified asphalt, a high shear mixer was used to shear and mix the GO in the asphalt. Finally, the GO-modified asphalt was prepared.
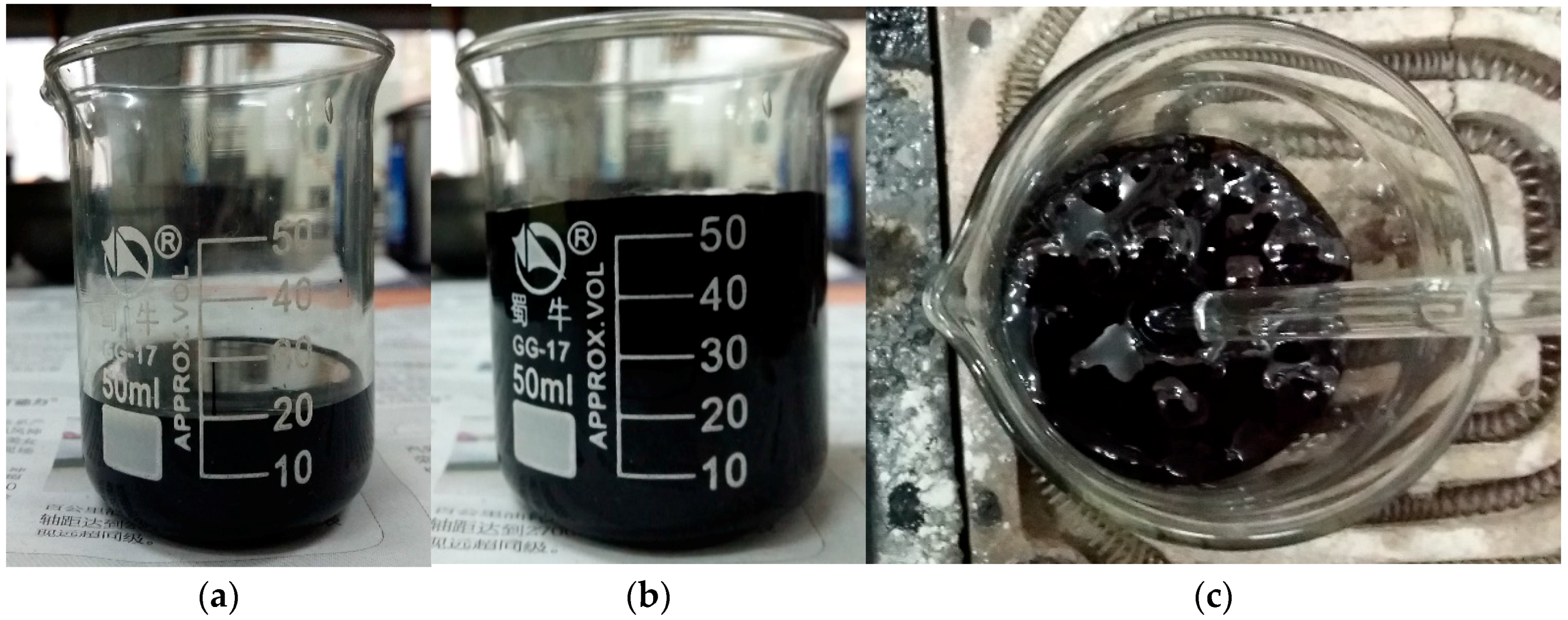
In the preparation process, an interesting phenomenon was found. When heating the GO-modified asphalt, a large amount of gas was released above 115 °C.
Figure 1 shows the gas was released in the GO-modified asphalt and the volume of modified asphalt was increased by almost three times. Then the expansive volume was reduced to its initial state after cooling. In this paper, the boiling reaction is defined for this phenomenon because it is similar to boiling water.
2.4. Test Method
2.4.1. Dynamic Shear Rheometer (DSR) Tests
The DSR (MCR101, Anton Paar, Graz, Austria) was used to investigate the rheological properties of both GO-modified asphalts and base asphalts before and after TFOT and PAV aging. Both high and low temperature sweep tests were performed under strain-controlled mode with a constant frequency of 10 rad/s. The testing temperature ranged from −10 °C to 30 °C and 30 °C to 80 °C with temperature increment of 2 °C per minute. When the temperature is below 30 °C, plates with 8 mm diameter and a 2 mm gap were applied. When the temperature is above 30 °C, plates with 25 mm diameter and a 1 mm gap were used.
2.4.2. Softening and Penetration Tests
The penetration (25 °C, 0.1 mm) and softening point (°C) of the binders were tested in accordance with standards ASTM D564 and ASTM D3626, respectively.
2.4.3. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS) Test
Pyrolysis-gas chromatography/mass spectrometry is a very useful method to investigate the volatile gases of organic materials at certain temperatures. In this research, a Py-GC/MS machine (Agilent 6890N/5975, Agilent, Santa Clara, CA, USA) was used to determine what kinds of gases were released from GO modified asphalt. In this test, GO modified asphalt was pyrolyzed at 115 °C. Then the released gases were analyzed by gas chromatography and mass spectrometry to investigate the gas components.
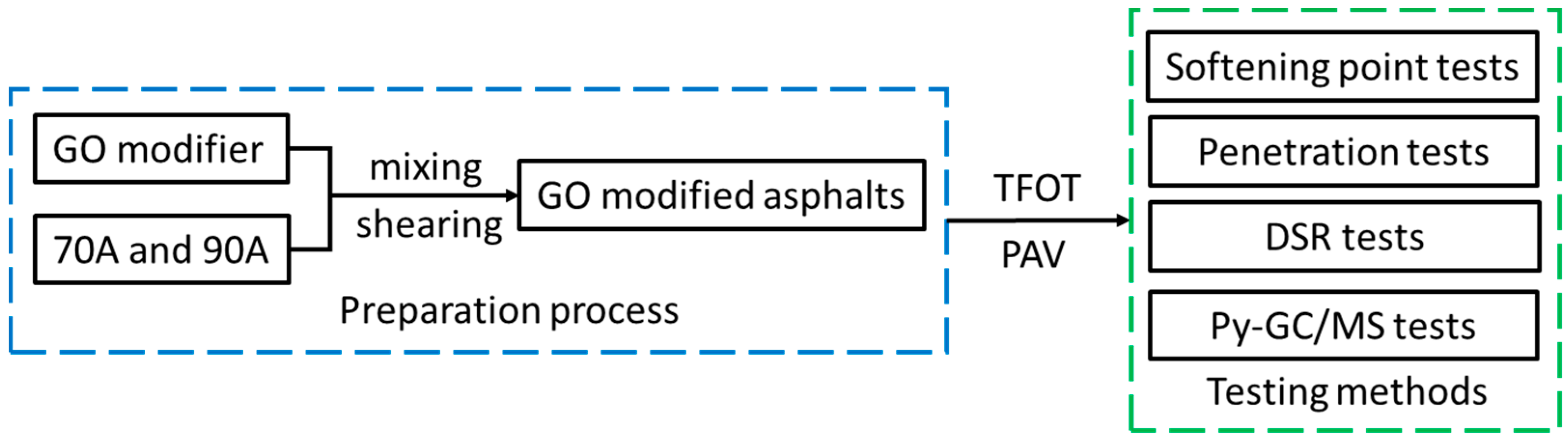
2.5. Experimental Plan
The experimental plan is shown in
Figure 2. Firstly, the GO modifier and base asphalts were mixed and sheared to prepare GO-modified asphalts. Then, TFOT and TFOT+PAV aging were applied to simulate the aging process. Finally, softening point tests, penetration tests, DSR tests, and Py-GC/MS tests were used to evaluate how GO would influence the properties of asphalts.
3. Results and Discussion
3.1. Softening Point Tests
The softening point could be used to evaluate the soft and hard degree of asphalt. Once asphalt is oxidized, the softening point of it increases. Thus, a greater increase in the softening point indicates a more serious aging index of the asphalt after aging.
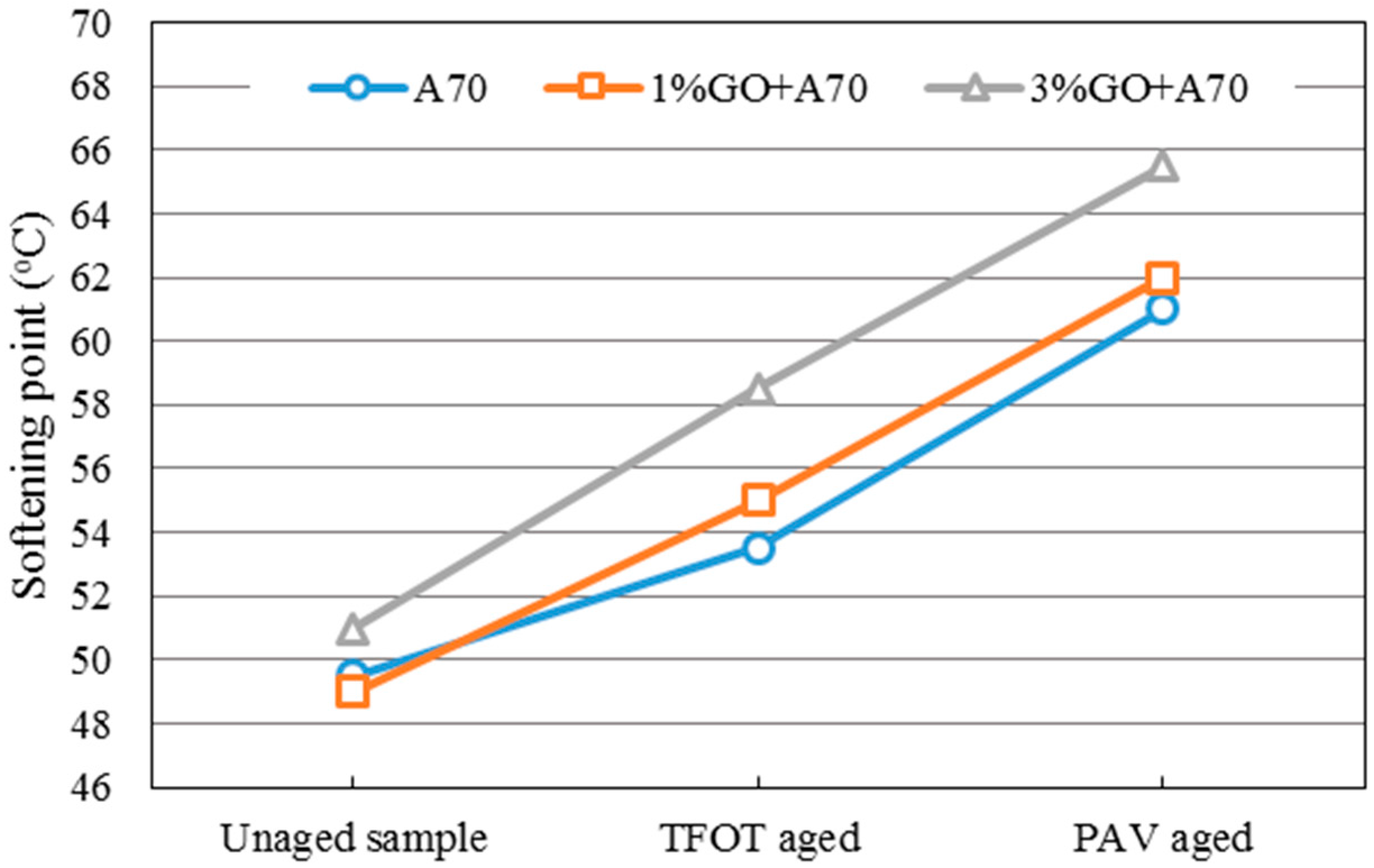
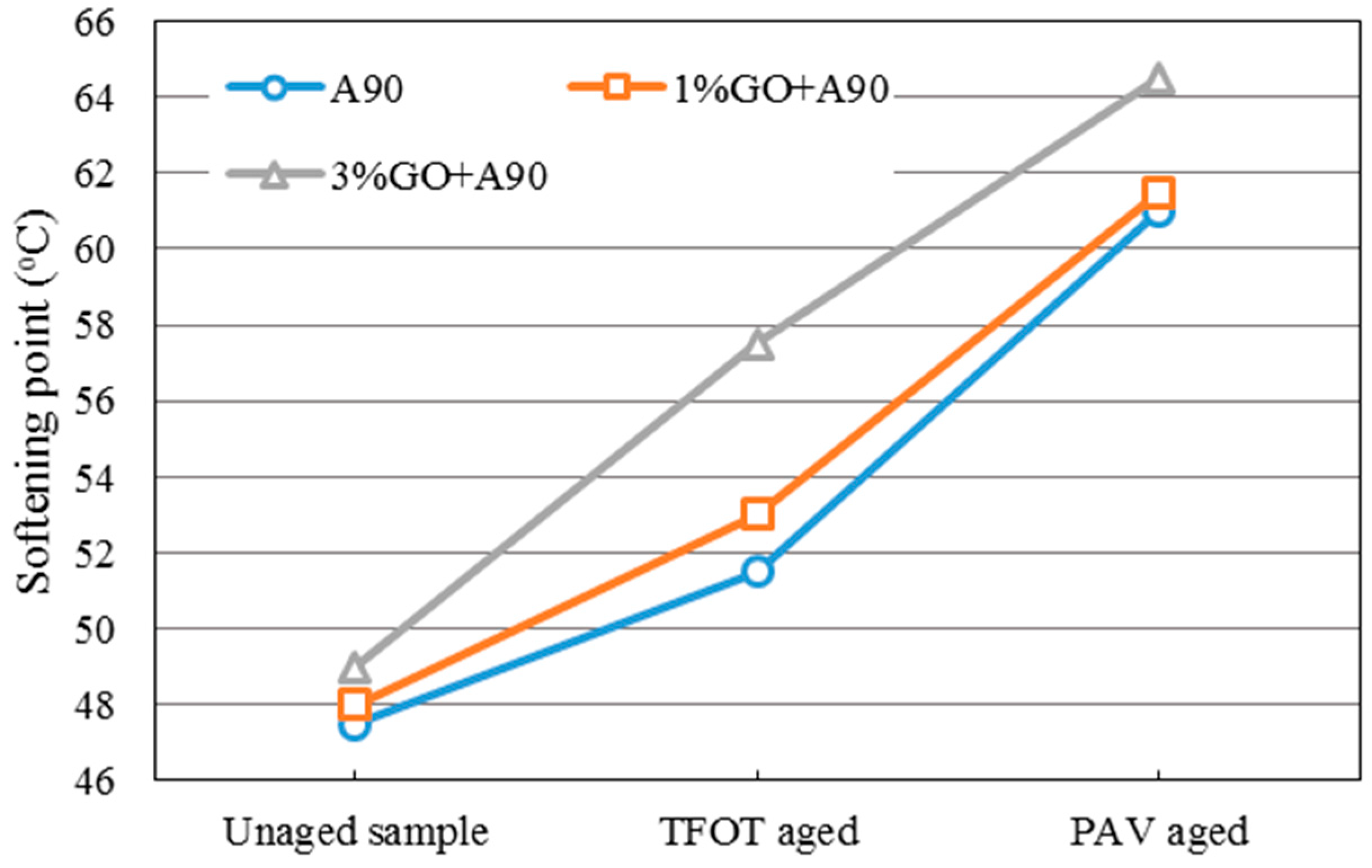
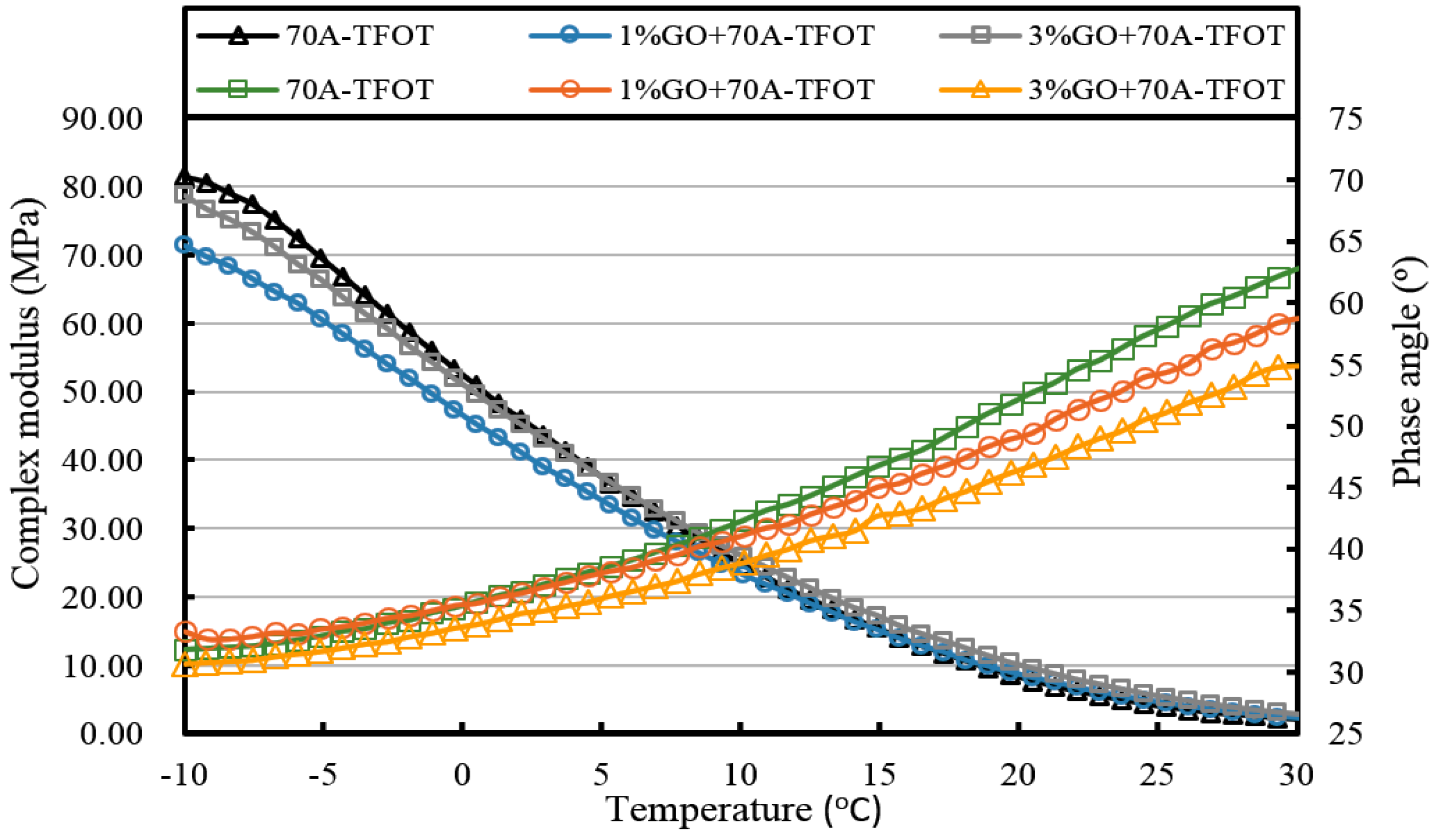
Figure 3 and
Figure 4 showed the changing tendency of the softening point of GO-modified 70A, GO-modified 90A, and their matrix before and after aging.
From both graphs, it was obvious that the softening point (SP) of GO-modified asphalts and the base asphalts were almost the same before aging when the GO content is 1%. In addition, 1% GO-modified asphalts showed greater increments compared to both base asphalts after TFOT aging process. However, softening points of both modified asphalts with 1% GO were almost the same as the base asphalt after the PAV aging process. This phenomenon could be attributed to the boiling reaction when the temperature was above 115 °C. Gas was released in the TFOT aging process in which the aging temperature was 163 °C. The GO-modified asphalts were boiled by gas in the TFOT oven and it resulted in a similar stir action in the inner part of asphalts. So more air was exposed to asphalt, more serious aging index of asphalt happened. Moreover, the aging temperature was only 90 °C in the PAV aging process, at which temperature the boiling reaction would not occur. The sheet structure and impermeable property of GO could block the permeation of air. It could slow down the oxidation rate of asphalt and lead to a similar SP value of both modified and base asphalts after PAV aging.
When the content of GO was 3% in 70A and 90A, the softening point was much higher than that of matrix asphalt before and after aging, compared to 1% GO-modified asphalt. Before aging, it was easily understandable that inorganic powder (GO) enhanced the hardness of asphalt. In addition, with more GO content, the boiling reaction was more severe in the TFOT aging process. Thus, a much higher SP value of 3% GO-modified asphalt is shown in
Figure 3 and
Figure 4 after TFOT aging. For PAV aging, the increasing slope of the 3% modified asphalt between TFOT and PAV aging was slightly smaller than that of the 1% modified asphalt. This might mean the anti-aging property was improved slightly.
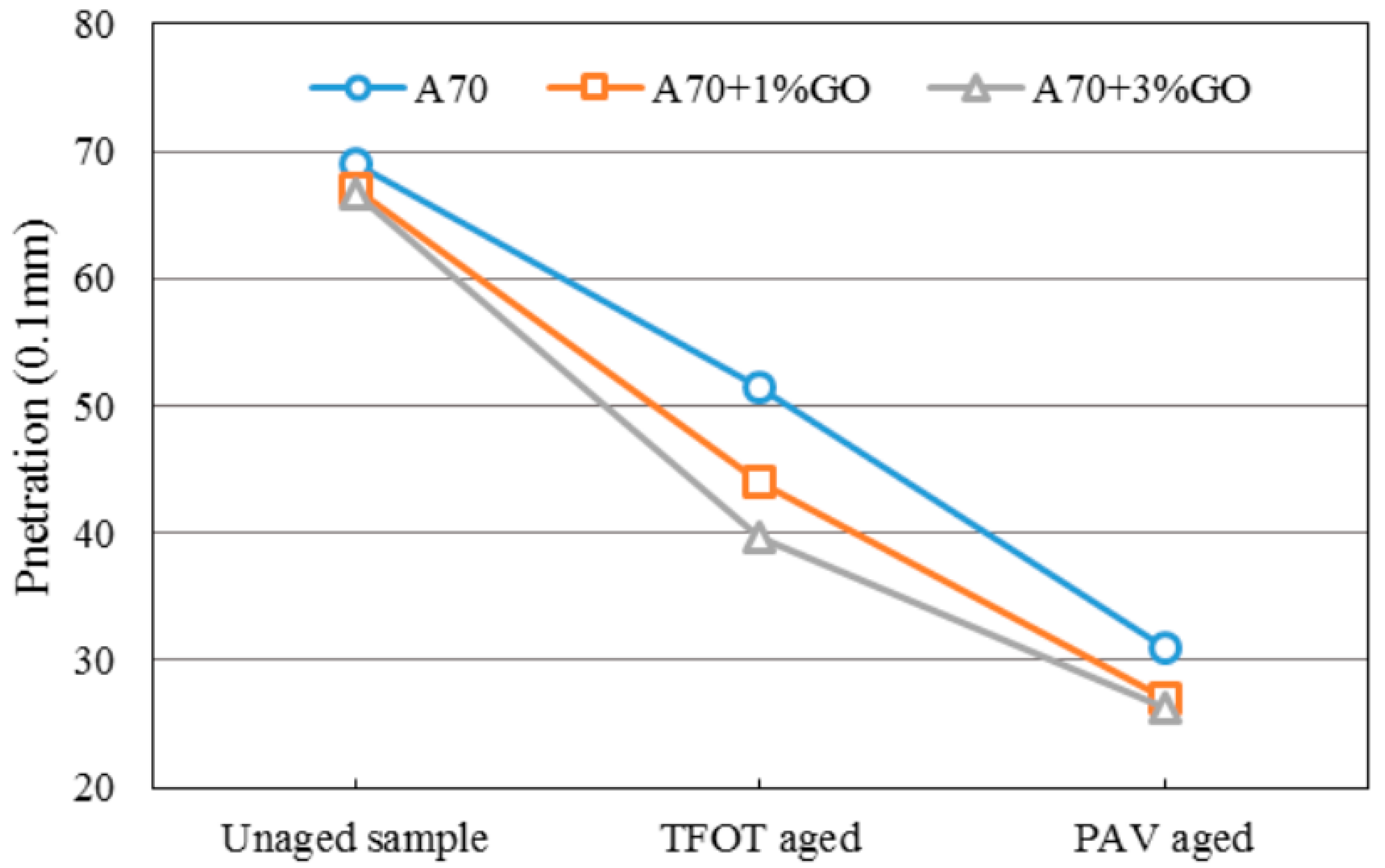
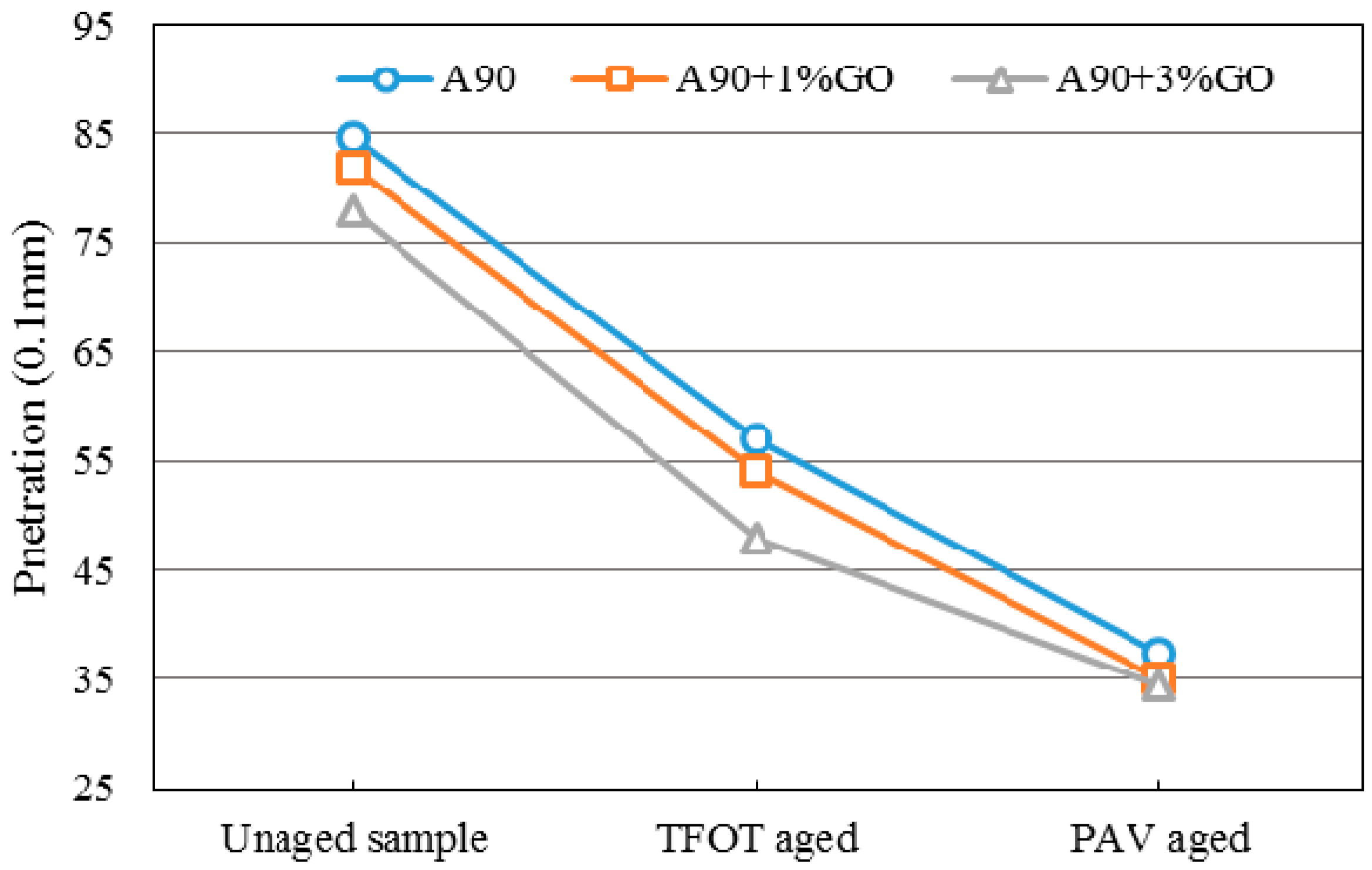
3.2. Penetration Tests
Penetration presents a degree of consistency for asphalt, indicating the ability of resisting shear failure. Under certain conditions, it could reflect the relative softness and hardness degree of asphalt. A larger penetration indicates that asphalt is softer; conversely, a smaller penetration means that asphalt is harder.
Figure 5 and
Figure 6 display the penetration changes of modified and base asphalts before and after aging. With the addition of GO, the penetration (Pen) of modified asphalts all decreased slightly before aging, and the Pen decrement of GO-modified asphalt was significantly larger and slightly smaller than base asphalt after TOFT aging and PAV aging, respectively. The changing tendency of Pen was the same as the SP.
The penetration and softening point results showed GO might promote the anti-aging property of asphalts. However, the anti-aging effects were not so obvious.
3.3. Temperature Sweep
3.3.1. High-Temperature Sweep
In high temperature conditions, the viscosity of asphalt reduces and its flowability increases. This is the main cause of asphalt pavement rutting damage at high temperatures. Thus, the larger complex modulus means the better high temperature performance, which leads to a better ability to resist rutting in a high temperature environment. At the same time, phase angle can also be used to evaluate the ratio of the viscous component and the elastic component. The lower phase angle shows better deformation recovery ability of asphalts.
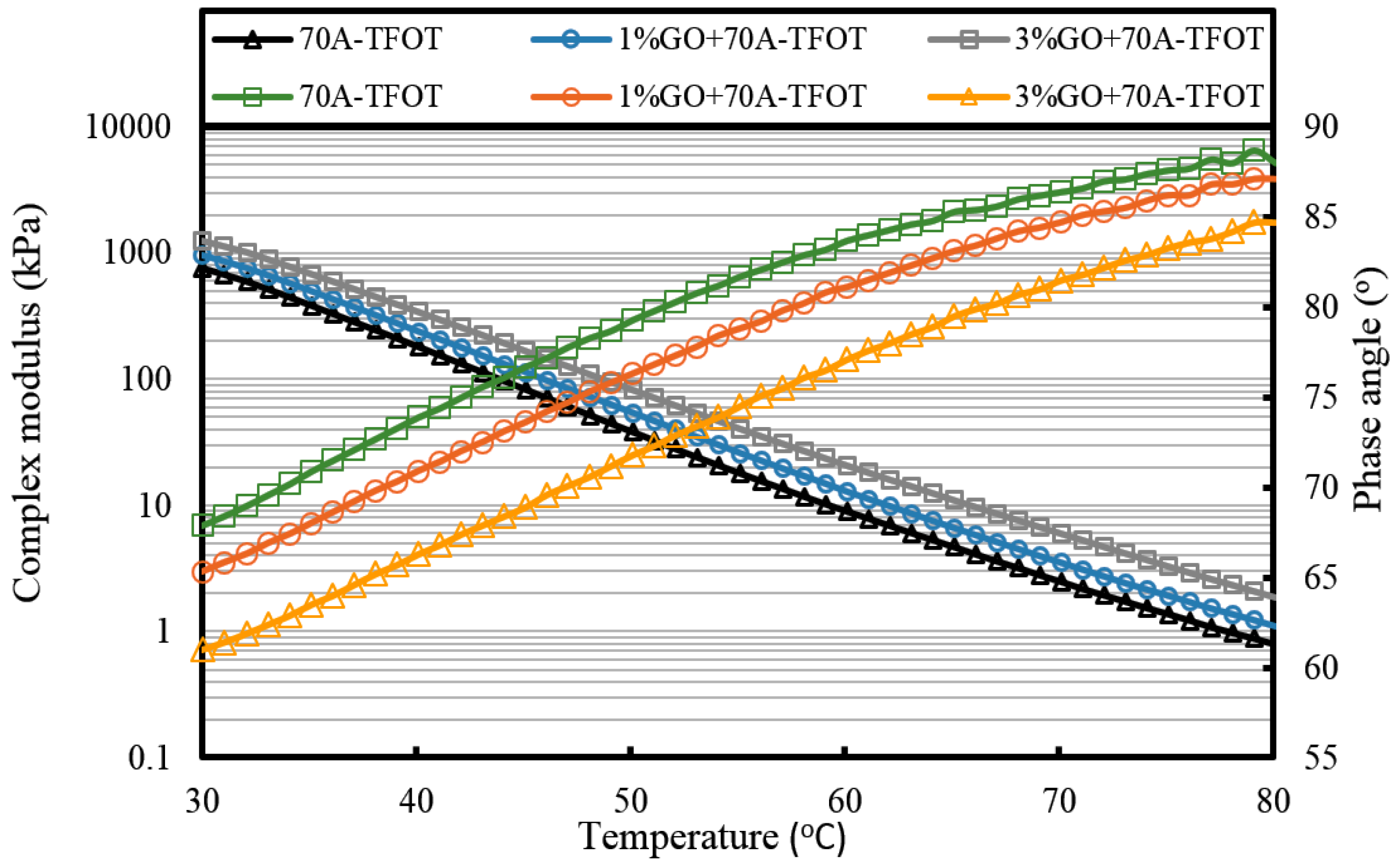
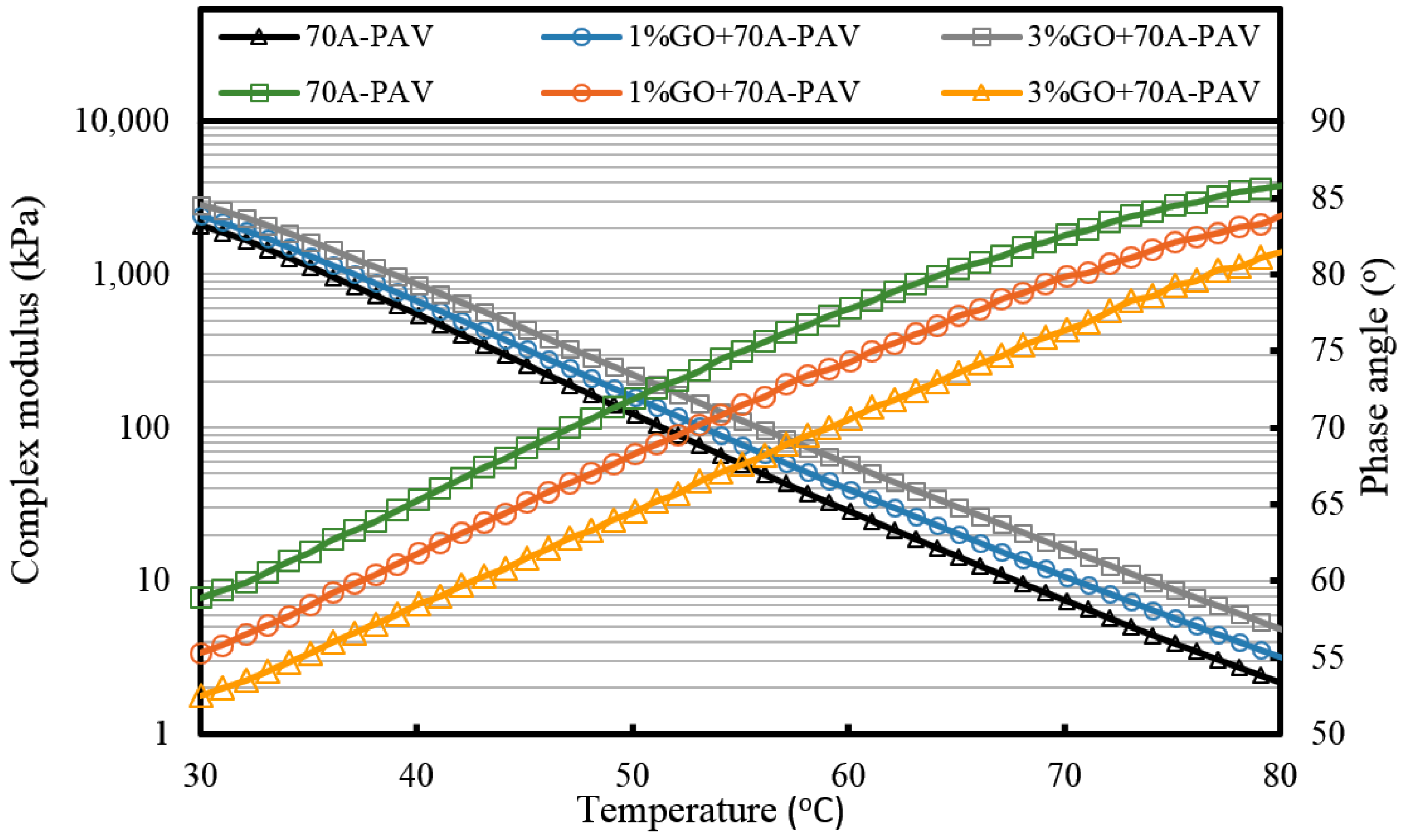
It can be seen from
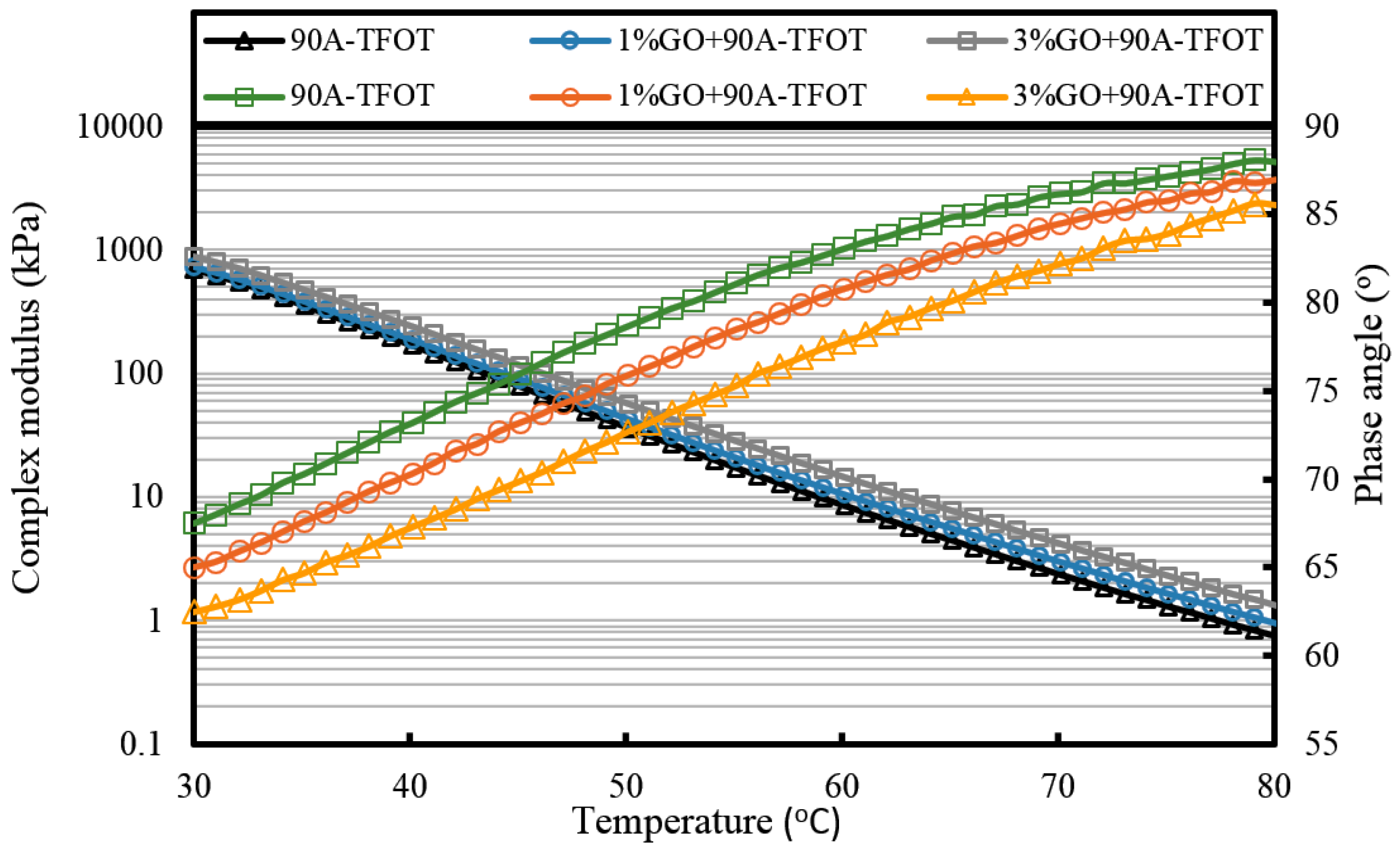
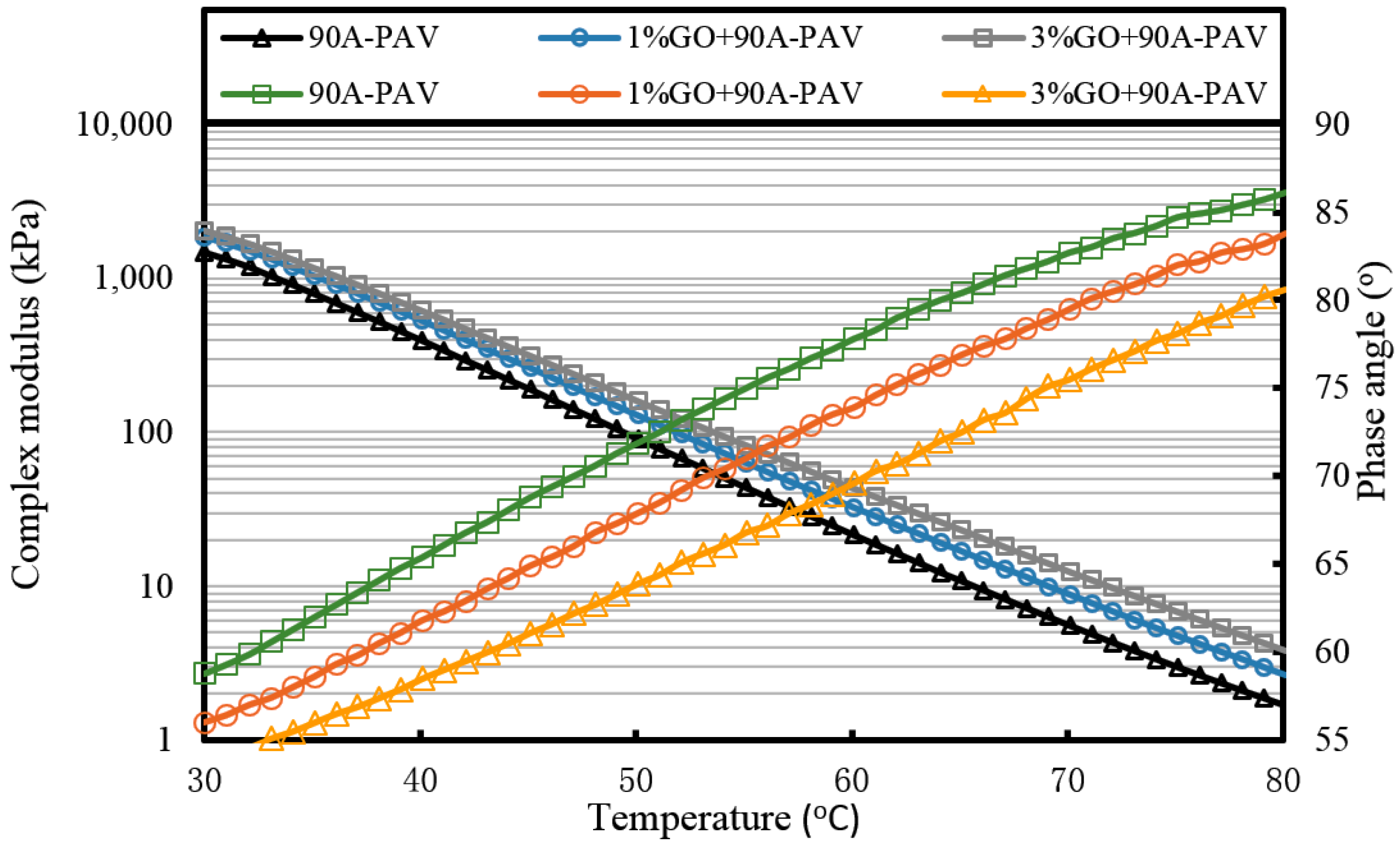
Figure 7 that the complex modulus gradually increases and the phase angle reduces with the increasing weight ratio of GO. This means the introduction of GO can improve the high temperature properties of asphalt. After TFOT and PAV aging (seen from
Figure 8 and
Figure 9, and
Table 2), the complex modulus and the phase angle exhibited the same regularity as samples before aging. The complex modulus of 3% GO-modified 70A was the largest, 1% GO-modified 70A was at the median, and 70A was the least. On the contrary, the phase angle of 3% GO-modified 70A was the least, 1% GO-modified 70A was at the median, and 70A was the largest. From
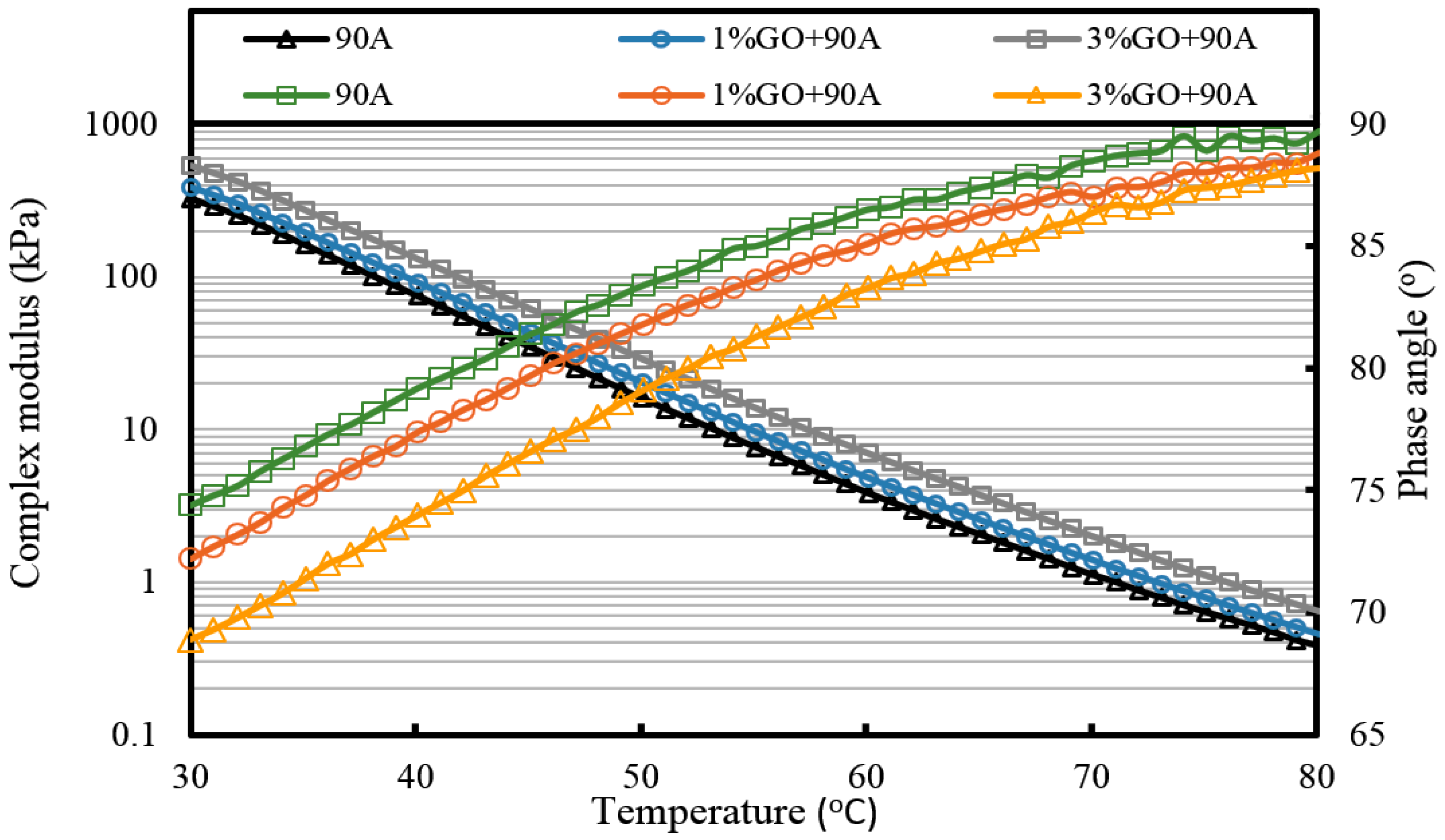
Table 2, it can be seen that the complex modulus of 3% GO-modified 70A and 90A were about 1.8 times larger than 70A and 90A at 50 °C, respectively, after PAV aging. Moreover, the complex modulus of 3% GO-modified asphalts were 1.2 times larger than the base asphalts at 80 °C. The distinguished modulus of GO sheet could surely increase the complex modulus and reduce the phase angle. However, the anti-aging properties seemed unimproved by the introduction of GO sheet. The aging of asphalt leads to the increasing modulus and reducing phase angle. If GO slows down the oxidation of asphalt, the modulus of GO-modified 70A is lower than that of neat 70A after TFOT and PAV aging, or the modulus increment of GO-modified 70A is obviously smaller than neat 70A after aging. However, the increasing tendency of the modulus and reducing tendency of the phase angle for GO-modified 70A was almost the same as 70A after TFOT and PAV aging. Thus, from these statistics, GO could only improve the high-temperature properties, but not the anti-aging ability.
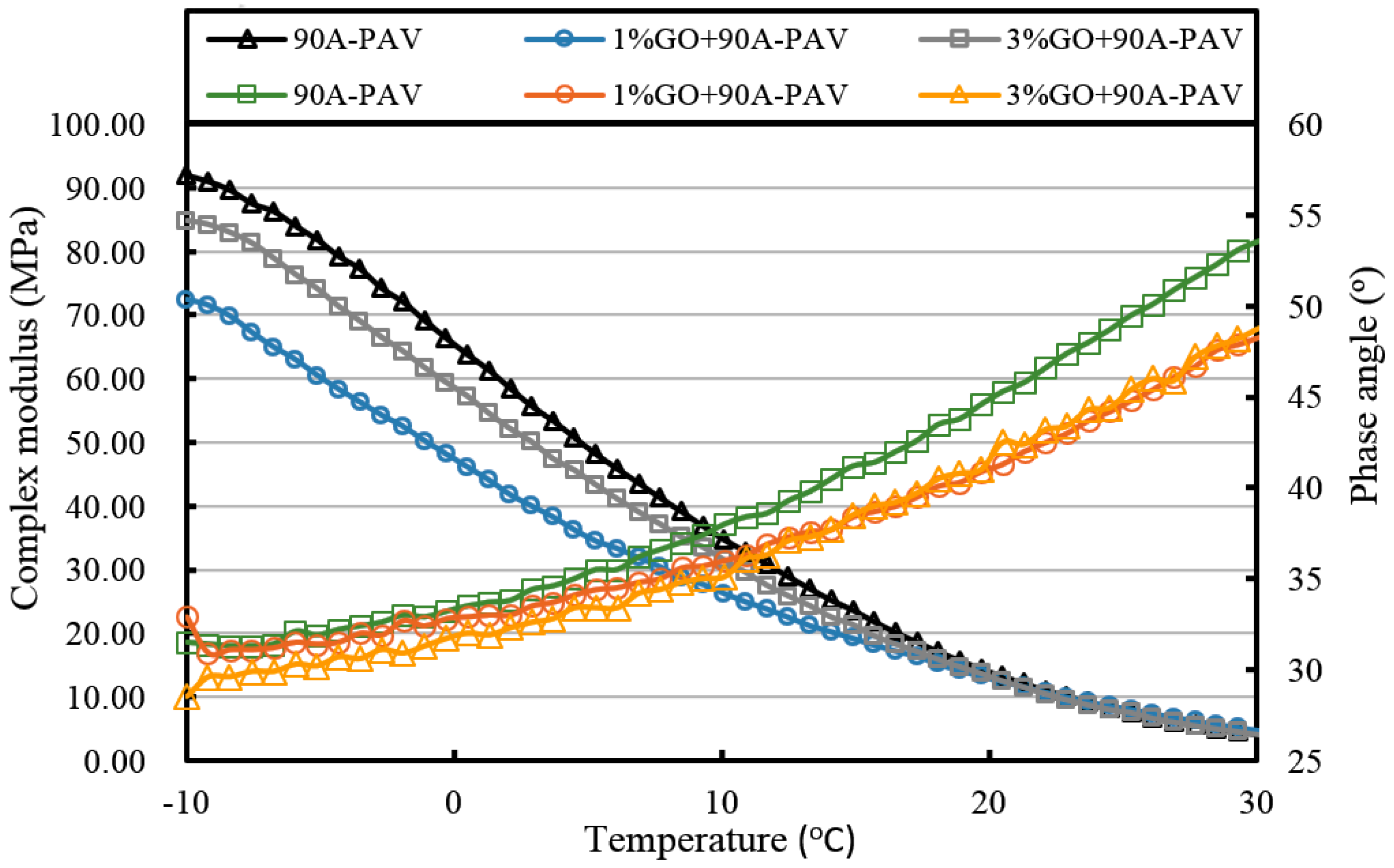
For GO-modified 90A (seen from
Figure 10,
Figure 11 and
Figure 12, and
Table 2), the results of the high-temperature sweep were the same as that of GO-modified 70A. The increasing modulus and reducing phase angle of all samples with the increasing addition of GO were also presented after aging. Thus, for both 70A and 90A, GO showed the same physical enhancement at high temperature. However, anti-aging improvement was not shown.
3.3.2. Low-Temperature Sweep
At low temperature, asphalt is relatively like elastic materials. It becomes hard and brittle, which is the main reason that causes the cracking of asphalt pavement in winter. Therefore, the smaller complex modulus and larger phase angle mean better viscosity at low temperature.
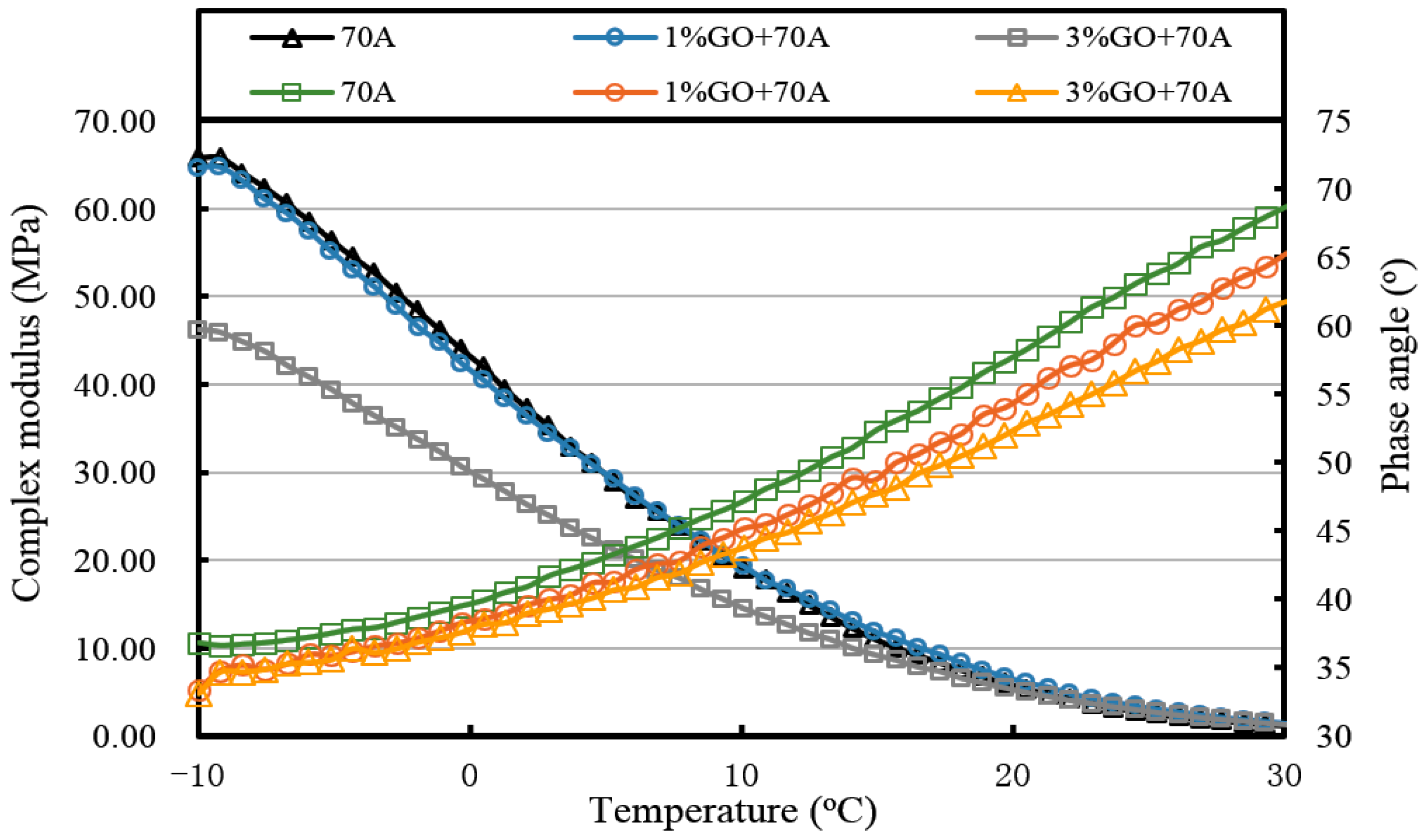
From
Figure 13 and
Figure 14 and
Table 3, the introduction of 1% GO had a minor effect on the base asphalt. The complex modulus of 1% GO-modified 70A and 90A were almost overlapped compared to both base asphalts. When the content of GO rose to 3%, the complex modulus of modified 70A and 90A greatly reduced to 46.2 MPa and 41.0 MPa, respectively. For the phase angle, it was decreased by the addition of GO. However, the phase angle of 1% GO-modified 90A was slightly higher than the base asphalt below 10 °C (seen from
Figure 14).
After TFOT aging (seen from
Figure 15 and
Figure 16, and
Table 3), the complex modulus of 3% GO-modified 70A was slightly smaller than 70A below 5 °C and its phase angle was larger below 0 °C. Meanwhile, the complex modulus of 1% GO-modified 70A were significantly smaller and the phase angle was higher than that of base asphalt when temperature below 0 °C. From
Figure 16, the complex modulus and phase angle of 1% and 3% GO-modified 90A were almost the same. Compared to 90A, the complex modulus of both modified asphalts was significantly smaller and the phase angle of them was slightly higher when the temperature was below 0 °C.
From
Figure 17 and
Figure 18, it can be seen clearly that the complex modulus of the both 1% GO-modified asphalts was lower than that of the both 3% GO-modified asphalts after PAV aging. Moreover, both base asphalts held the largest modulus. The phase angle of 70A was lower than 1% modified 70A under 10 °C, and lower than 3% modified asphalt under 0 °C. Meanwhile, the phase angle of 90A was larger than 1% GO-modified 90A, but the difference between them was not so obvious under 0 °C.
At the same time, it can be seen from statistics of
Table 3 that the complex modulus of both 1% and 3% GO-modified 70A and 90A were almost the same as the two base asphalts at 20 °C. However, the complex modulus of GO-modified asphalts was much lower than the base asphalts at −10 °C. This means that the temperature sensitivity of GO-modified asphalts was better.
All of the complex modulus and phase angles of the modified asphalts and base asphalts showed the same fact that GO could improve the low temperature properties of asphalt before and after aging.
3.4. Py-GC/MS Test
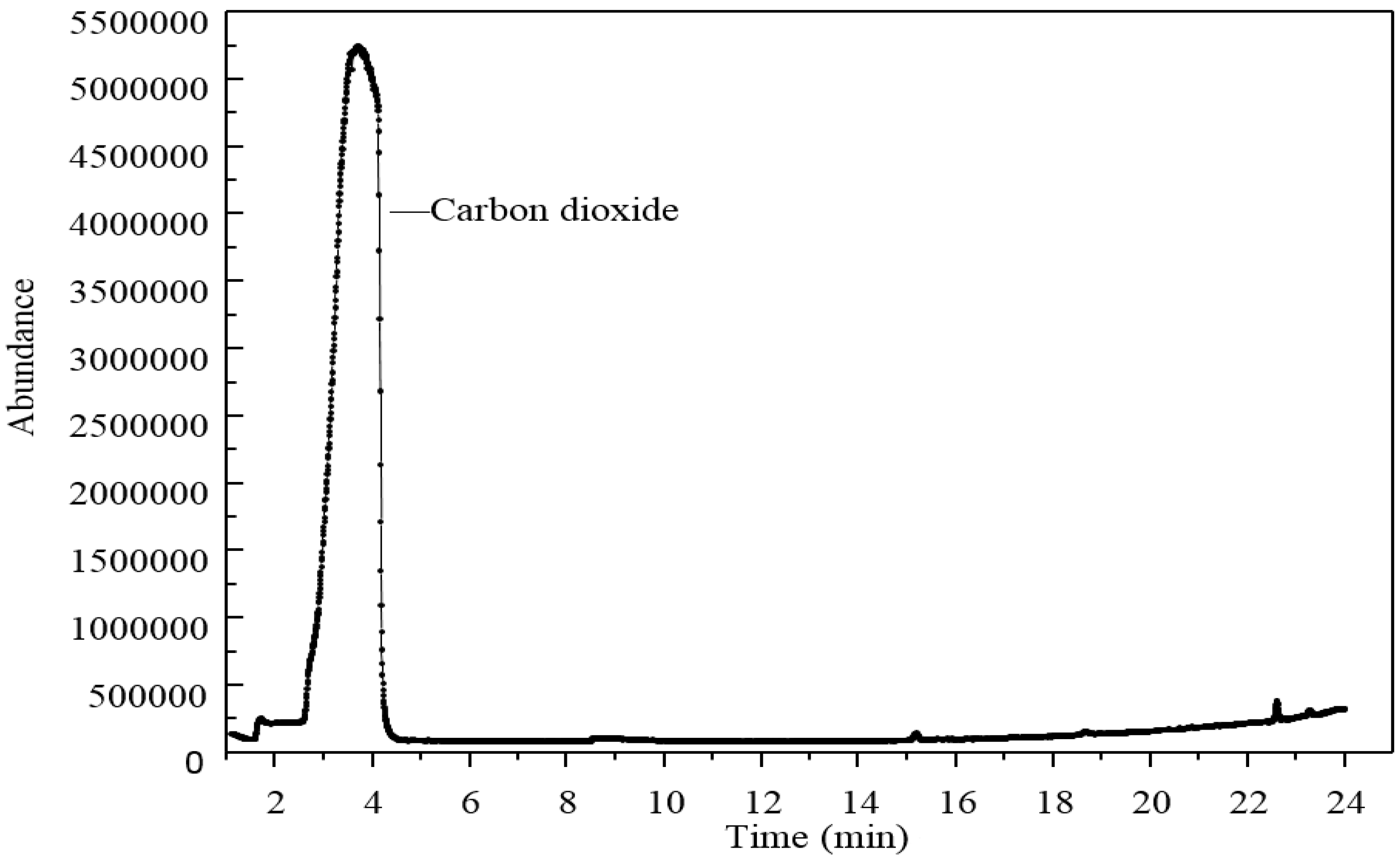
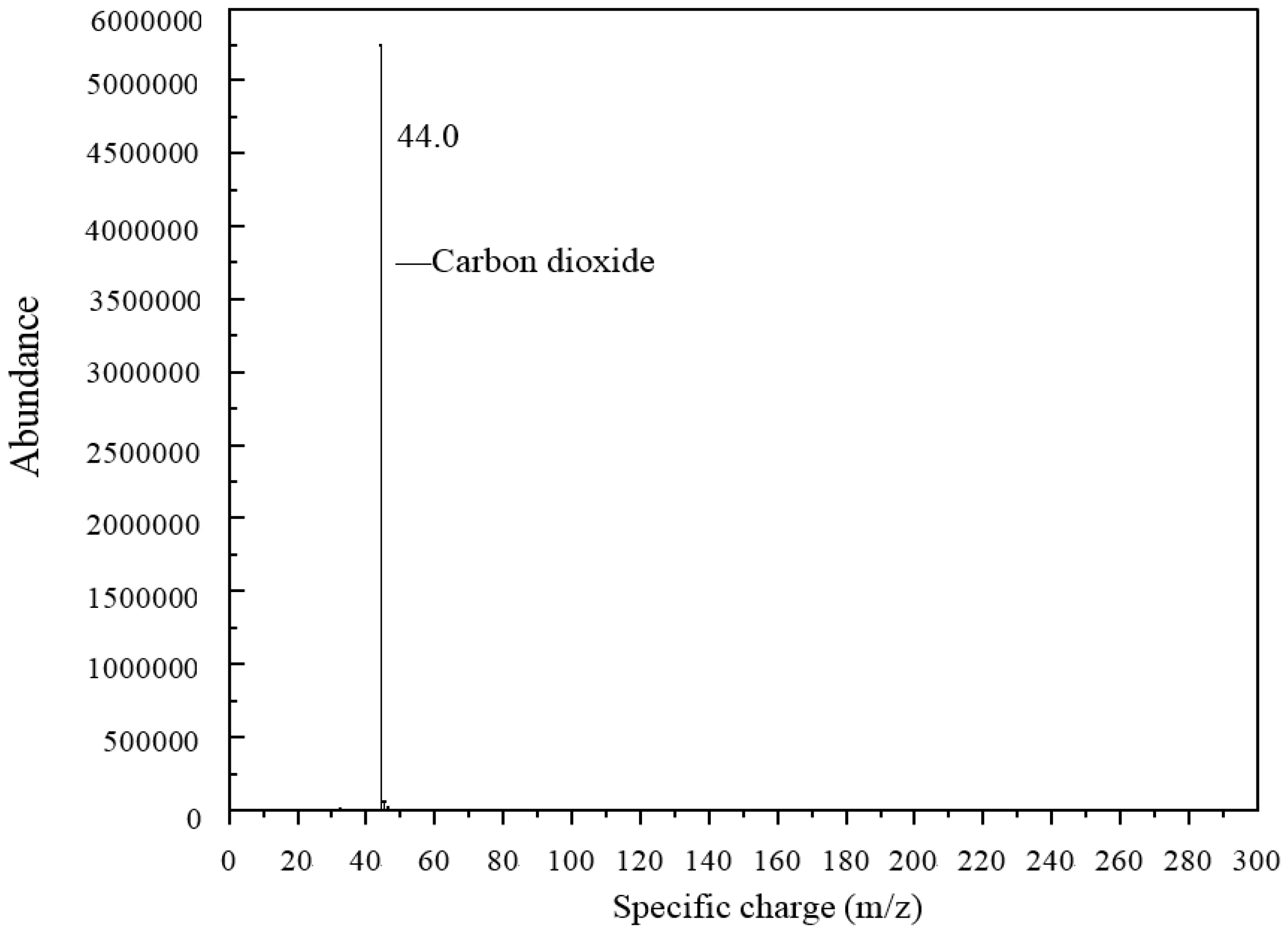
Due to the boiling reaction that happened during the heating process, Py-GC/MS was applied to find out what types of gases were released from the 3% GO-modified asphalt. The results of the Py-GC/MS are shown in
Figure 19 and
Figure 20.
Figure 19 shows the gas chromatography results. Only one clear peak was displayed in this graph. This phenomenon meant only one type of gas was released when GO-modified asphalt was heated to 115 °C.
Figure 20 shows the mass spectrometry results. The gas released at 3.73 min at Py-GC/MS was applied to a mass spectrometry test. The specific charge of the tested gas was 44, and according to a mass spectrometry database, the gas was considered as carbon dioxide (CO
2). This means that CO
2 was released when GO-modified asphalt was heated to 115 °C.
3.5. Potential Application of GO-Modified Asphalt
The released CO2 gives the GO-modified asphalt some potential applications, like warm mix asphalt and flame retardant asphalt.
For example, water-containing technology and water-base technology are commonly applied to complete the foaming process in order to reduce the viscosity of asphalt [
26,
27,
28]. The working principle of warm mix GO asphalt is just like the water-base technology. When asphalt is heated above 115 °C, a large amount of CO
2 will be released. This can increase the volume of asphalt and decrease the mix viscosity of asphalt. Thus, the mixing temperature can be reduced.
In addition, GO holds the potential application as a flame retardant material on surface pavement. Thus, the reheating test of GO-modified asphalt was completed to investigate whether the release of CO2 would happen in this test. Firstly, the GO-modified asphalt was heated at 115 °C at which the boiling reaction occurred. Then, it was cooled to room temperature and the volume of the GO-modified asphalt returned to its original level. Thirdly, the sample was heated to 115 °C again and it was found that the CO2 gas was released again. Finally, the heat-cool-heat process was conducted for three cycles and the CO2 could still be released at the third cycle. Thus, supposing self-ignition of a car occurred, the fire would raise the surface pavement to a relatively high temperature. Once the temperature has reached above 115 °C, the GO-modified asphalt would release CO2 from the bottom of the enflamed car. Like a fire extinguisher set off from the bottom, it could retard the flame.
From the working principle, these two potential applications could work. However, more research should be completed to investigation and confirm this hypothesis.