The Adsorption Capacity of GONs/CMC/Fe3O4 Magnetic Composite Microspheres and Applications for Purifying Dye Wastewater
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
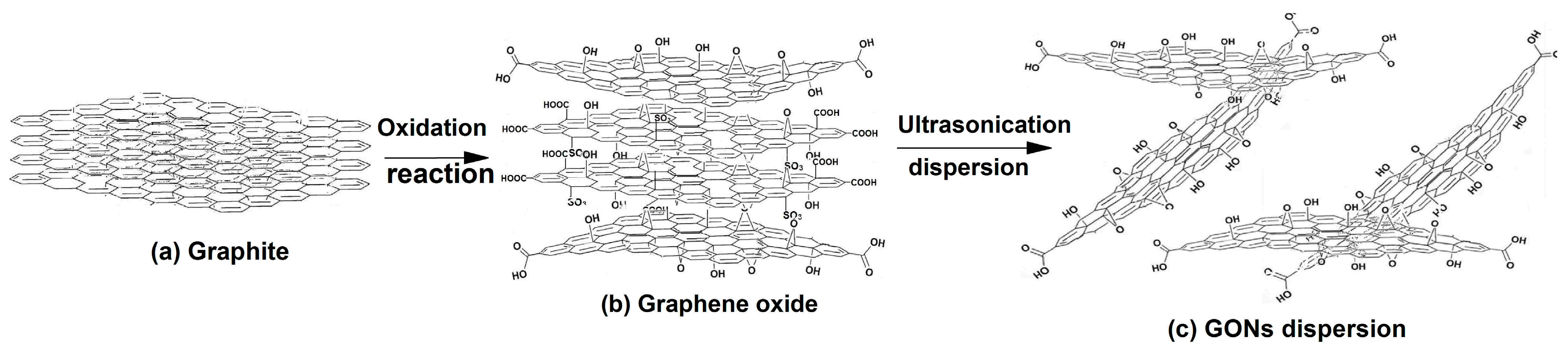
2.2. Preparation of GONs
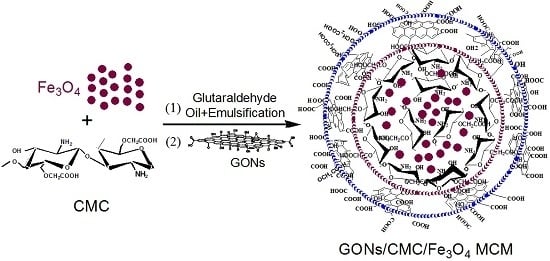
2.3. Preparation of GONs/CMC/Fe3O4 MCMs
2.4. Investigation of Adsorption Capacity of GONs/CMC/Fe3O4 MCMs
2.4.1. Testing Data Processing Method
2.4.2. Adsorption Capacity and Affect Factors
2.4.3. Regenerative Ability
2.5. Investigation of Adsorption Mechanism
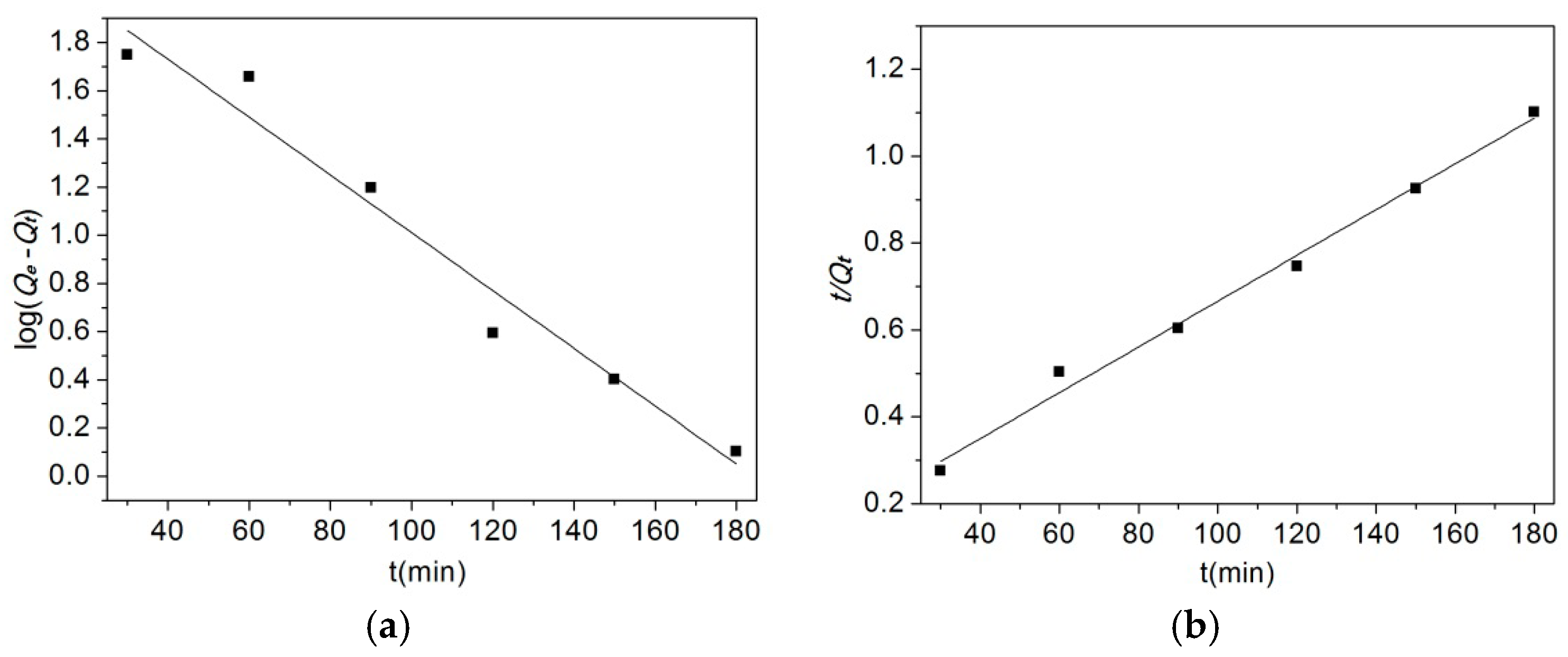
2.5.1. Adsorption Kinetics
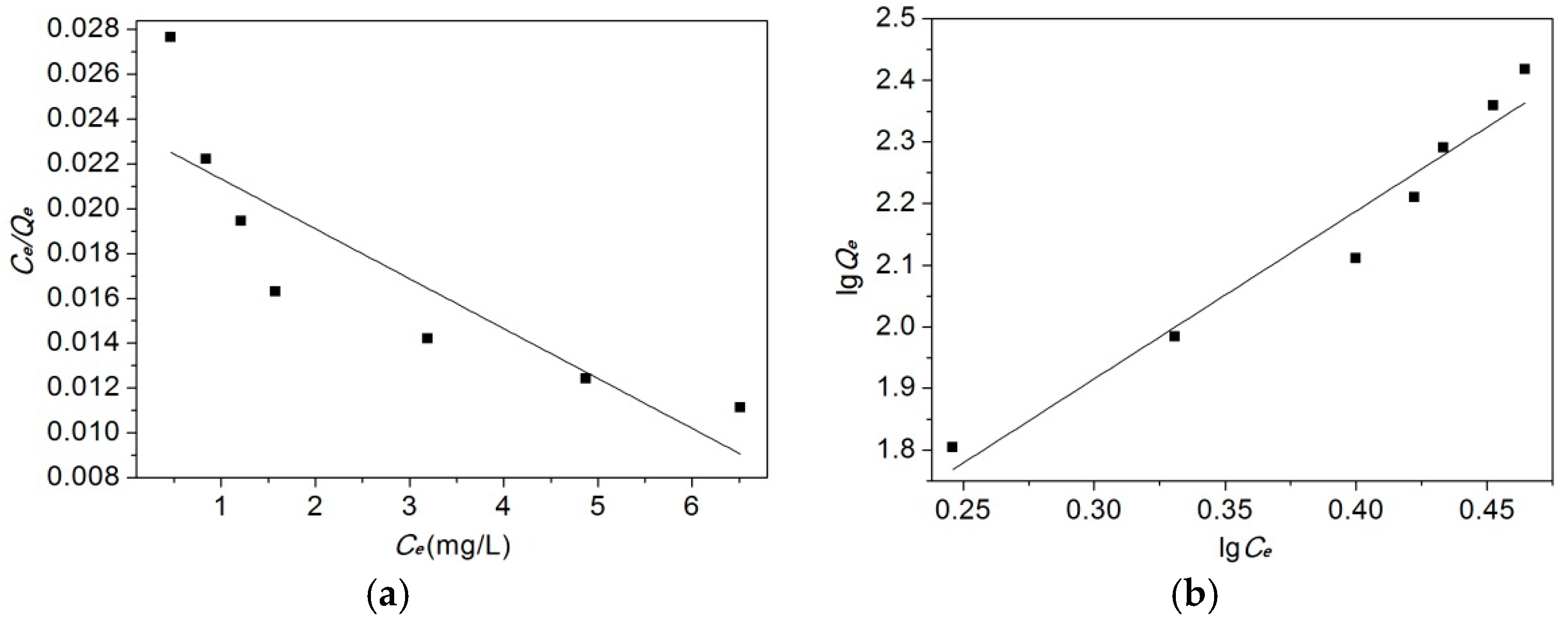
2.5.2. Adsorption Isotherms
2.6. Characterization Methods
3. Results and Discussion
3.1. Structural Characterization of GONs
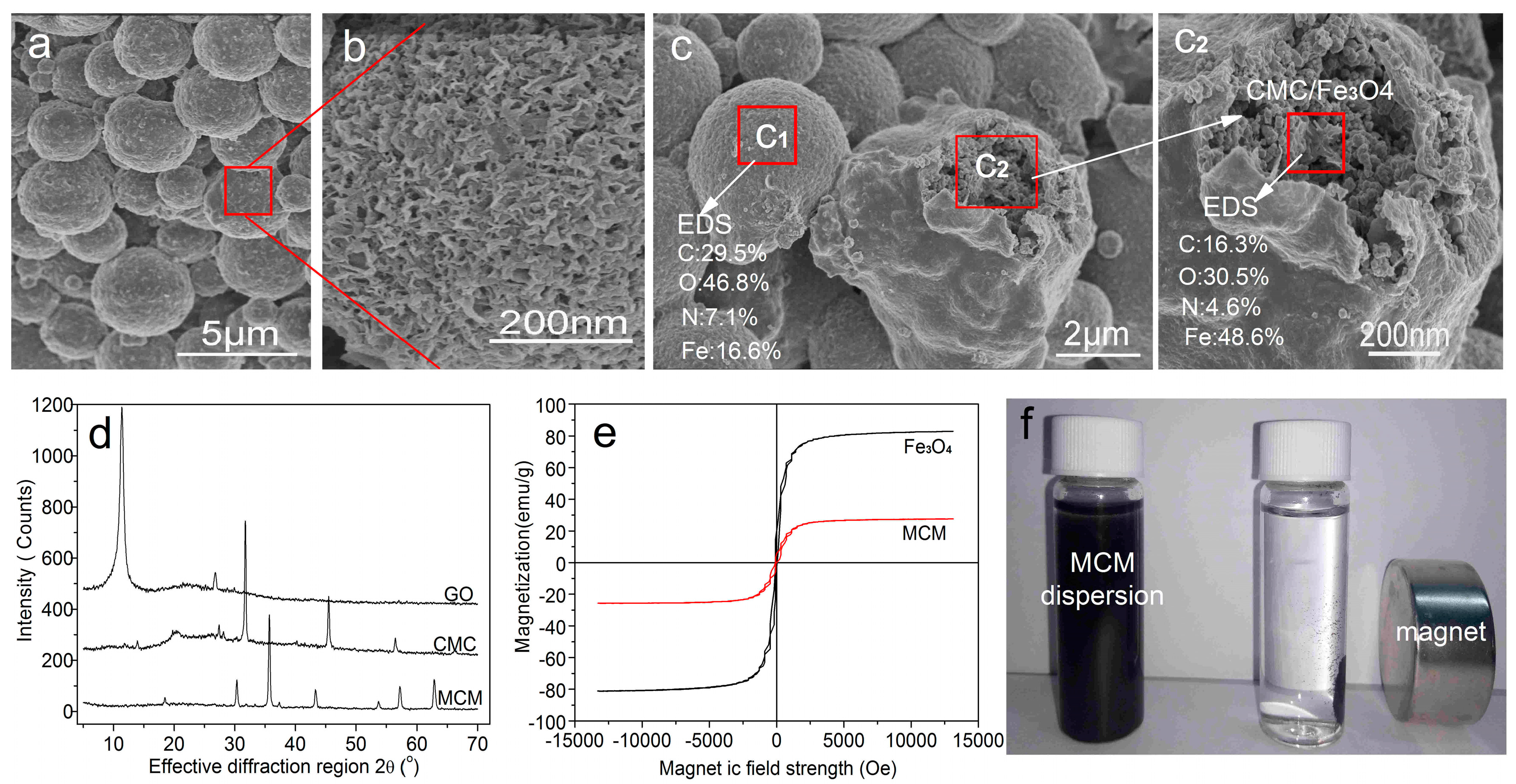
3.2. Structural Characterization of GONs/CMC/Fe3O4 MCMs
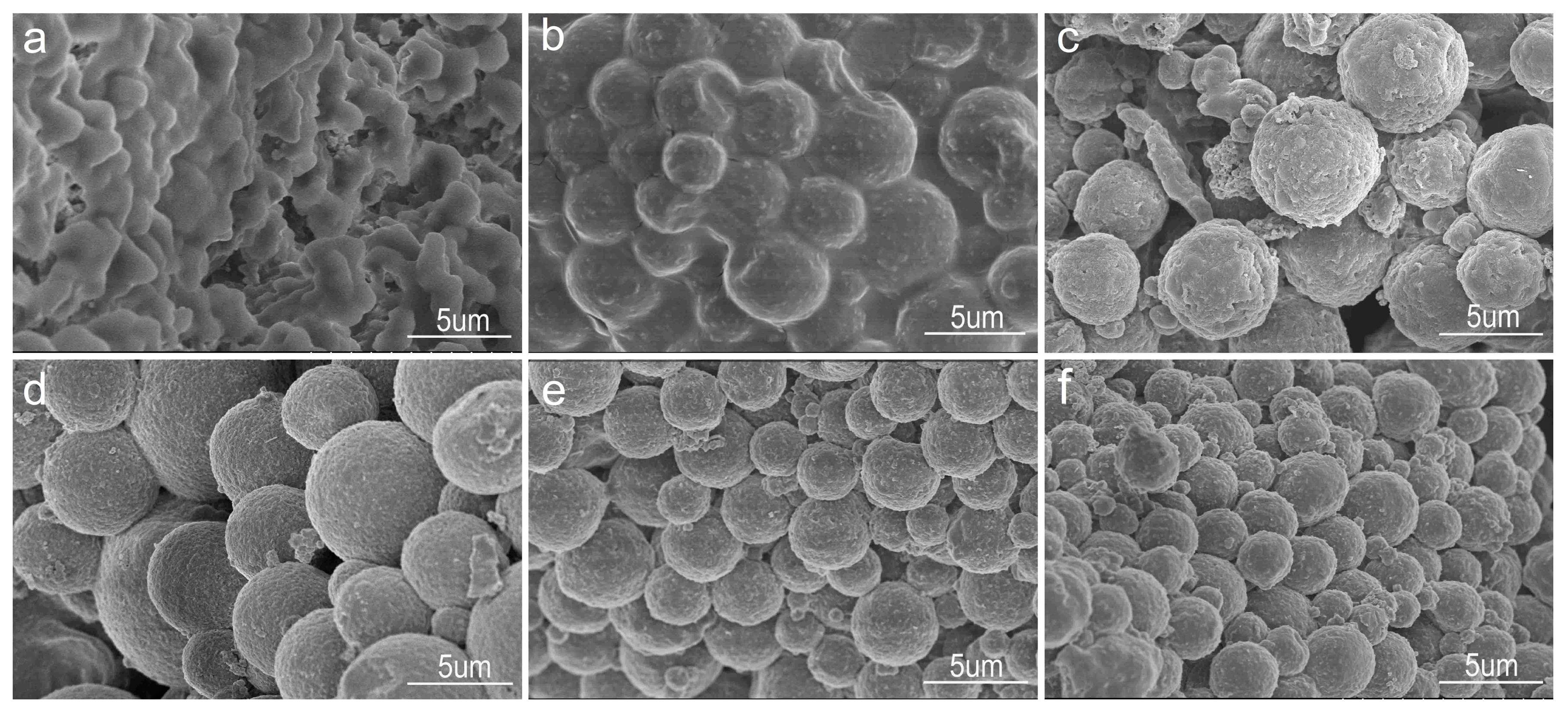
3.3. Effect of the Oil–Water Ratio on the Shape of GONs/CMC/Fe3O4 MCMs
3.4. Effect of GON Content on Special Surface Area and Adsorption Capacity of GONs/CMC/Fe3O4 MCMs
3.5. Factors Affecting the Adsorption Capacity of GONs/CMC/Fe3O4 MCMs
3.6. Adsorption Mechanism of MCMs
3.6.1. Adsorption Kinetics
3.6.2. Adsorption Isotherm
3.7. Adsorption Capacity of the MCMs for Other Dyes and Metal Ions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Guo, D.H.; Zhou, Y.; Wu, Z.Y.; Li, W.Y.; Zhao, F.; Zheng, X.M. Identification of ancient textiles from Yingpan, Xinjiang, by multiple analytical techniques. J. Archaeol. Sci. 2011, 38, 1763–1770. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A novel nanohybrid nanofibrous adsorbent for water purification from dye pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef]
- Fan, M.Y.; Li, T.J.; Hu, J.W.; Cao, R.S.; Wu, Q.; Wei, X.H.; Li, L.Y.; Shi, X.D.; Ruan, W.Q. Synthesis and characterization of reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites used for Pb(II) Removal. Materials 2016, 9, 687. [Google Scholar] [CrossRef]
- Chu, H.C.; Chen, K.M. Reuse of activated sludge biomass: I. Removal of basic dyes from wastewater by biomass. Process Biochem. 2002, 37, 595–600. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Tanvir, A.; Tam, K.C. Application of the central composite design to study the flocculation of an anionic azo dye using quaternized cellulose nanofibrils. Carbohyd. Polym. 2015, 133, 80–89. [Google Scholar] [CrossRef]
- Ou, W.S.; Zhang, G.Q.; Yuan, X.J.; Su, P. Experimental study on coupling photocatalytic oxidation process and membrane separation for the reuse of dye wastewater. J. Water Process Eng. 2015, 6, 120–128. [Google Scholar] [CrossRef]
- Wong, K.S.; Huang, Q.L.; Au, C.H.; Wang, J.; Kwan, H.S. Biodegradation of dyes and polyaromatic hydrocarbons by two allelic forms of Lentinula edodes laccase expressed from Pichia pastoris. Bioresour. Technol. 2012, 104, 157–164. [Google Scholar] [CrossRef] [PubMed]
- George, J.S.; Ramos, A.; Shipley, H.J. Tanning facility wastewater treatment: Analysis of physical–chemical and reverse osmosis methods. J. Environ. Chem. Eng. 2015, 3, 969–976. [Google Scholar] [CrossRef]
- Yang, C.; Li, L.; Shi, J.L.; Long, C.; Li, A.M. Advanced treatment of textile dyeing secondary effluent using magnetic anion exchange resin and its effect on organic fouling in subsequent RO membrane. J. Hazard. Mater. 2015, 284, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N. Adsorption of dyes from aqueous solutions on activated charcoal. J. Hazard. Mater. B 2007, 139, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198, 219–227. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.M.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Gong, R.M.; Ding, Y.; Li, M.; Yang, C.; Liu, H.J.; Sun, Y.Z. Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes Pigments 2005, 64, 187–192. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Chen, X.G.; Wang, Z.; Liu, W.S.; Park, H.J. The effect of carboxymethyl-chitosan on proliferation and collagen secretion of normal and keloid skin fibroblasts. Biomaterials 2002, 23, 4609–4614. [Google Scholar] [CrossRef]
- Zhu, A.P.; Chan-Park, M.B.; Dai, S.; Li, L. The aggregation behavior of O-carboxymethylchitosan in dilute aqueous solution. Colloids Surf. B 2005, 43, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.G.; Park, H.J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohyd. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Mi, F.L.; Wu, S.J.; Chen, Y.C. Combination of carboxymethyl chitosan-coated magnetic nanoparticles and chitosan-citrate complex gel beads as a novel magnetic adsorbent. Carbohyd. Polym. 2015, 131, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Dang, Q.F.; Liu, C.S.; Yan, J.Q.; Fan, B.; Cai, J.P.; Li, J.J. Preparation and characterization of carboxyl-functionalized chitosan magnetic microspheres and submicrospheres for Pb2+ removal. Colloids Surf. A 2015, 482, 353–364. [Google Scholar] [CrossRef]
- Kadam, A.A.; Lee, D.S. Glutaraldehyde cross-linked magnetic chitosan nanocomposites: Reduction precipitation synthesis, characterization, and application for removal of hazardous textile dyes. Bioresour. Technol. 2015, 193, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Park, S.S.; Chao, S.Y. Adsorption characteristics of Reactive Black 5 onto chitosan beads cross-linked with epichlorohydrin. J. Ind. Eng. Chem. 2012, 18, 1458–1464. [Google Scholar] [CrossRef]
- Zheng, E.D.; Dang, Q.F.; Liu, C.S.; Fan, B.; Yan, J.Q.; Yu, Z.Z.; Zhang, H.F. Preparation and evaluation of adipic acid dihydrazide cross-linked carboxymethyl chitosan microspheres for copper ion adsorption. Colloids Surf. A 2016, 502, 34–43. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.J.; Zhang, S.B. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Lv, S.H.; Ma, Y.J.; Qiu, C.C.; Sun, T.; Liu, J.J.; Zhou, Q.F. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Zhang, N.; Qiu, H.X.; Si, Y.M.; Wang, W.; Gao, J.P. Fabrication of highly porous biodegradable monoliths strengthened by graphene oxide and their adsorption of metal ions. Carbon 2011, 49, 827–837. [Google Scholar] [CrossRef]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W.D. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Fan, L.L.; Luo, C.N.; Li, X.J.; Lu, F.G.; Qiu, H.M.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Fan, L.L.; Sun, M. Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin–chitosan. Colloids Surf. B 2013, 107, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Luo, C.N.; Sun, M. Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/grapheme oxide composites. Colloids Surf. B 2013, 103, 523–529. [Google Scholar] [CrossRef] [PubMed]



| Ref. | Adsorbents | Adsorbates | Adsorption Conditions | Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| [11] | Activated carbon | Methylene blue | C* = 600 mg/L, m✦ = 0.2 g/L, pH = 7.4, t✹ = 35 min | 25.3 |
| [12] | Modified clay | Methylene blue | C = 100 mg/L, m = 0.2 g/L, pH = 12, t = 750 min | 34.6 |
| [13] | Kaolin | Congo red | C = 150 mg/L, m = 0.5 g/L, pH = 7.5, t = 500 min | 5.6 |
| [14] | Peanut hull | Amaranth | C = 50 mg/L, m = 0.5 g/L, pH = 2, t = 15 h | 14.9 |
| [15] | Cashew nutshells | Congo red | C = 50 mg/L, m = 0.2 g/L, pH = 2, t = 2 h | 5.2 |
| GONs Content (g) | Specific Surface Area (m2/g) | Average Pore Diameter (nm) | Adsorption Capacity (mg/g) |
|---|---|---|---|
| 0 | 46.35 | 52.5 | 115.5 ± 0.4 |
| 0.02 | 62.6 | 31.4 | 146.3 ± 0.6 |
| 0.04 | 95.2 | 29.5 | 156.7 ± 0.7 |
| 0.06 | 126.4 | 28.3 | 164.6 ± 0.7 |
| 0.08 | 125.6 | 28.5 | 164.4 ± 0.6 |
| 0.10 | 126.4 | 28.2 | 164.3 ± 0.8 |
| Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | ||||
|---|---|---|---|---|---|
| k1 (min−1) | Q’e (mg/g) | k2 (L/g) | Q’e (mg/g) | ||
| 0.0276 | 0.9671 | 162.144 | 0.000202 | 0.9917 | 188.68 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| Qm (mg/g) | B (L/g) | kF (L/g) | n | ||
| 454.55 | 0.0932 | 0.7495 | 12.517 | 0.367 | 0.955 |
| Adsorbate | Adsorption Conditions | Adsorption Capacity (mg/g) | |||
|---|---|---|---|---|---|
| Temperature (°C) | pH | Adsorption Time (min) | CMC/Fe3O4 MCMs | GONs/CMC/Fe3O4 MCMs | |
| Acid red (14720) * | 30 | 5.5 | 90 | 115.3 ± 0.5 | 168.6 ± 0.7 |
| Based black (20470) | 30 | 5.5 | 90 | 108.6 ± 0.5 | 166.5 ± 0.7 |
| Direct black (35255) | 30 | 6.0 | 90 | 121.4 ± 0.6 | 174.2 ± 0.8 |
| Reactive black 8 (2050) | 30 | 6.5 | 90 | 118.9 ± 0.5 | 166.3 ± 0.6 |
| Cr3+ ✦ | 30 | 6.5 | 90 | 21.4 ± 0.4 | 71.6 ± 0.5 |
| Hg2+ | 30 | 6.5 | 90 | 17.7 ± 0.4 | 65.3 ± 0.5 |
| Pb2+ | 30 | 6.5 | 90 | 18.6 ± 0.4 | 58.7 ± 0.4 |
| Cu2+ | 30 | 6.5 | 90 | 15.4 ± 0.4 | 46.4 ± 0.4 |
| Refs. | Adsorbents | Adsorbates | Adsorption Capacity (mg/g) | Cycle Times |
|---|---|---|---|---|
| [27] | GO | Methylene blue | 43.5 | – |
| [28] | Natural chitosan membrane | Methylene blue | 46.23 | <5 |
| [27] | Magnetic chitosan | Methylene blue | 60.4 | 5–10 |
| [27] | GO/Magnetic chitosan composite | Methylene blue | 95.16 | 5–10 |
| [29] | GO/magnetic cyclodextrin–chitosan | Cr6+ | 67.66 | 5–10 |
| [30] | GO/magnetic chitosan composites | Pb2+ | 76.94 | 5–10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Zhu, L.; Li, Y.; Jia, C.; Sun, S. The Adsorption Capacity of GONs/CMC/Fe3O4 Magnetic Composite Microspheres and Applications for Purifying Dye Wastewater. Materials 2017, 10, 58. https://doi.org/10.3390/ma10010058
Lv S, Zhu L, Li Y, Jia C, Sun S. The Adsorption Capacity of GONs/CMC/Fe3O4 Magnetic Composite Microspheres and Applications for Purifying Dye Wastewater. Materials. 2017; 10(1):58. https://doi.org/10.3390/ma10010058
Chicago/Turabian StyleLv, Shenghua, Linlin Zhu, Ying Li, Chunmao Jia, and Shiyu Sun. 2017. "The Adsorption Capacity of GONs/CMC/Fe3O4 Magnetic Composite Microspheres and Applications for Purifying Dye Wastewater" Materials 10, no. 1: 58. https://doi.org/10.3390/ma10010058