Gel/Space Ratio Evolution in Ternary Composite System Consisting of Portland Cement, Silica Fume, and Fly Ash
Abstract
:1. Introduction
2. Experimental Procedure
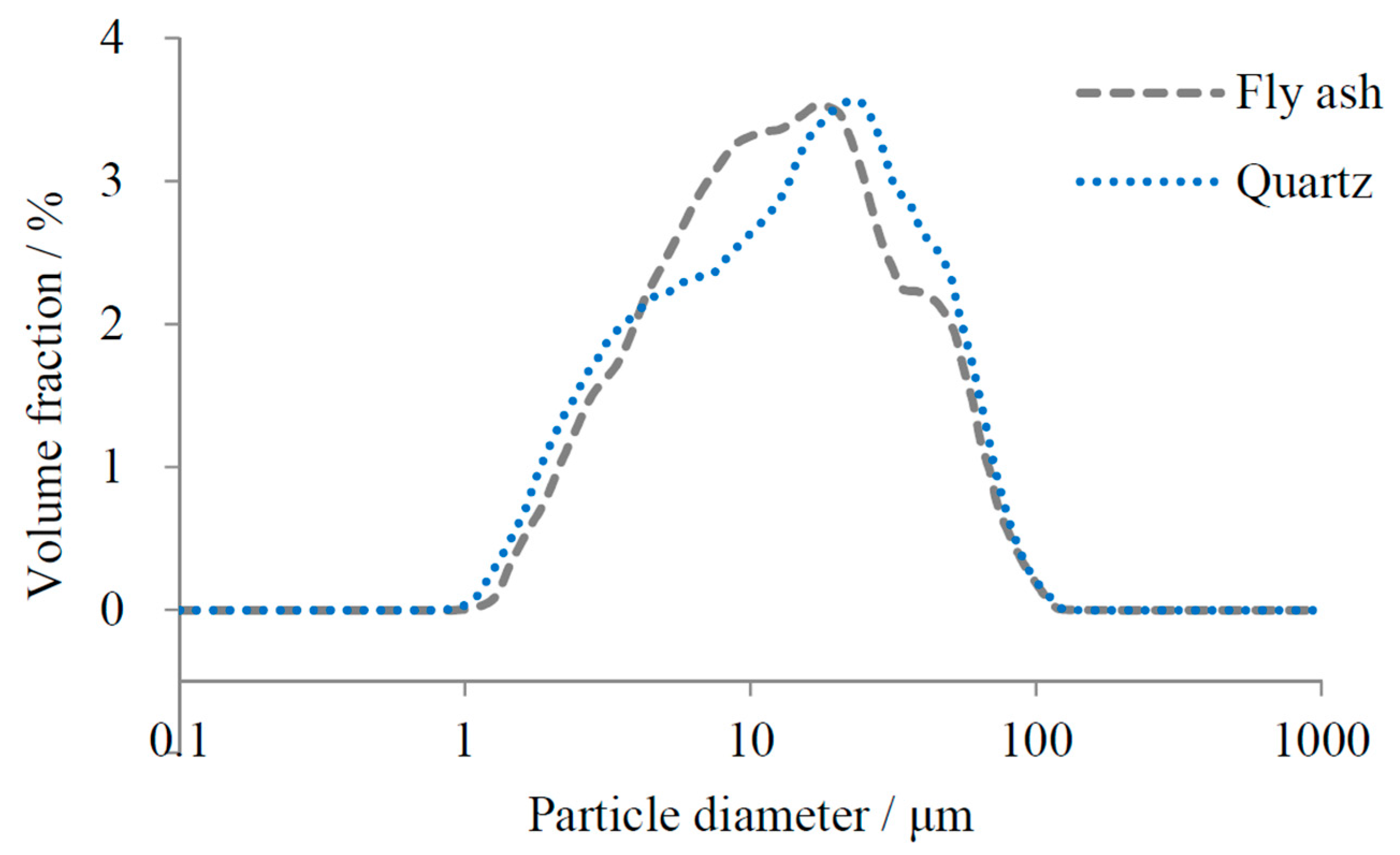
2.1. Materials
2.2. Sample Preparation
2.3. Compression Test
2.4. Determination of SF and FA Reaction Degrees
2.5. Determination of Cement Reaction Degree
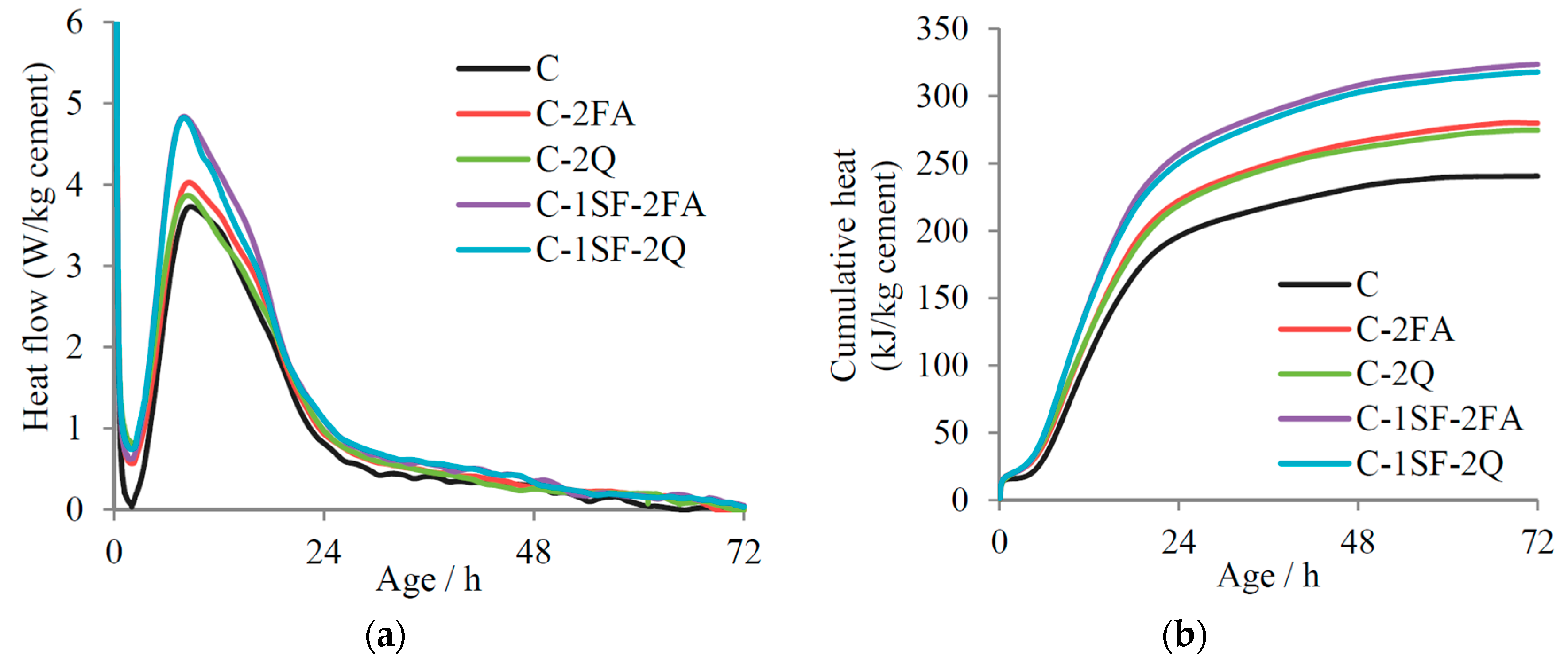
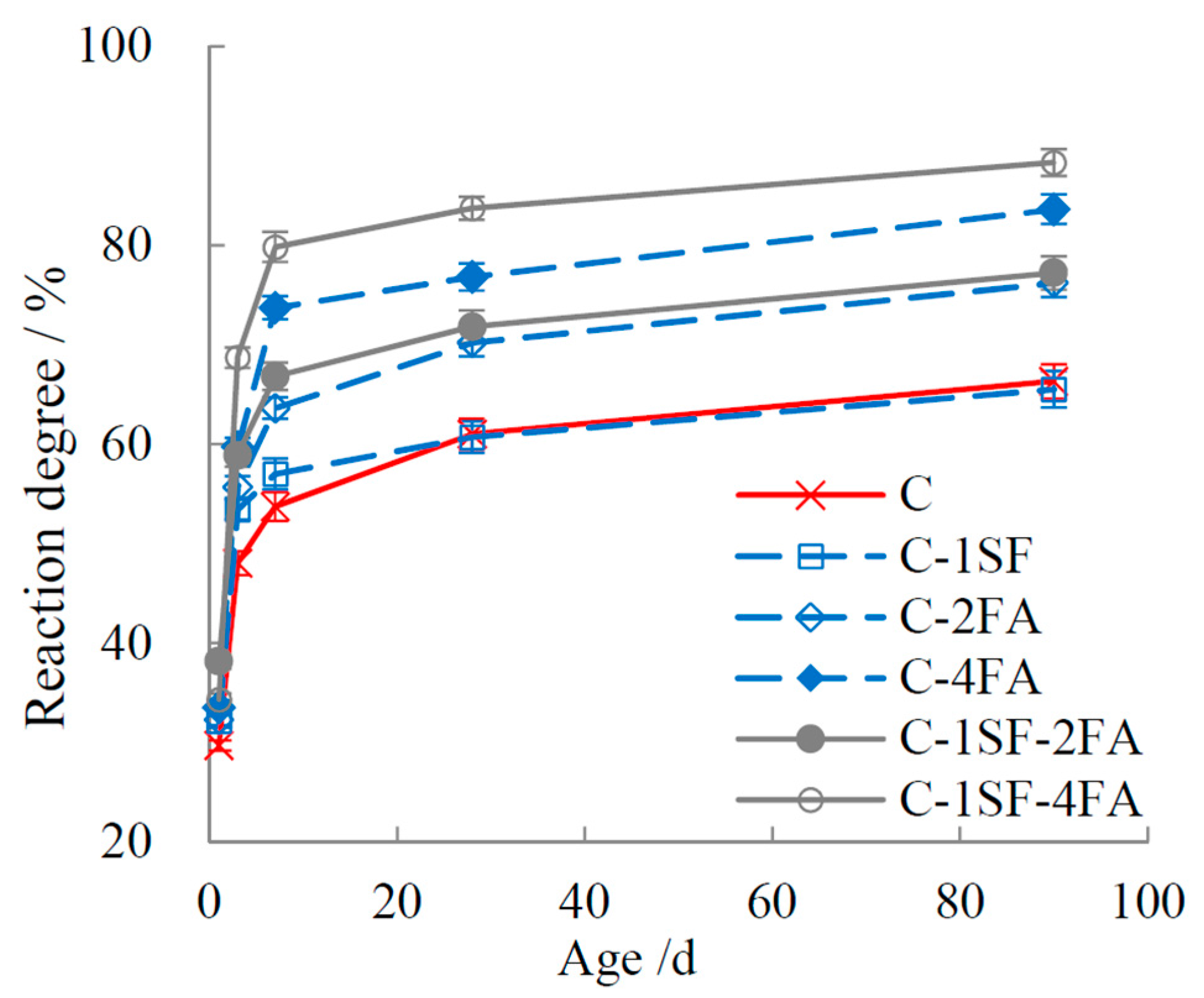
3. Results
3.1. Compressive Strength
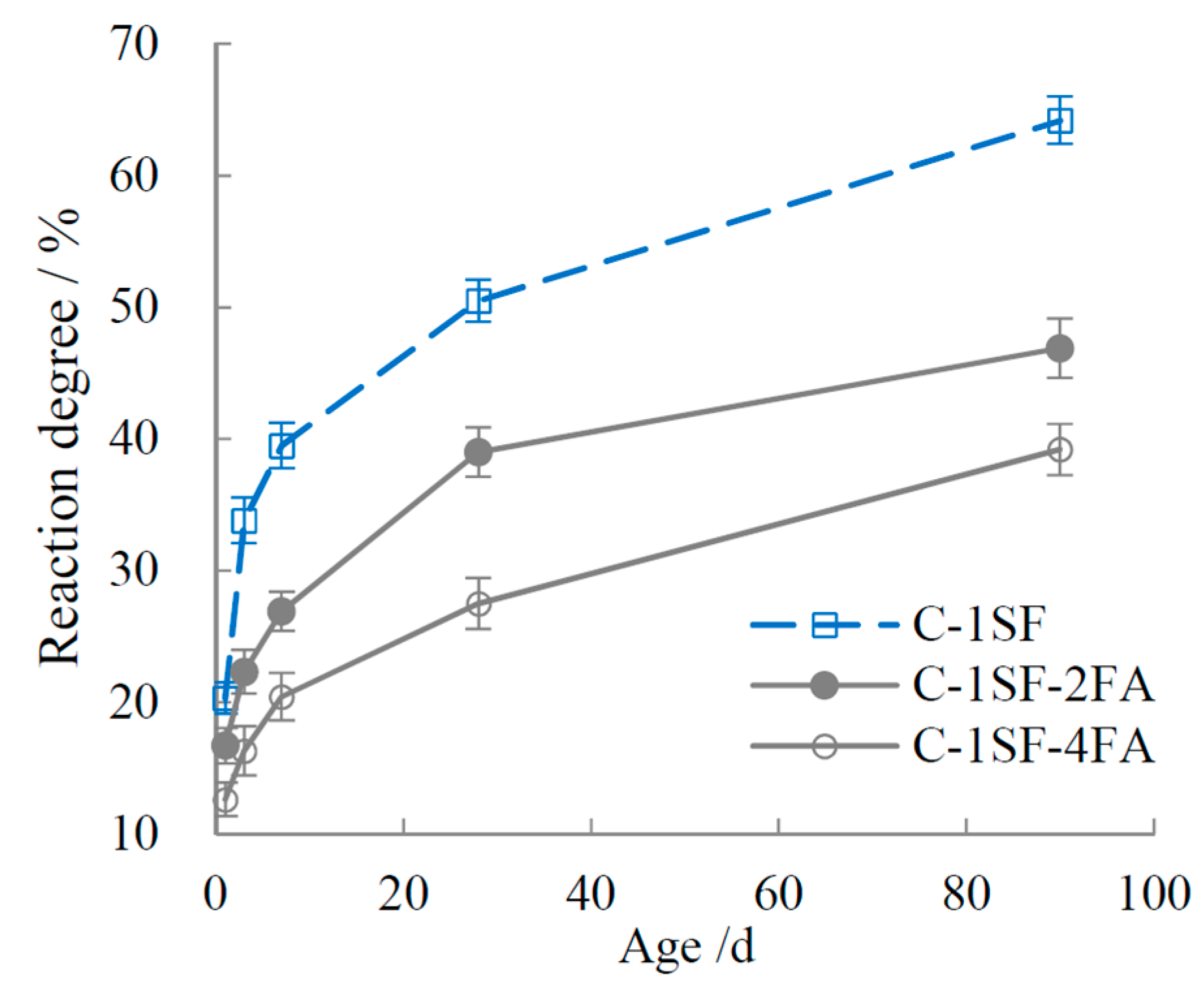
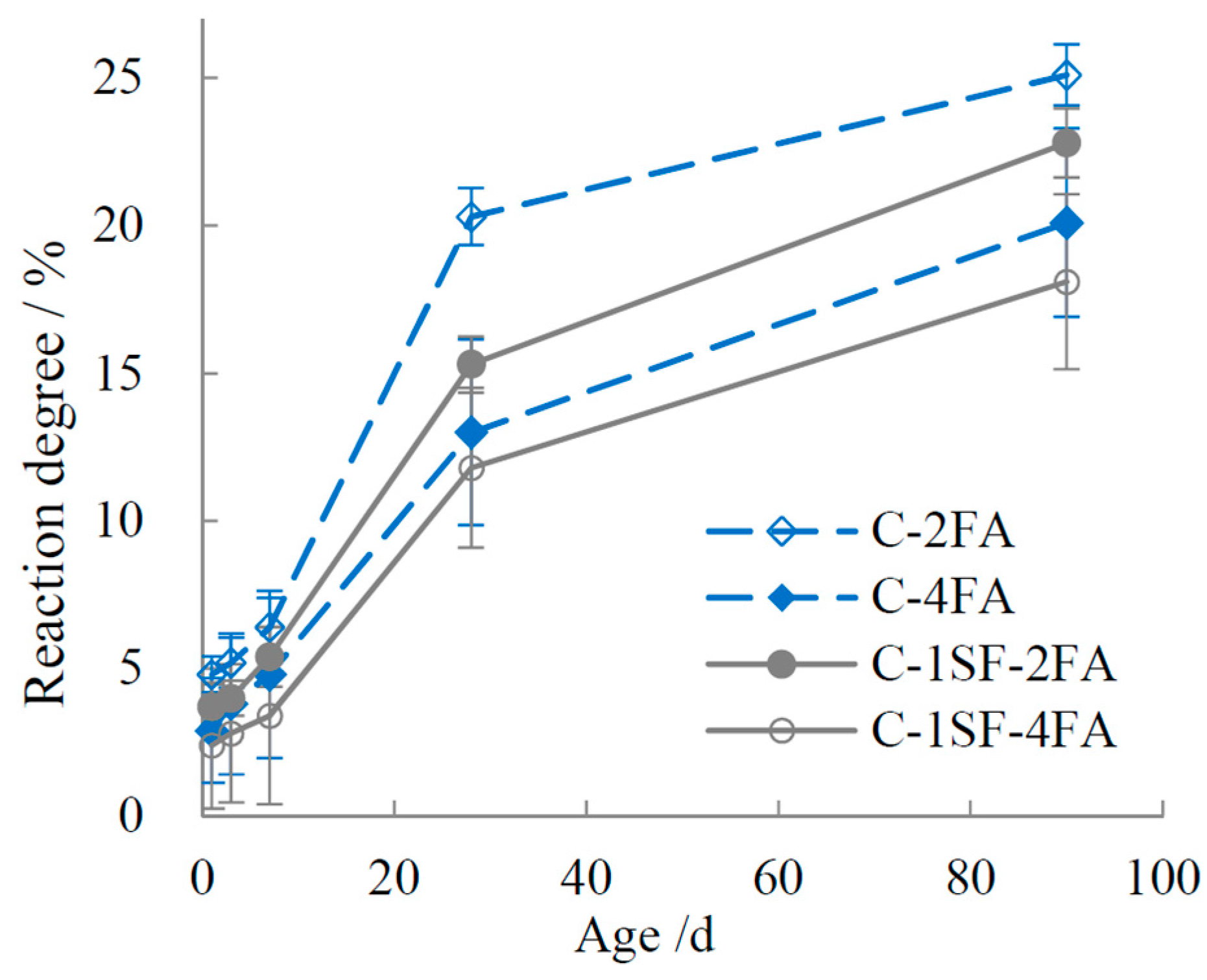
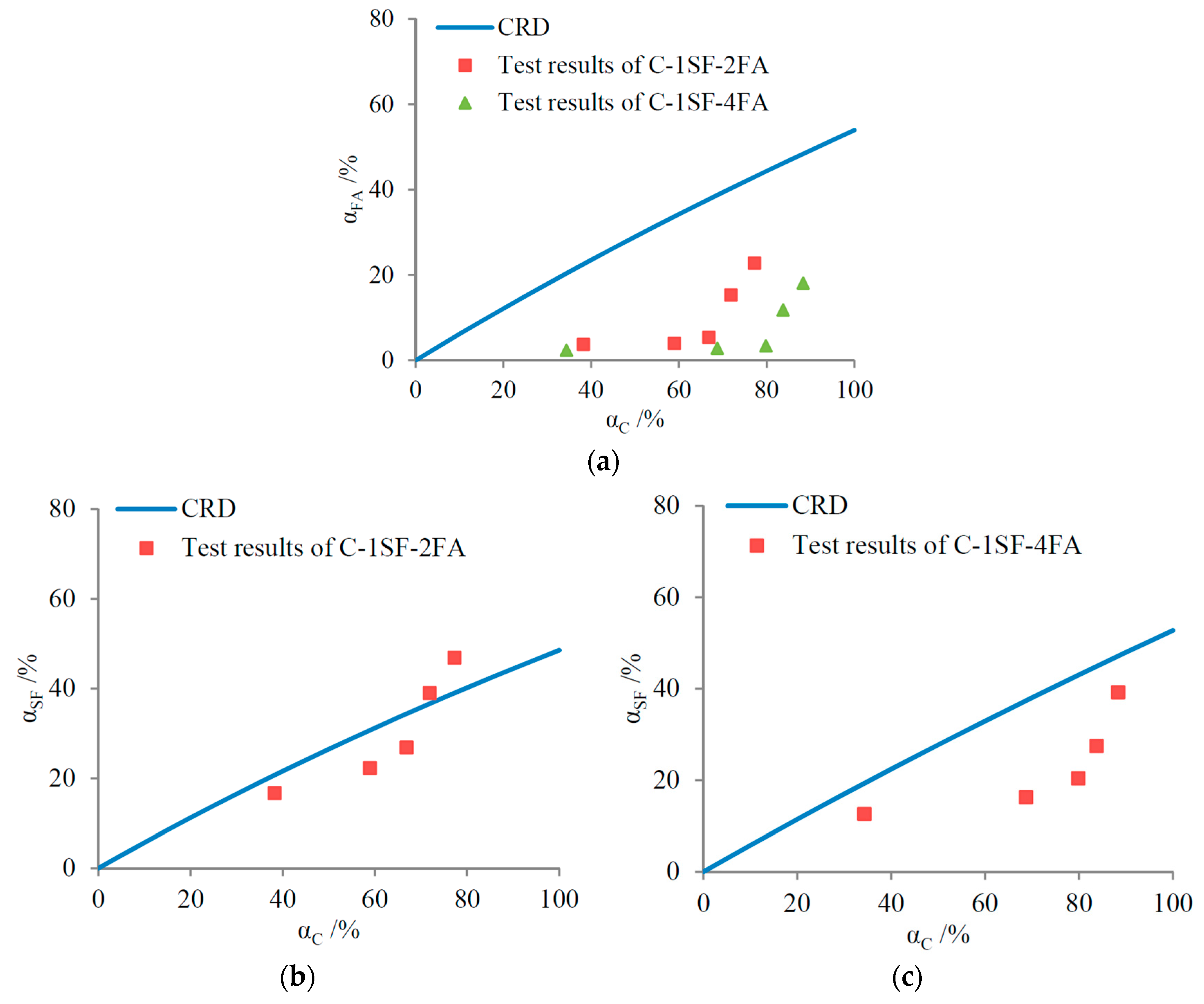
3.2. Reaction Degrees of SF, FA, and Cement
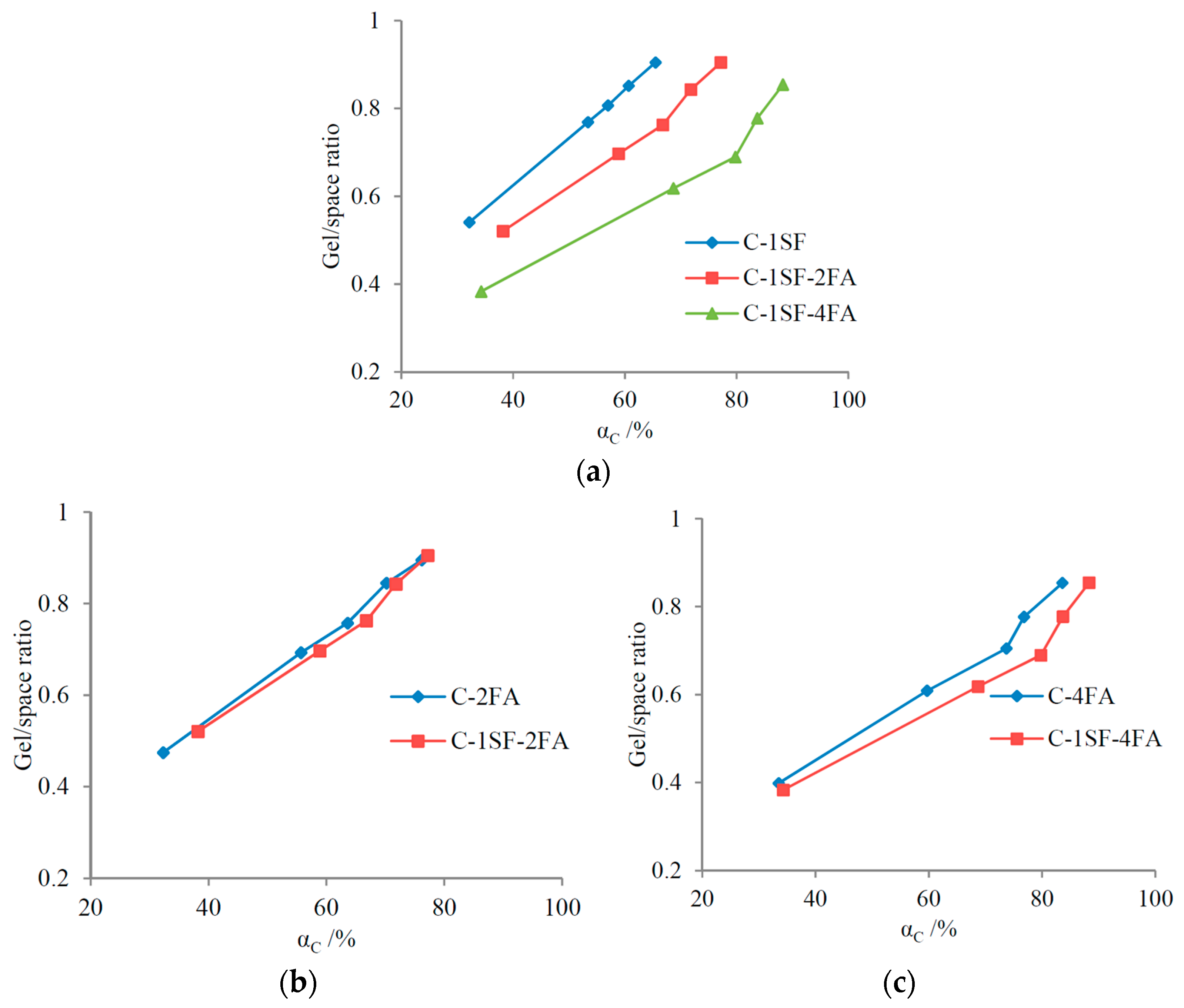
3.3. Gel/Space Ratio
4. Discussion
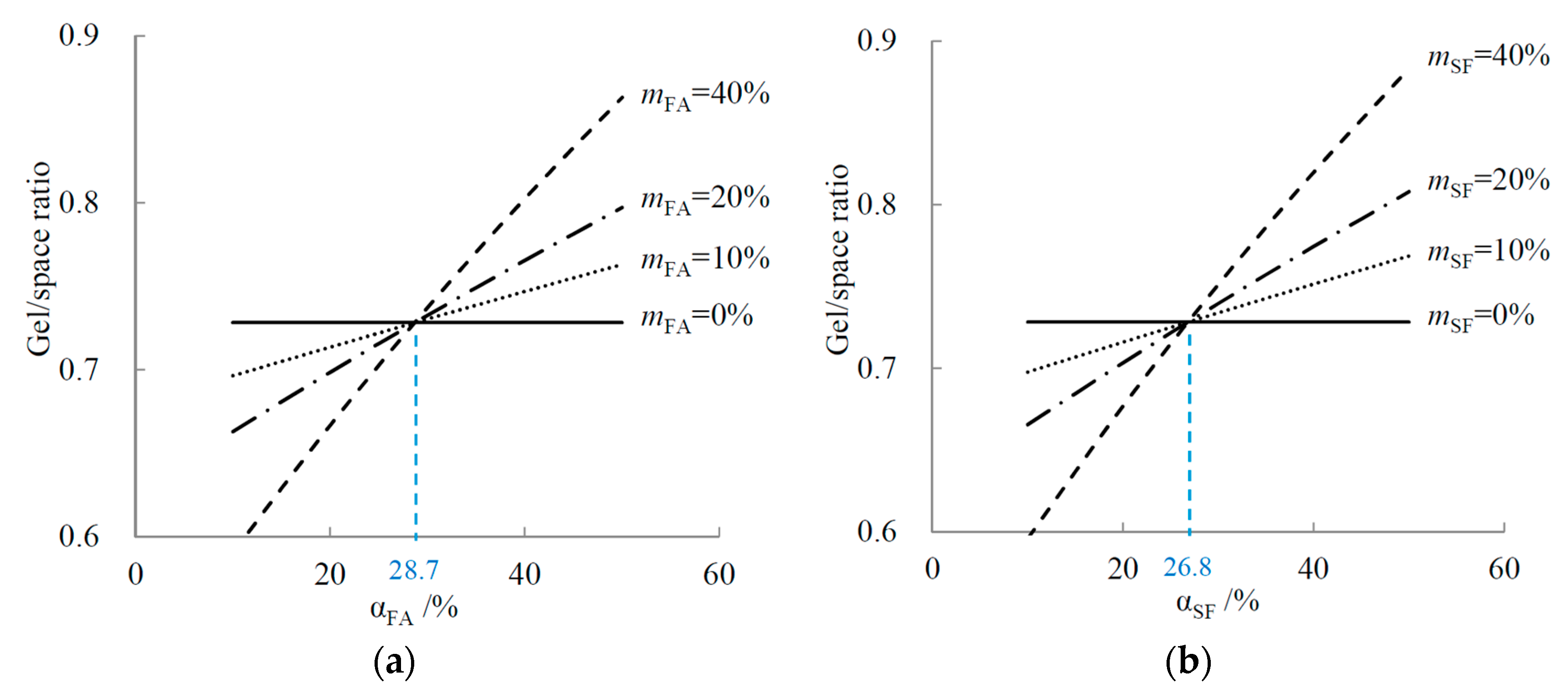
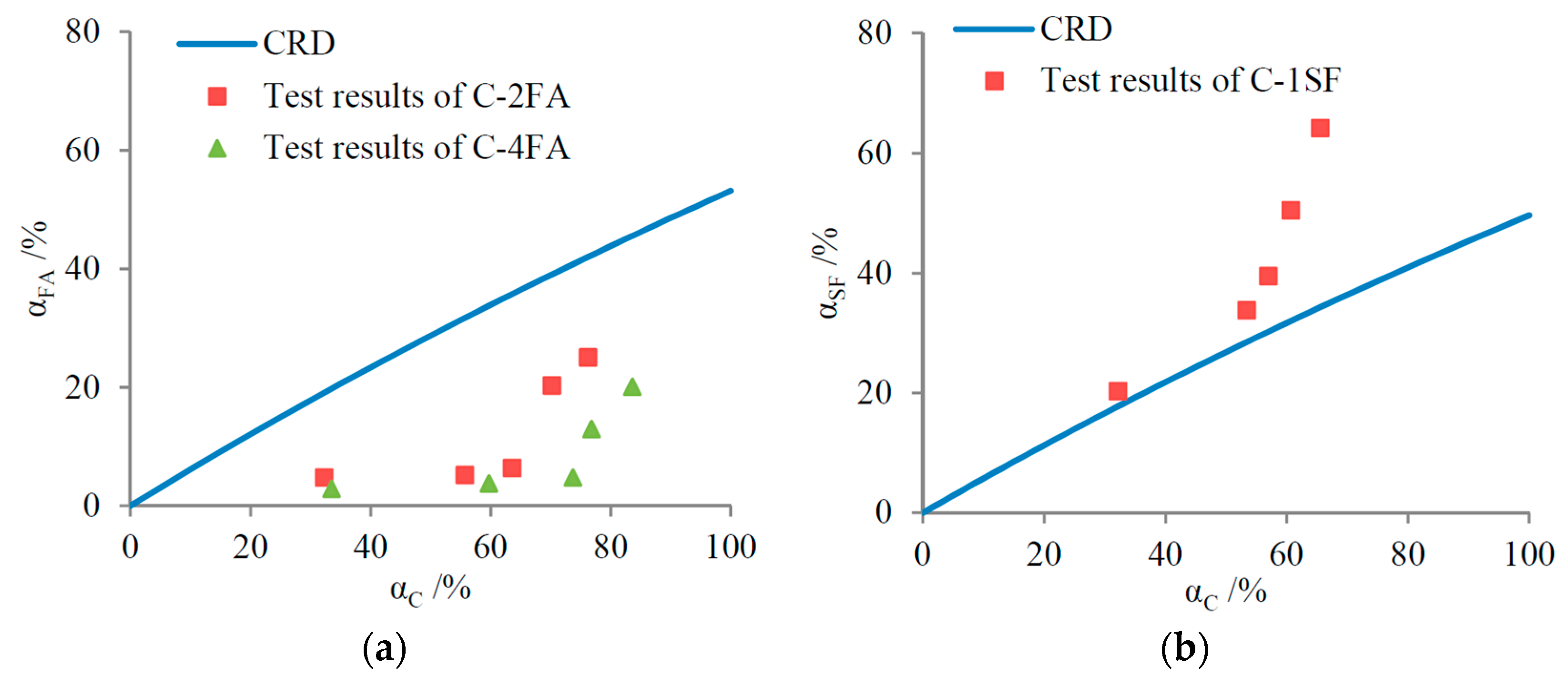
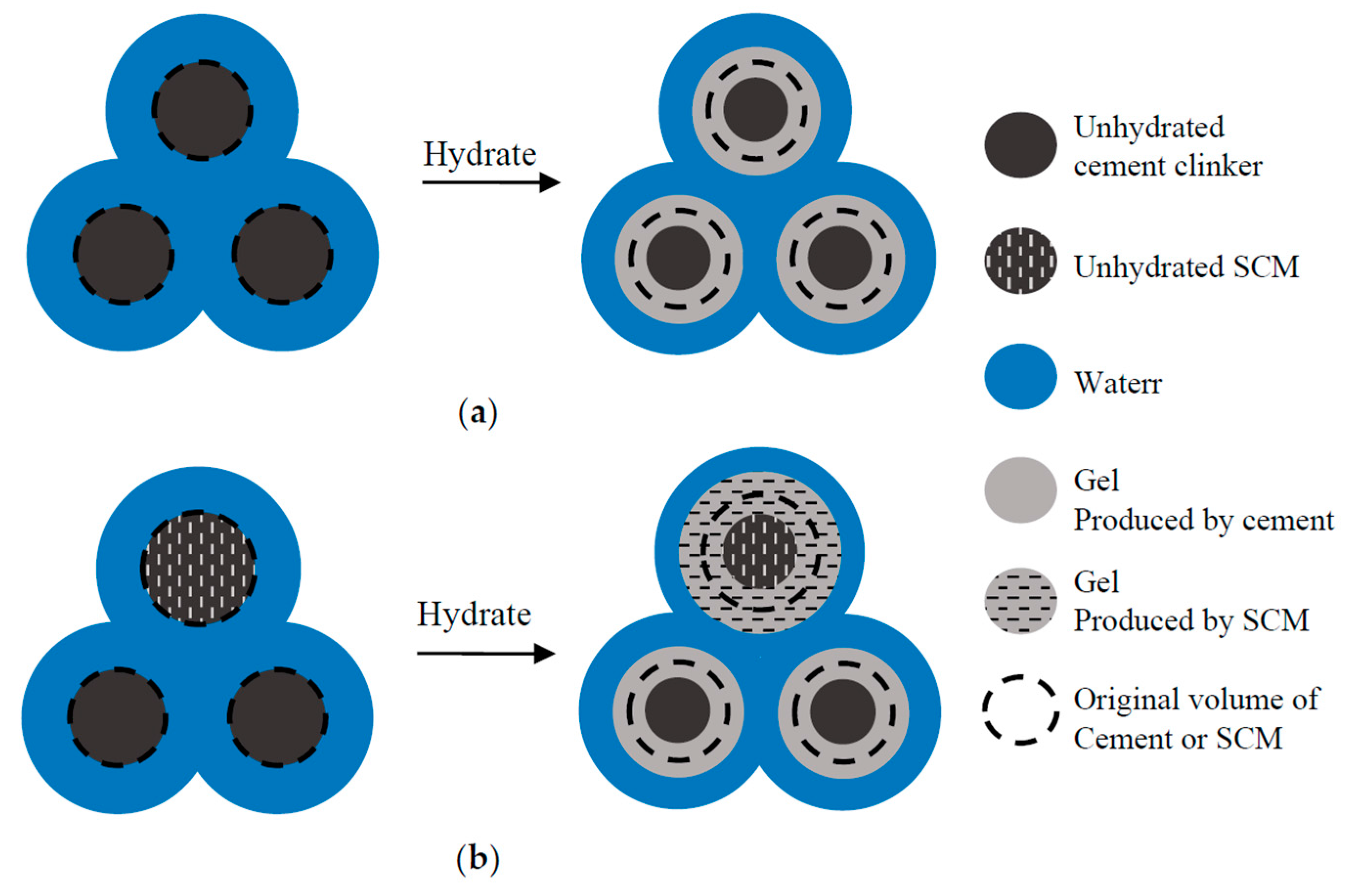
4.1. Relationship between Reaction Degree and Gel/Space Ratio
4.1.1. Binary Systems
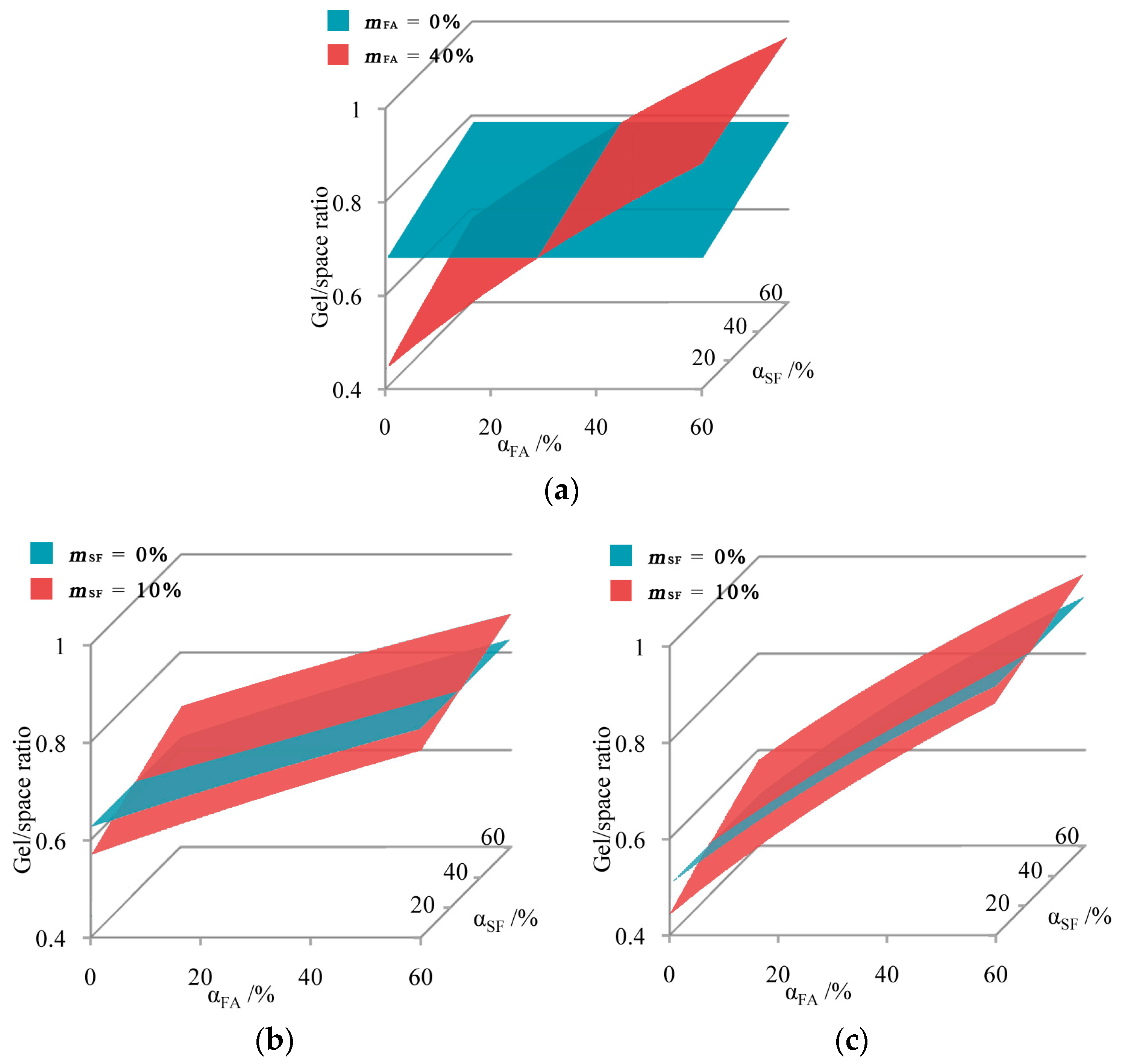
4.1.2. C-SF-FA System
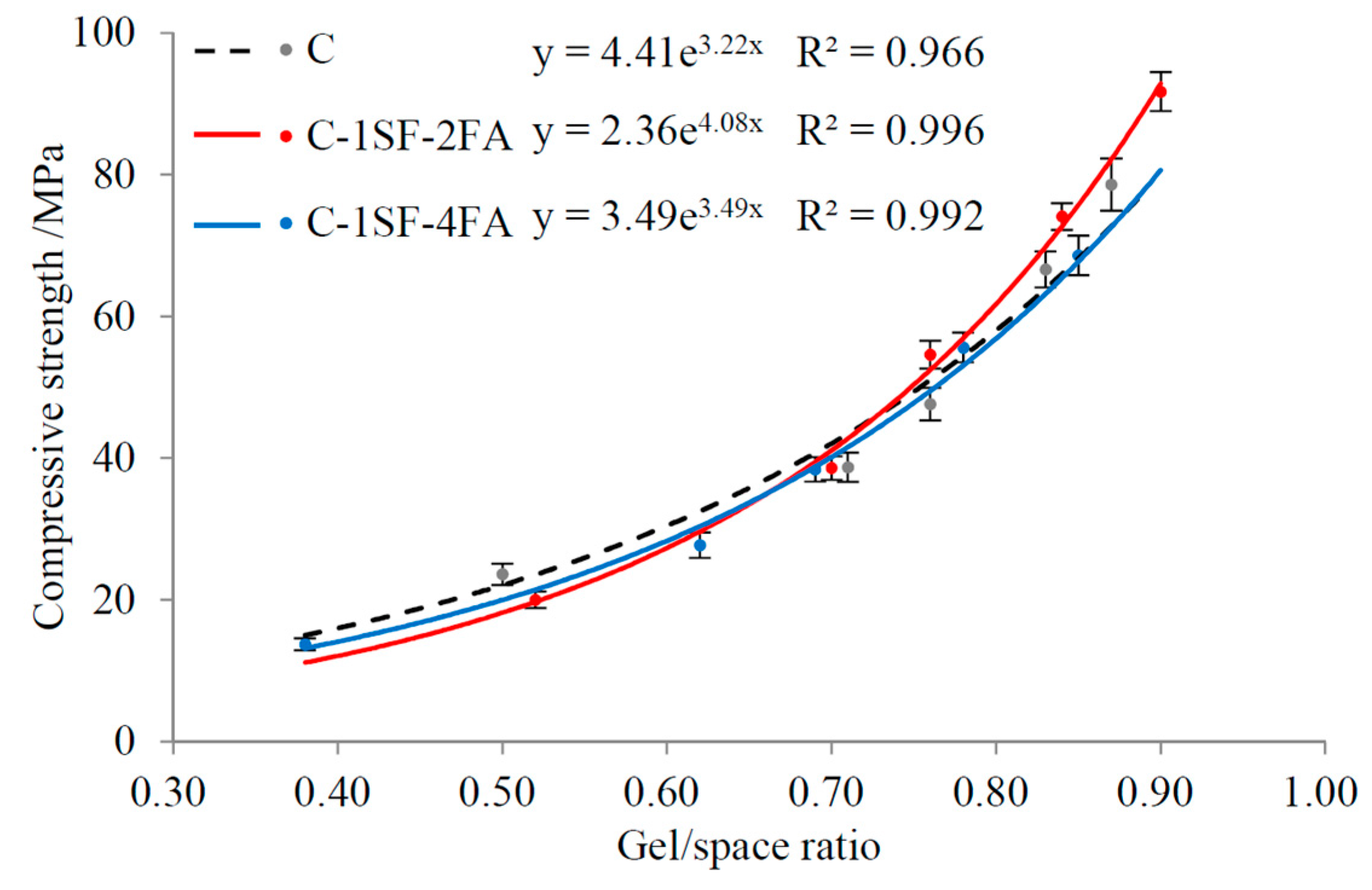
4.2. Relationship between Gel/Space Ratio and Compressive Strength
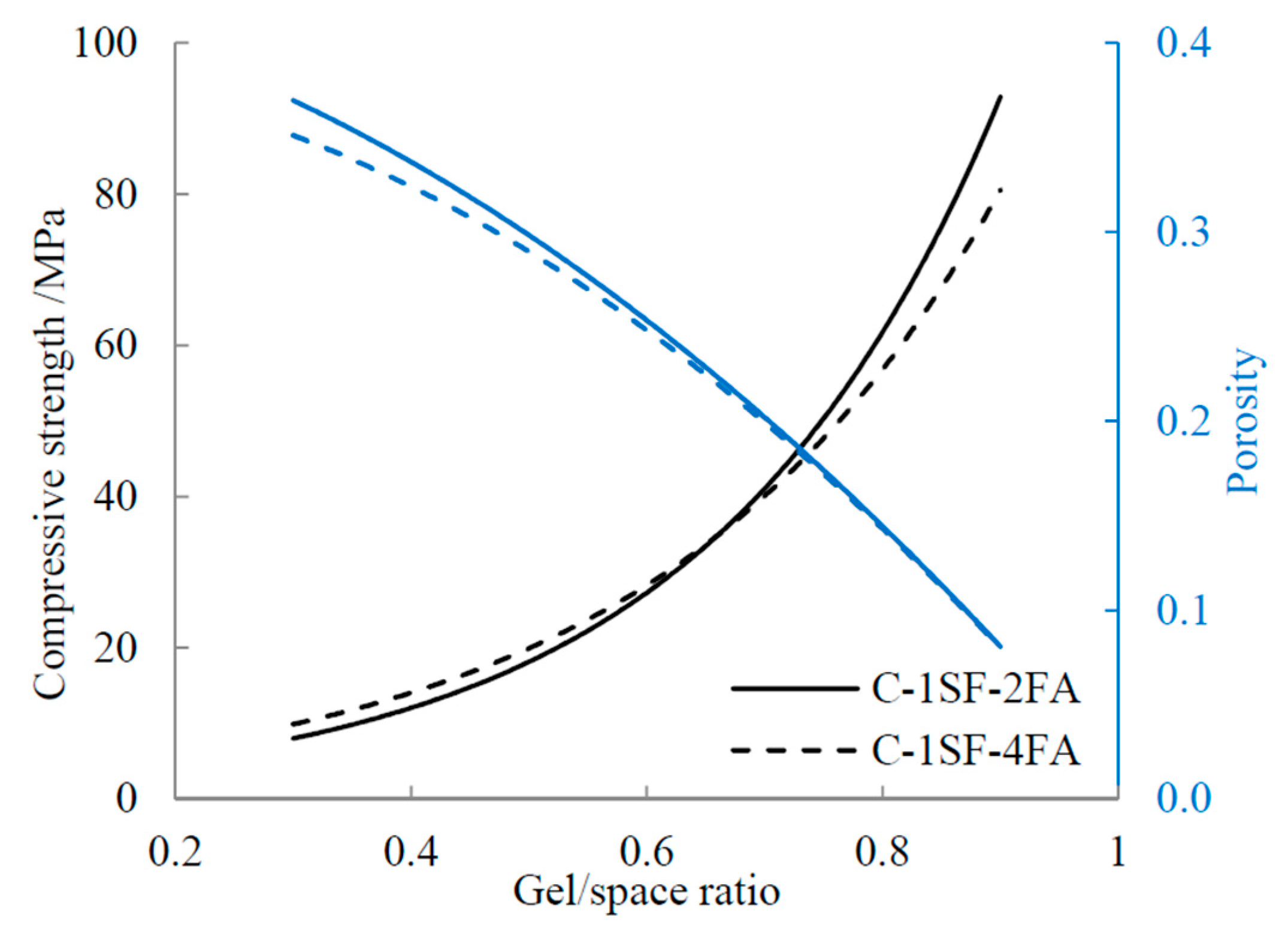
4.2.1. Influence of Porosity
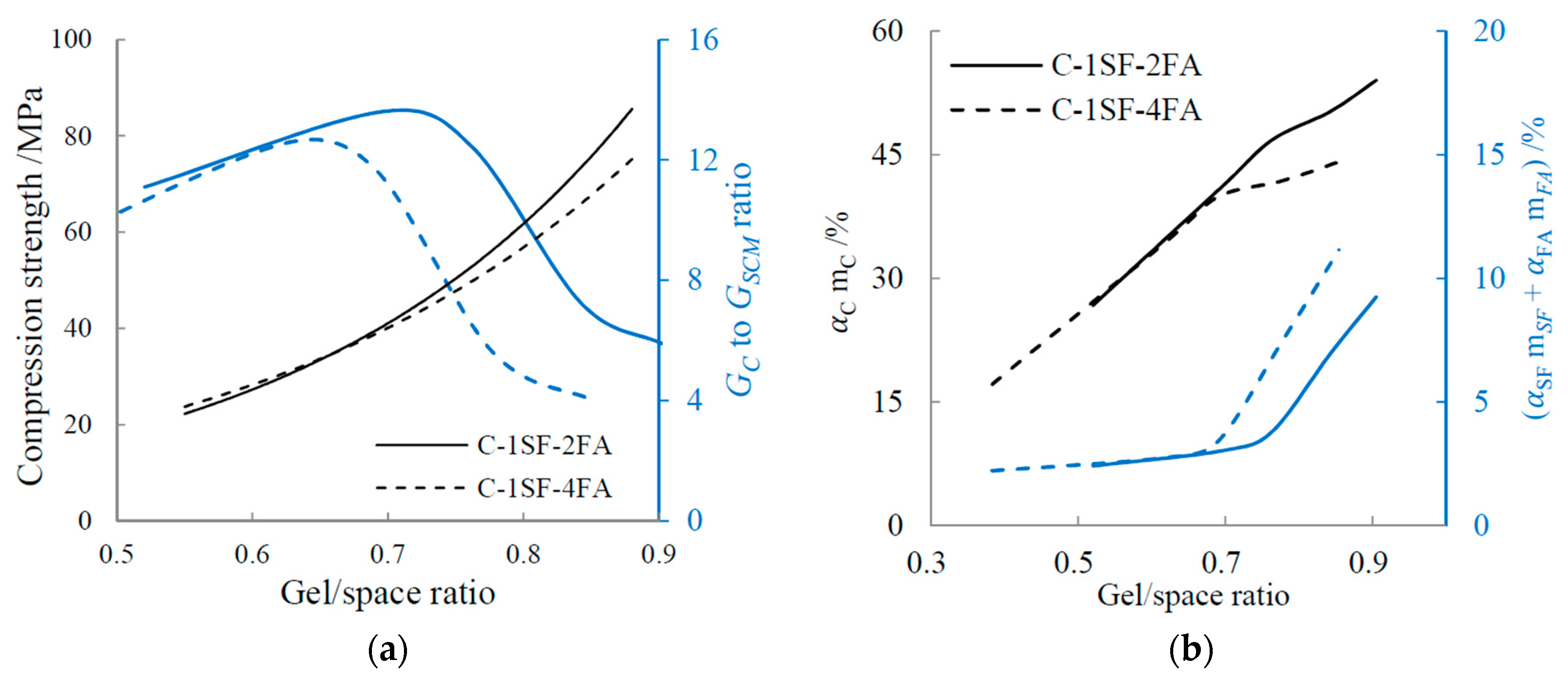
4.2.2. Influence of Ratio of Cement-Produced Gel to SCM-Produced Gel
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr. Build. Mater. 2012, 27, 560–569. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, W.; Xing, X.; Wu, M. Quantitative evaluation of hydrated cement modified by silica fume using QXRD, 27Al MAS NMR, TG-DSC and selective dissolution techniques. Constr. Build. Mater. 2012, 36, 925–932. [Google Scholar] [CrossRef]
- Kocaba, V.; Gallucci, E.; Scrivener, K.L. Methods for determination of degree of reaction of slag in blended cement pastes. Cem. Concr. Res. 2012, 42, 511–525. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F.; Alder, C.; Wieland, E.; Lunk, P. Effect of temperature on the pore solution, microstructure and hydration products of Portland cement pastes. Cem. Concr. Res. 2007, 37, 483–491. [Google Scholar] [CrossRef]
- De Schutter, G.; Taerwe, L. Degree of hydration-based description of mechanical properties of early age concrete. Mater. Struct. 1996, 29, 335–344. [Google Scholar] [CrossRef]
- De Schutter, G. Finite element simulation of thermal cracking in massive hardening concrete elements using degree of hydration based material laws. Comput. Struct. 2002, 80, 2035–2042. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Boumiz, A.; Vernet, C.; Tenoudji, F.C. Mechanical properties of cement pastes and mortars at early ages: Evolution with time and degree of hydration. Adv. Cem. Based Mater. 1996, 3, 94–106. [Google Scholar] [CrossRef]
- Odler, I.; Rößler, M. Investigations on the relationship between porosity, structure and strength of hydrated Portland cement pastes. II. Effect of pore structure and of degree of hydration. Cem. Concr. Res. 1985, 15, 401–410. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Kendall, K.; Howard, A.J.; Birchall, J.D.; Pratt, P.L.; Proctor, B.A.; Jefferis, S.A. The relation between porosity, microstructure and strength, and the approach to advanced cement-based materials [and discussion]. Philos. Trans. R. Soc. Lond. A 1983, 310, 139–153. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rukzon, S. Strength, porosity and corrosion resistance of ternary blend Portland cement, rice husk ash and fly ash mortar. Constr. Build. Mater. 2008, 22, 1601–1606. [Google Scholar] [CrossRef]
- Powers, T.C.; Brownyard, T.L. Studies of the physical properties of hardened Portland cement paste. J. Proc. 1946, 43, 101–132. [Google Scholar]
- Powers, T.C. Structure and physical properties of hardened Portland cement paste. J. Am. Ceram. Soc. 1958, 41, 1–6. [Google Scholar] [CrossRef]
- Mccarter, W.J. Gel formation during early hydration. Cem. Concr. Res. 1987, 17, 55–64. [Google Scholar] [CrossRef]
- Lam, L.; Wong, Y.L.; Poon, C.S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem. Concr. Compos. 2000, 30, 747–756. [Google Scholar] [CrossRef]
- Bonavetti, V.; Donza, H.; Menendez, G.; Cabrera, O.; Irassar, E.F. Limestone filler cement in low w/c concrete: A rational use of energy. Cem. Concr. Res. 2003, 33, 865–871. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jespersen, M.H.S.; Jensen, O.M. Mechanical properties of concrete with SAP. Part I: Development of compressive strength. In Use of Superabsorbent Polymers and Other New Additives in Concrete; RILEM Publications SARL: Paris, France, 2010; pp. 117–126. [Google Scholar]
- Termkhajornkit, P.; Vu, Q.H.; Barbarulo, R.; Daronnat, S.; Chanvillard, G. Dependence of compressive strength on phase assemblage in cement pastes: Beyond gel-space ratio—Experimental evidence and micromechanical modeling. Cem. Concr. Res. 2014, 56, 1–11. [Google Scholar] [CrossRef]
- Pichler, B.; Hellmich, C.; Eberhardsteiner, J.; Wasserbauer, J.; Termkhajornkit, P.; Barbarulo, R.; Chanvillard, G. Effect of gel-space ratio and microstructure on strength of hydrating cementitious materials: An engineering micromechanics approach. Cem. Concr. Res. 2013, 45, 55–68. [Google Scholar] [CrossRef]
- Suprenant, B.A.; Papadopoulos, G. Selective dissolution of Portland-fly-ash cements. J. Mater. Civil. Eng. 1991, 3, 48–59. [Google Scholar] [CrossRef]
- Dyson, H.M. Early Hydration in Binary and Ternary Blended Cement Systems. Ph.D. Thesis, University of Leeds, Leeds, UK, 2005. [Google Scholar]
- Scrivener, K.; Lothenbach, B.; De Belie, N.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and microstructure of concrete with SCMs State of the art on methods to determine degree of reaction of SCMs. Mater. Struct. 2015, 48, 835–861. [Google Scholar] [CrossRef] [Green Version]
- Nocuñ-Wczelik, W. Heat evolution in hydrated cementitious systems admixture with fly ash. J. Therm. Anal. Calorim. 2001, 65, 613–619. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Saout, G.L.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Dyson, H.M.; Richardson, I.G.; Brough, A.R. A combined 29Si MAS NMR and selective dissolution technique for the quantitative evaluation of hydrated blast furnace slag cement blends. J. Am. Ceram. Soc. 2007, 90, 598–602. [Google Scholar] [CrossRef]
- Al Dulaijan, S.U.; Parry Jones, G.; Al Tayyib, A.H.J.; Al Mana, A.I. 29Si Magic-Angle-Spinning nuclear magnetic resonance study of hydrated cement paste and mortar. J. Am. Ceram. Soc. 1990, 73, 736–739. [Google Scholar] [CrossRef]
- Wei, Y. Quantitative Characterization and Evaluation of Hydration Progress of Composite Cementitious Systems. Ph.D. Thesis, Tongji University, Shanghai, China, 2013. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997; pp. 278–280. [Google Scholar]
- Papadakis, V.G. Experimental investigation and theoretical modeling of silica fume activity in concrete. Cem. Concr. Res. 1999, 29, 79–86. [Google Scholar] [CrossRef]
- Singh, N.B.; Kalra, M.; Kumar, M.; Rai, S. Hydration of ternary cementitious system: Portland cement, fly ash and silica fume. J. Therm. Anal. Calorim. 2015, 119, 381–389. [Google Scholar] [CrossRef]
- Schwarz, N.; Neithalath, N. Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cem. Concr. Res. 2008, 38, 429–436. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of fly ash on Portland cement systems: Part I. Low-calcium fly ash. Cem. Concr. Res. 1999, 29, 1727–1736. [Google Scholar] [CrossRef]
- Hobbs, S.V. A Study of Non-Evaporable Water Content in Cement-Based Mixtures with and without Pozzolanic Materials. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2000. [Google Scholar]
- Lei, D.; Guo, L.; Sun, W.; Liu, J.; Shu, X.; Guo, X. A new dispersing method on silica fume and its influence on the performance of cement-based materials. Constr. Build. Mater. 2016, 115, 716–726. [Google Scholar] [CrossRef]
- Wang, X. Effect of fly ash on properties evolution of cement based materials. Constr. Build. Mater. 2014, 69, 32–40. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Argiz, C.; Gálvez, J.C.; Moragues, A. Effect of silica fume fineness on the improvement of Portland cement strength performance. Constr. Build. Mater. 2015, 96, 55–64. [Google Scholar] [CrossRef]
- Antiohos, S.K.; Papageorgiou, A.; Papadakis, V.G.; Tsimas, S. Influence of quicklime addition on the mechanical properties and hydration degree of blended cements containing different fly ashes. Constr. Build. Mater. 2008, 22, 1191–1200. [Google Scholar] [CrossRef]
- Oltulu, M.; Şahin, R. Pore structure analysis of hardened cement mortars containing silica fume and different nano-powders. Constr. Build. Mater. 2014, 53, 658–664. [Google Scholar] [CrossRef]
- Kim, K.M.; Heo, Y.S.; Kang, S.P.; Lee, J. Effect of sodium silicate- and ethyl silicate-based nano-silica on pore structure of cement composites. Cem. Concr. Compos. 2014, 49, 84–91. [Google Scholar] [CrossRef]
- Constantinides, G.; Ulm, F.J. The effect of two types of C-S-H on the elasticity of cement-based materials: Results from nanoindentation and micromechanical modeling. Cem. Concr. Res. 2004, 34, 67–80. [Google Scholar] [CrossRef]
- Constantinides, G.; Ulm, F.J.; van Vliet, K. On the use of nanoindentation for cementitious materials. Mater. Struct. 2003, 36, 191–196. [Google Scholar] [CrossRef]
- Němeček, J.; Šmilauer, V.; Kopecký, L. Characterization of Alkali-Activated Fly-Ash by Nanoindentation. Nanotechnology in Construction 3; Springer: Berlin/Heidelberg, Germany, 2009; pp. 337–343. [Google Scholar]

| Binders | Chemical Compositions (wt %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | MnO | Fe2O3 | LOI | |
| Cement clinker | 0.61 | 0.92 | 5.48 | 23.1 | 0.07 | 0.48 | 0.76 | 64.8 | 0.08 | 3.15 | 0.55 |
| SF | 0.37 | 0.70 | 0.30 | 94.5 | 0.13 | 0.40 | 1.16 | 0.94 | 0.02 | 0.06 | 1.42 |
| FA | 0.45 | 1.23 | 28.98 | 54.70 | 0.18 | 0.58 | 1.65 | 4.48 | 0.06 | 5.24 | 2.45 |
| Sample No. | Mix Proportions (g) | ||||
|---|---|---|---|---|---|
| Cement | SF | FA | Quartz | Water | |
| C | 100 | - | - | - | 30 |
| C-1SF | 90 | 10 | - | - | 30 |
| C-2FA | 80 | - | 20 | - | 30 |
| C-4FA | 60 | - | 40 | - | 30 |
| C-1SF-2FA | 70 | 10 | 20 | - | 30 |
| C-1SF-2Q | 70 | 10 | - | 20 | 30 |
| C-1SF-4FA | 60 | 10 | 40 | - | 30 |
| C-1SF-4Q | 60 | 10 | - | 40 | 30 |
| Sample No. | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|
| 1 d | 3 d | 7 d | 28 d | 90 d | |
| C | 23.6 ± 1.5 | 38.7 ± 2.1 | 47.6 ± 2.3 | 66.6 ± 2.6 | 78.6 ± 3.7 |
| C-1SF | 27.1 ± 1.3 | 45.6 ± 1.9 | 57.9 ± 2.0 | 73.3 ± 2.6 | 88.5 ± 2.7 |
| C-2FA | 22.9 ± 1.0 | 36.5 ± 1.3 | 49.4 ± 1.7 | 70.9 ± 2.2 | 93.8 ± 2.9 |
| C-4FA | 12.8 ± 1.6 | 31.9 ± 1.8 | 43.8 ± 2.2 | 58.0 ± 2.4 | 71.2 ± 3.1 |
| C-1SF-2FA | 20.0 ± 1.2 | 38.6 ± 1.7 | 54.6 ± 2.0 | 74.1 ± 1.9 | 91.7 ± 2.7 |
| C-1SF-4FA | 13.7 ± 0.9 | 27.7 ± 1.8 | 38.4 ± 1.7 | 55.6 ± 2.1 | 68.6 ± 2.8 |
| Sample No. | Gel/Space Ratio | ||||
|---|---|---|---|---|---|
| 1 d | 3 d | 7 d | 28 d | 90 d | |
| C | 0.50 | 0.71 | 0.76 | 0.83 | 0.87 |
| C-1SF | 0.54 | 0.77 | 0.81 | 0.85 | 0.90 |
| C-2FA | 0.47 | 0.69 | 0.76 | 0.85 | 0.90 |
| C-4FA | 0.40 | 0.61 | 0.70 | 0.78 | 0.85 |
| C-1SF-2FA | 0.52 | 0.70 | 0.76 | 0.84 | 0.90 |
| C-1SF-4FA | 0.38 | 0.62 | 0.69 | 0.78 | 0.85 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Li, C.; Yao, W. Gel/Space Ratio Evolution in Ternary Composite System Consisting of Portland Cement, Silica Fume, and Fly Ash. Materials 2017, 10, 59. https://doi.org/10.3390/ma10010059
Wu M, Li C, Yao W. Gel/Space Ratio Evolution in Ternary Composite System Consisting of Portland Cement, Silica Fume, and Fly Ash. Materials. 2017; 10(1):59. https://doi.org/10.3390/ma10010059
Chicago/Turabian StyleWu, Mengxue, Chen Li, and Wu Yao. 2017. "Gel/Space Ratio Evolution in Ternary Composite System Consisting of Portland Cement, Silica Fume, and Fly Ash" Materials 10, no. 1: 59. https://doi.org/10.3390/ma10010059





