Bonding to Different PEEK Compositions: The Impact of Dental Light Curing Units
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of Specimens
2.2. Fracture Type Anaysis
2.3. Statistical Analyses
3. Results
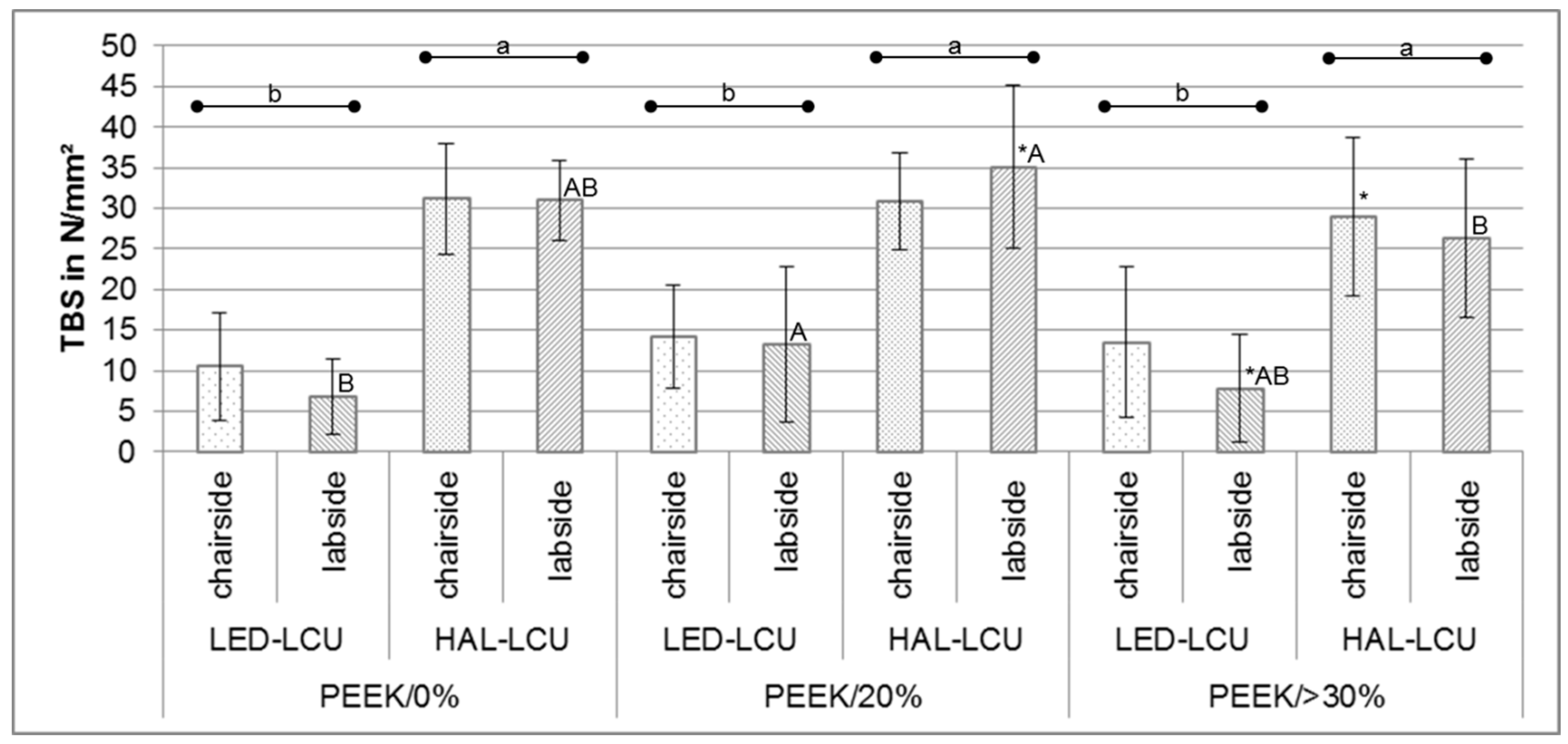
3.1. Tensile Bond Strength
3.2. Fracture Types
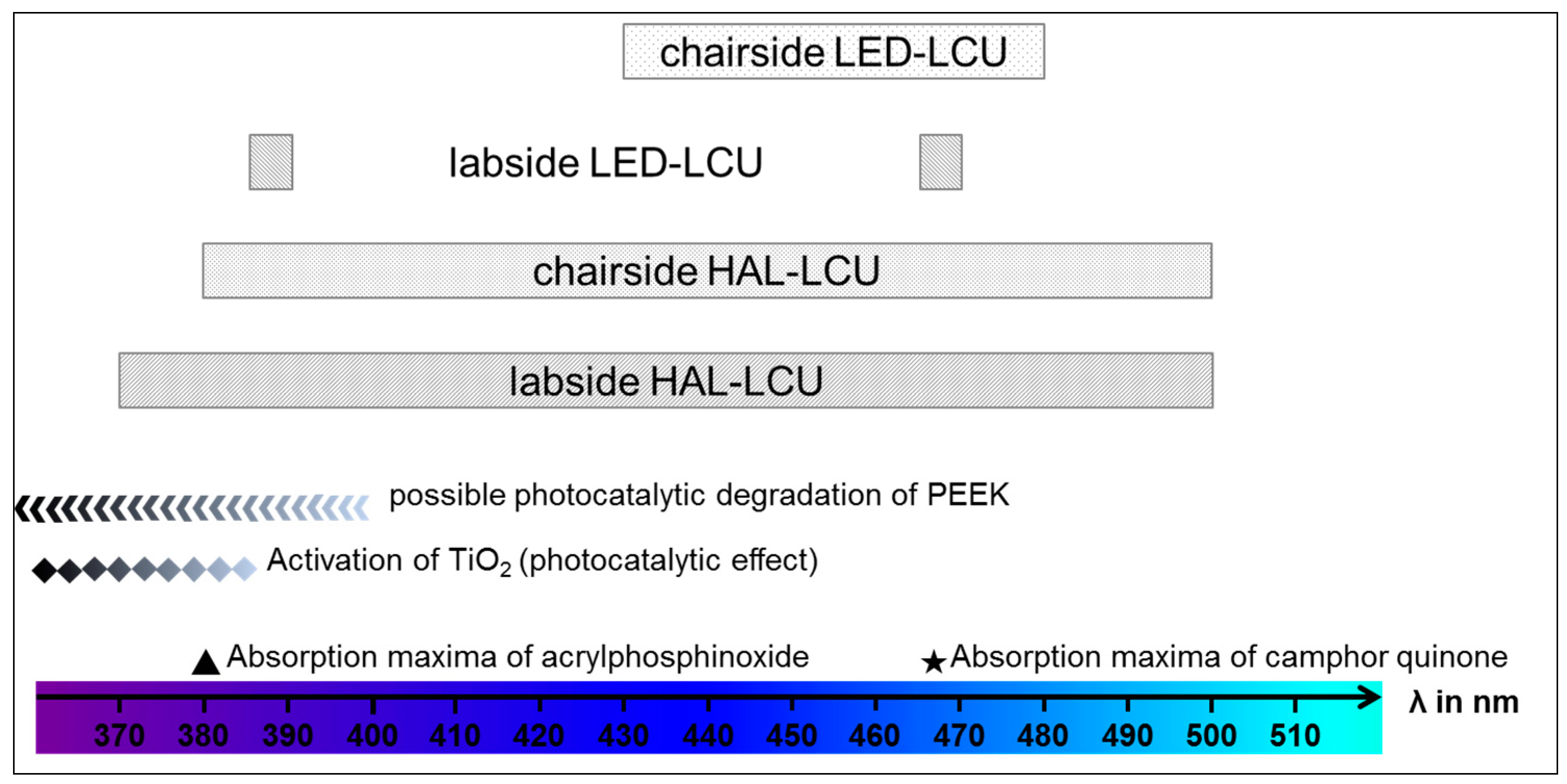
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PEEK | polyetheretherketone |
| MMA | methylmethacrylate |
| DMA | dimethacrlyate |
| TiO2 | titanium dioxide |
| LED | light-emitting diode |
| HAL | halogen |
| TBS | tensile bond strength |
| LCU | light curing unit |
| FDPs | fixed dental prostheses |
| VL | visio.link |
| Al2O3 | alumina oxide |
References
- Modjarrad, K.; Ebnesajjad, S. Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Nano-TiO2 reinforced PEEK/PEI blends as biomaterials for load-bearing implant applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, T.; Huffmann, A.M.S.; Eichberger, M.; Schmidlin, P.R.; Stawarczyk, B. Two-body wear rate of PEEK, CAD/CAM resin composite and PMMA: Effect of specimen geometries, antagonist materials and test set-up configuration. Dent. Mater. 2016, 32, e127–e136. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Thrun, H.; Eichberger, M.; Roos, M.; Edelhoff, D.; Schweiger, J.; Schmidlin, P.R. Effect of different surface pretreatments and adhesives on the load-bearing capacity of veneered 3-unit PEEK FDPs. J. Prosthet. Dent. 2015, 114, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.O.; Tsitrou, E.A.; Pollington, S. Comparative in vitro evaluation of CAD/CAM vs conventional provisional crowns. J. Appl. Oral Sci. Rev. FOB 2016, 24, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Taufall, S.; Eichberger, M.; Schmidlin, P.R.; Stawarczyk, B. Fracture load and failure types of different veneered polyetheretherketone fixed dental prostheses. Clin. Oral Investig. 2016, 20, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Schmidlin, P.; Merk, S.; Wagner, C.; Roos, M.; Eichberger, M.; Stawarczyk, B. PEEK primary crowns with cobalt-chromium, zirconia and galvanic secondary crowns with different tapers—A comparison of retention forces. Materials 2016, 9, 187. [Google Scholar] [CrossRef]
- Wagner, C.; Stock, V.; Merk, S.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Stawarczyk, B. Retention load of telescopic crowns with different taper angles between cobalt-chromium and polyetheretherketone made with three different manufacturing processes examined by pull-off test. J. Prosthodont. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, A.; Wimmer, T.; Schmidlin, P.R.; Scherer, H.; Loffler, P.; Roos, M.; Stawarczyk, B. Physicomechanical characterization of polyetheretherketone and current esthetic dental CAD/CAM polymers after aging in different storage media. J. Prosthet. Dent. 2016, 115, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.D.; Spintig, T.; Kallage, I.; Muller, W.-D. Flexural behavior of PEEK materials for dental application. Dent. Mater. 2015, 31, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Heimer, S.; Schmidlin, P.R.; Stawarczyk, B. Discoloration of PMMA, composite, and PEEK. Clin. Oral Investig. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, P.; Papathanasiou, I.; Polyzois, G. The use of a modified poly-ether-ether-ketone (PEEK) as an alternative framework material for removable dental prostheses. A clinical report. J. Prosthodont. 2016, 25, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, P.; Papathanasiou, I. Modified PEEK resin-bonded fixed dental prosthesis as an interim restoration after implant placement. J. Prosthet. Dent. 2016, 116, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, P.; Bakiri, E.; Polyzois, G. Using modified polyetheretherketone (PEEK) as an alternative material for endocrown restorations: A short-term clinical report. J. Prosthet. Dent. 2016. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Beuer, F.; Wimmer, T.; Jahn, D.; Sener, B.; Roos, M.; Schmidlin, P.R. Polyetheretherketone-a suitable material for fixed dental prostheses? J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Keul, C.; Liebermann, A.; Schmidlin, P.R.; Roos, M.; Sener, B.; Stawarczyk, B. Influence of PEEK surface modification on surface properties and bond strength to veneering resin composites. J. Adhes. Dent. 2014, 16, 383–392. [Google Scholar] [PubMed]
- Stawarczyk, B.; Keul, C.; Beuer, F.; Roos, M.; Schmidlin, P.R. Tensile bond strength of veneering resins to PEEK: Impact of different adhesives. Dent. Mater. J. 2013, 32, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Uhrenbacher, J.; Schmidlin, P.R.; Keul, C.; Eichberger, M.; Roos, M.; Gernet, W.; Stawarczyk, B. The effect of surface modification on the retention strength of polyetheretherketone crowns adhesively bonded to dentin abutments. J. Prosthet. Dent. 2014, 112, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Silthampitag, P.; Chaijareenont, P.; Tattakorn, K.; Banjongprasert, C.; Takahashi, H.; Arksornnukit, M. Effect of surface pretreatments on resin composite bonding to PEEK. Dent. Mater. J. 2016, 35, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Bahr, N.; Beuer, F.; Wimmer, T.; Eichberger, M.; Gernet, W.; Jahn, D.; Schmidlin, P.R. Influence of plasma pretreatment on shear bond strength of self-adhesive resin cements to polyetheretherketone. Clin. Oral Investig. 2014, 18, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Visai, L.; de Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Org. 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.L. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 2008, 17, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Gutwein, L.G.; Webster, T.J. Osteoblast and chrondrocyte proliferation in the presence of alumina and titania nanoparticles. J. Nanopart. Res. 2002, 4, 231–238. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, T.; Noguchi, T.; Imagawa, K. Photodegradation of PEEK sheets under tensile stress. Polym. Degrad. Stab. 2006, 91, 740–746. [Google Scholar] [CrossRef]
- Patel, P.; Hull, T.R.; McCabe, R.W.; Flath, D.; Grasmeder, J.; Percy, M. Mechanism of thermal decomposition of poly(ether ether ketone) (PEEK) from a review of decomposition studies. Polym. Degrad. Stab. 2010, 95, 709–718. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
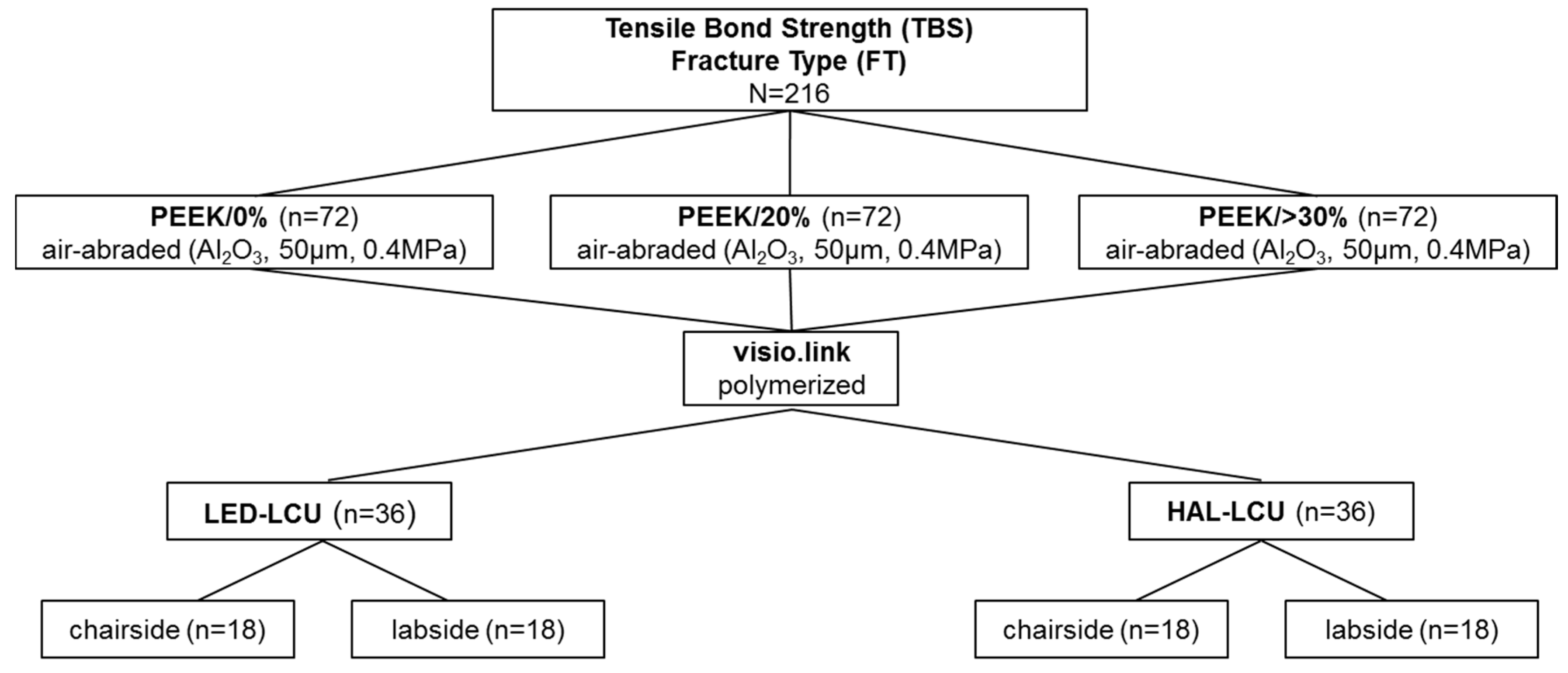
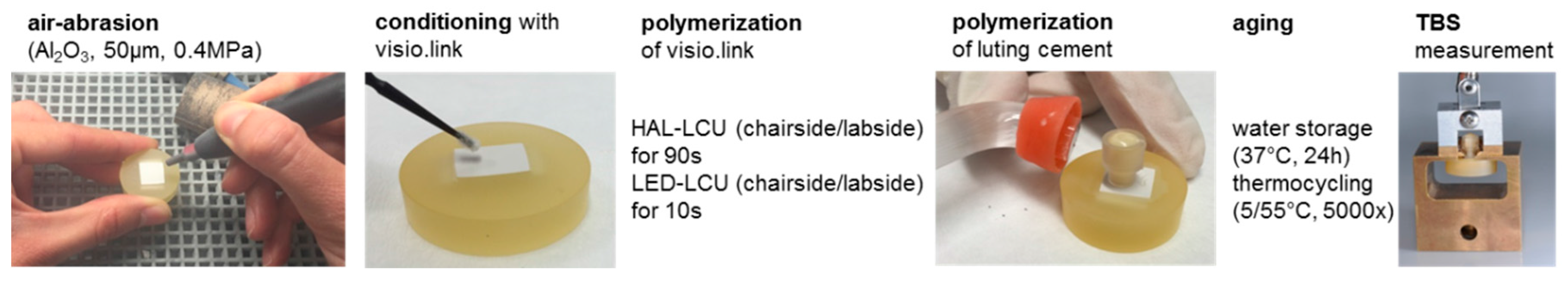
| Material Groups | Product Name Abbreviation | Manufacturer | LOT No. | ||
|---|---|---|---|---|---|
| PEEK | Tizian PEEK PEEK/0% | Schütz Dental Group, Rosbach, Germany | 2014004126 | ||
| Dentokeep PEEK PEEK/20% | nt-trading, Karlsruhe, Germany | 11DK14001 | |||
| bre.CAM. BioHPP dentine shade 2 PEEK/>30% | bredent, Senden, Germany | 438251 | |||
| Adhesive system | visio.link (VL) | bredent, Senden, Germany | 135071 | ||
| Luting cement | Panavia SA Cement | Kuraray Medical Inc., Tokyo, Japan | 058AAA | ||
| Light curing unit (LCU) | Wavelength/Light Intensity | ||||
| LED | chairside | Elipar S10 | 3M, Seefeld, Germany | 430–480 nm 1200 mW/cm2 | |
| labside | EyeVolution MAX | Dreve, Unna, Germany | 1 × 385−390 nm 6 × 465−470 nm | ||
| Halogen | chairside | Translux CL | Heraeus Kulzer, Hanau, Germany | 380–500 nm 450 mW/cm2 | |
| labside | bre.Lux Power Unit | bredent, Senden, Germany | 370–500 nm | ||
| TBS | |||||
|---|---|---|---|---|---|
| PEEK | Light Curing Unit | Mean ± SD | 95% CI | Min/Median/Max | |
| PEEK/0% | LED-LCU | chair | 10.5 ± 6.7 b | 7.0; 13.8 | 0.0/9.0/ 23.8 |
| lab | 6.8 ± 4.7 B,b | 4.3; 9.2 | 0.0/6.4/14.7 | ||
| HAL-LCU | chair | 31.2 ± 6.8 a | 27.7; 34.6 | 12.1/31.0/41.2 | |
| lab | 31.0 ± 4.9 A,B,a | 28.4; 33.5 | 22.3/31.6/38.6 | ||
| PEEK/20% | LED-LCU | chair | 14.2 ± 6.4 b | 10.8; 17.4 | 0.0/15.6/23.6 |
| lab | 13.2 ± 9.6 A,b | 8.3; 18.0 | 0.0/10.9/35.3 | ||
| HAL-LCU | chair | 30.9 ± 6.0 a | 27.7; 33.9 | 15.3/32.5/39.1 | |
| lab | 35.1 ± 10.0 *,A,a | 30.0; 40.2 | 0.0/37.3/43.9 | ||
| PEEK/>30% | LED-LCU | chair | 13.5 ± 9.2 b | 8.8; 17.9 | 0.0/12.9/34.5 |
| lab | 7.8 ± 6.6 *,A,B,b | 4.4; 11.2 | 0.0/8.2/17.3 | ||
| HAL-LCU | chair | 29.0 ± 9.8 *,a | 24.0; 33.9 | 4.6/30.7/40.6 | |
| lab | 26.3 ± 9.8 B,a | 21.3; 31.3 | 2.6/28.3/39.7 | ||
| Fracture Types | ||||
|---|---|---|---|---|
| PEEK | Light Curing Unit | Adhesive | Cohesive | |
| PEEK/0% | LED-LCU | chair | 100 (80; 100) | 0 (0; 19) |
| lab | 94 (72; 100) | 6 (0; 28) | ||
| HAL-LCU | chair | 0 (0; 19) | 100 (80; 100) | |
| lab | 6 (0; 28) | 94 (72; 100) | ||
| PEEK/20% | LED-LCU | chair | 83 (57; 97) | 17 (2; 42) |
| lab | 100 (80; 100) | 0 (0; 19) | ||
| HAL-LCU | chair | 0 (0; 19) | 100 (80; 100) | |
| lab | 0 (0; 19) | 100 (80; 100) | ||
| PEEK/>30% | LED-LCU | chair | 89 (64; 99) | 11 (0; 35) |
| lab | 89 (64; 99) | 11 (0; 35) | ||
| HAL-LCU | chair | 11 (0; 35) | 89 (64; 99) | |
| lab | 6 (0; 28) | 94 (72; 100) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Bonding to Different PEEK Compositions: The Impact of Dental Light Curing Units. Materials 2017, 10, 67. https://doi.org/10.3390/ma10010067
Lümkemann N, Eichberger M, Stawarczyk B. Bonding to Different PEEK Compositions: The Impact of Dental Light Curing Units. Materials. 2017; 10(1):67. https://doi.org/10.3390/ma10010067
Chicago/Turabian StyleLümkemann, Nina, Marlis Eichberger, and Bogna Stawarczyk. 2017. "Bonding to Different PEEK Compositions: The Impact of Dental Light Curing Units" Materials 10, no. 1: 67. https://doi.org/10.3390/ma10010067







