Comparison of Maraging Steel Micro- and Nanostructure Produced Conventionally and by Laser Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Additive Manufacturing
2.2. Microstructural Analysis
3. Results
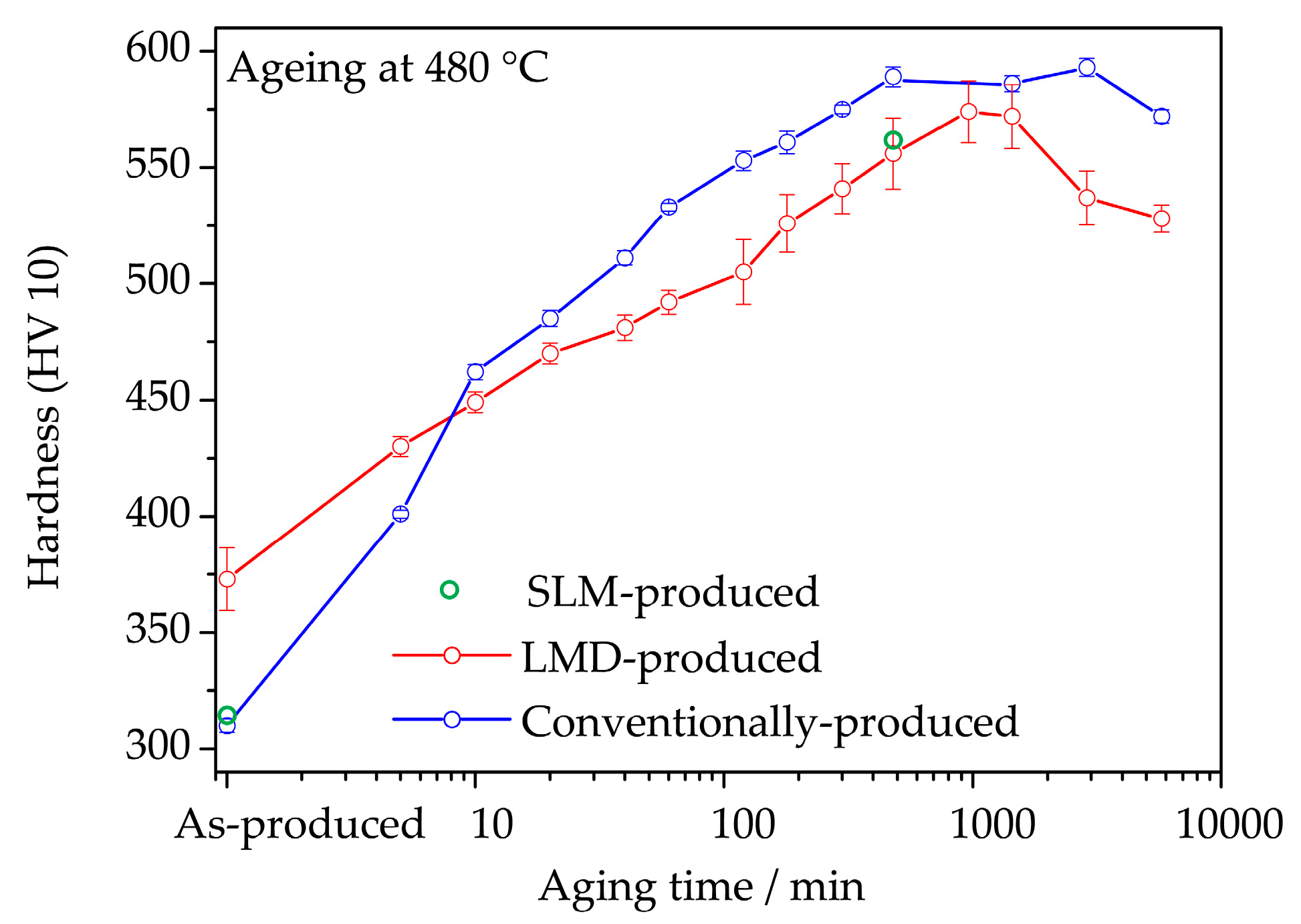
3.1. Ageing Behavior
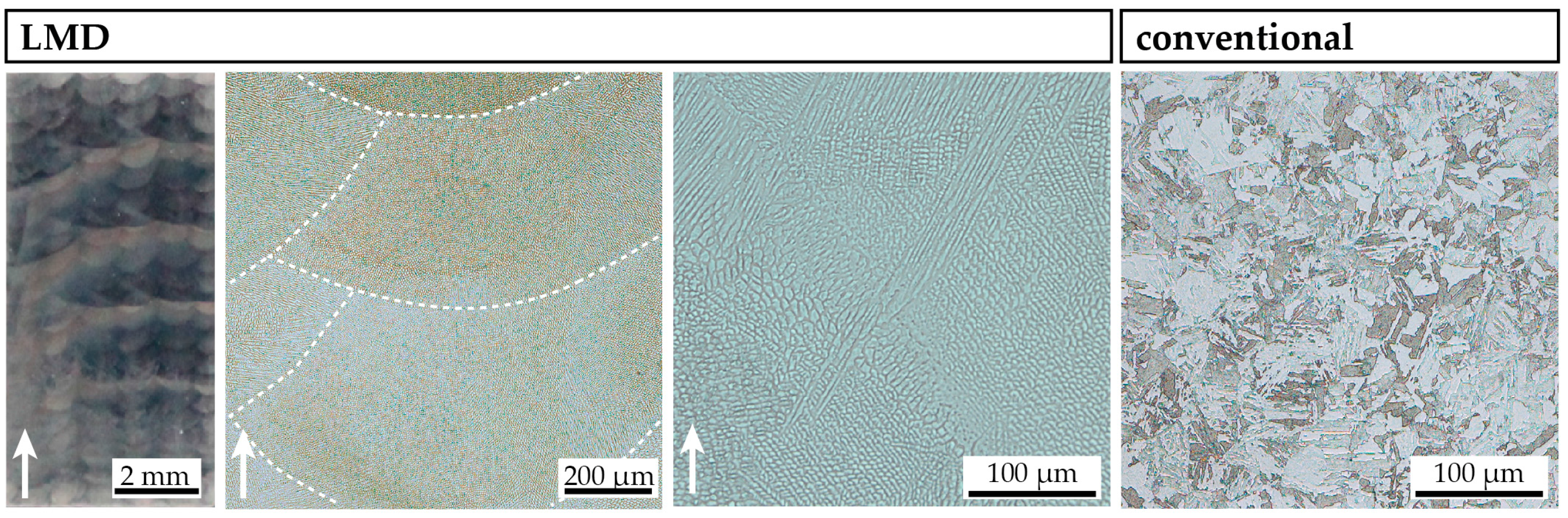
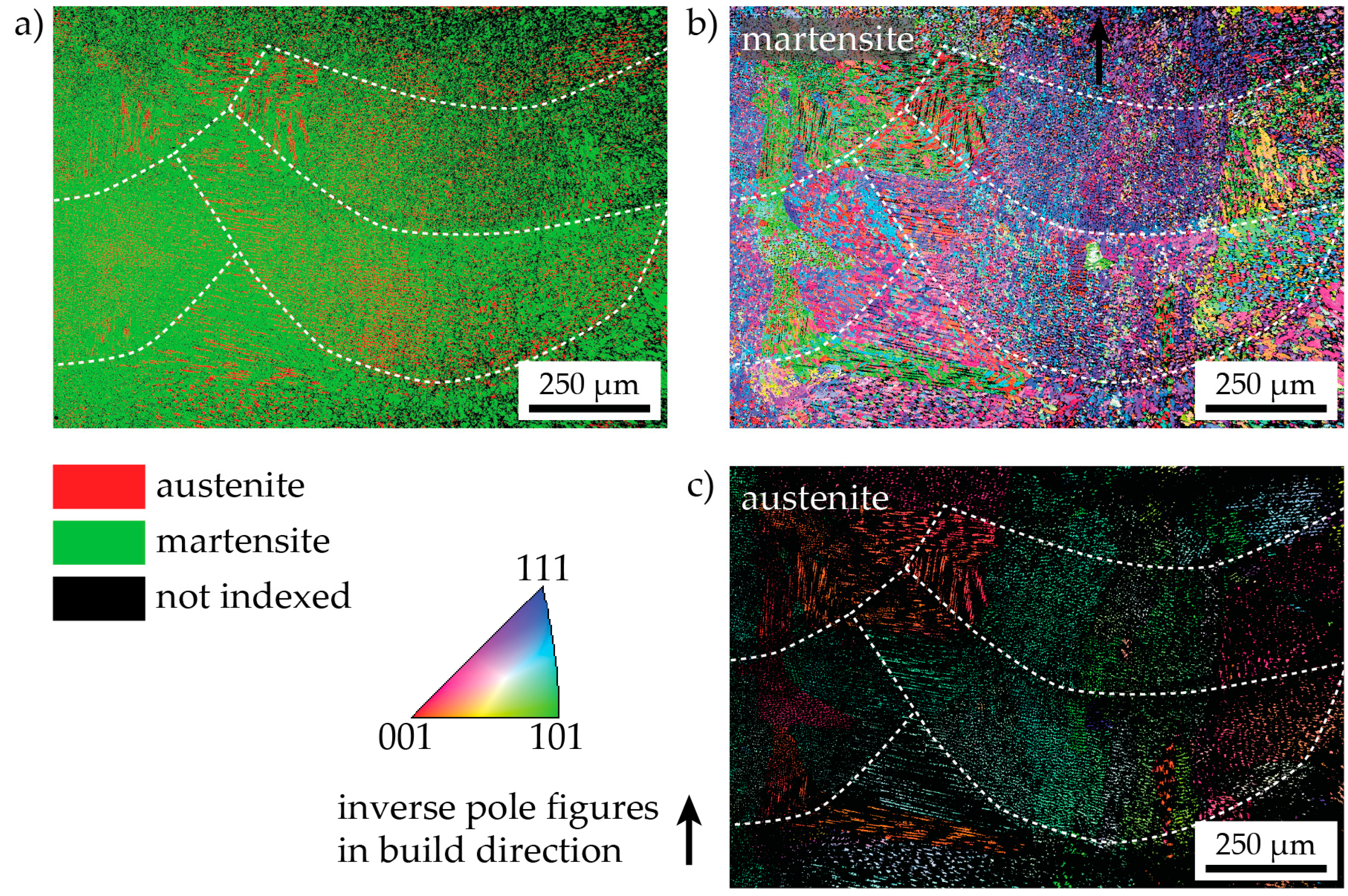
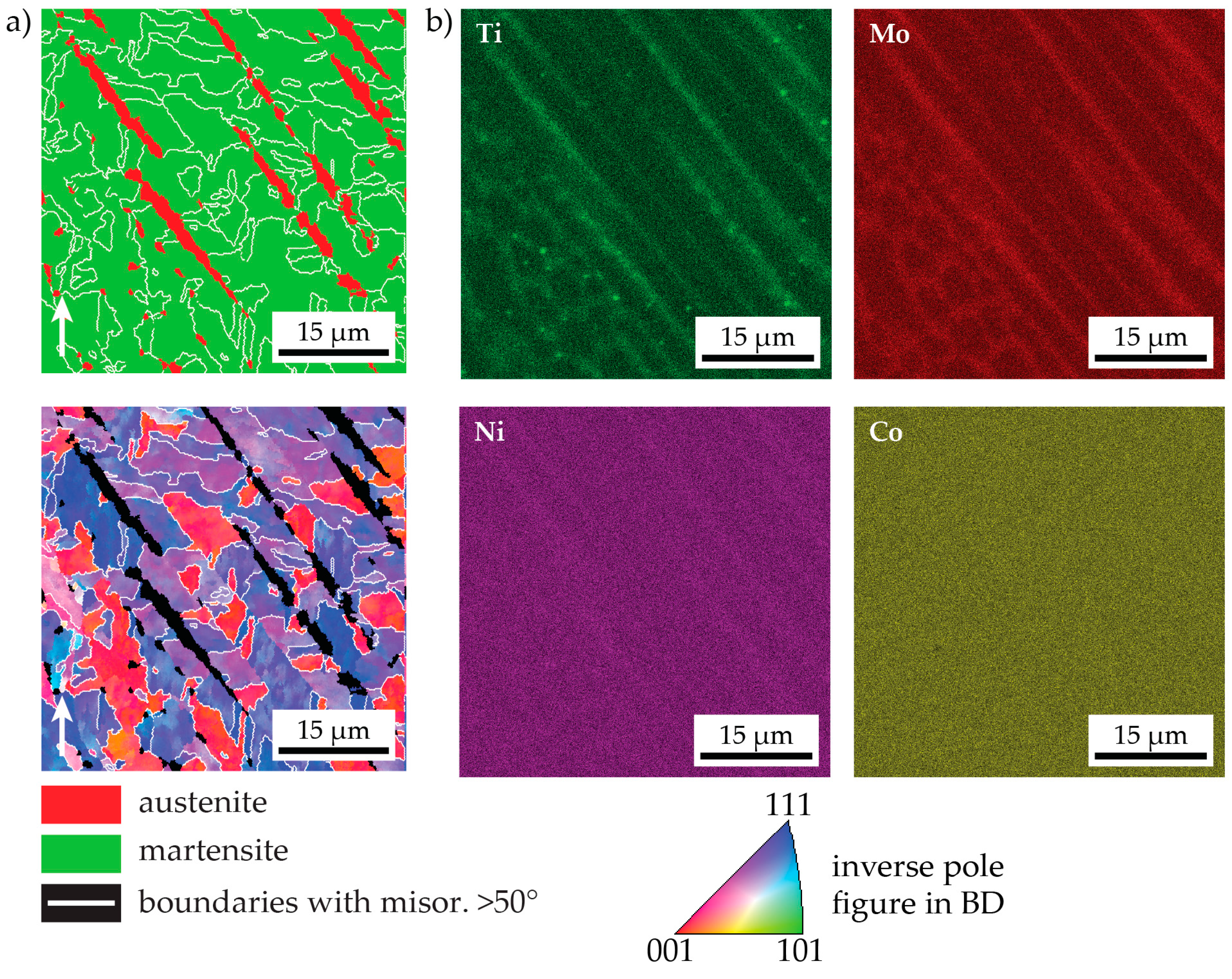
3.2. Microstructure in the As-Produced/As-Received State State
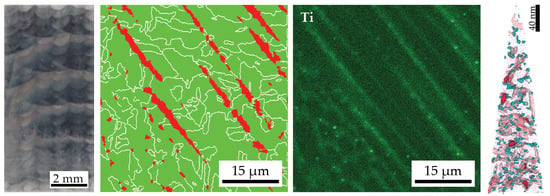
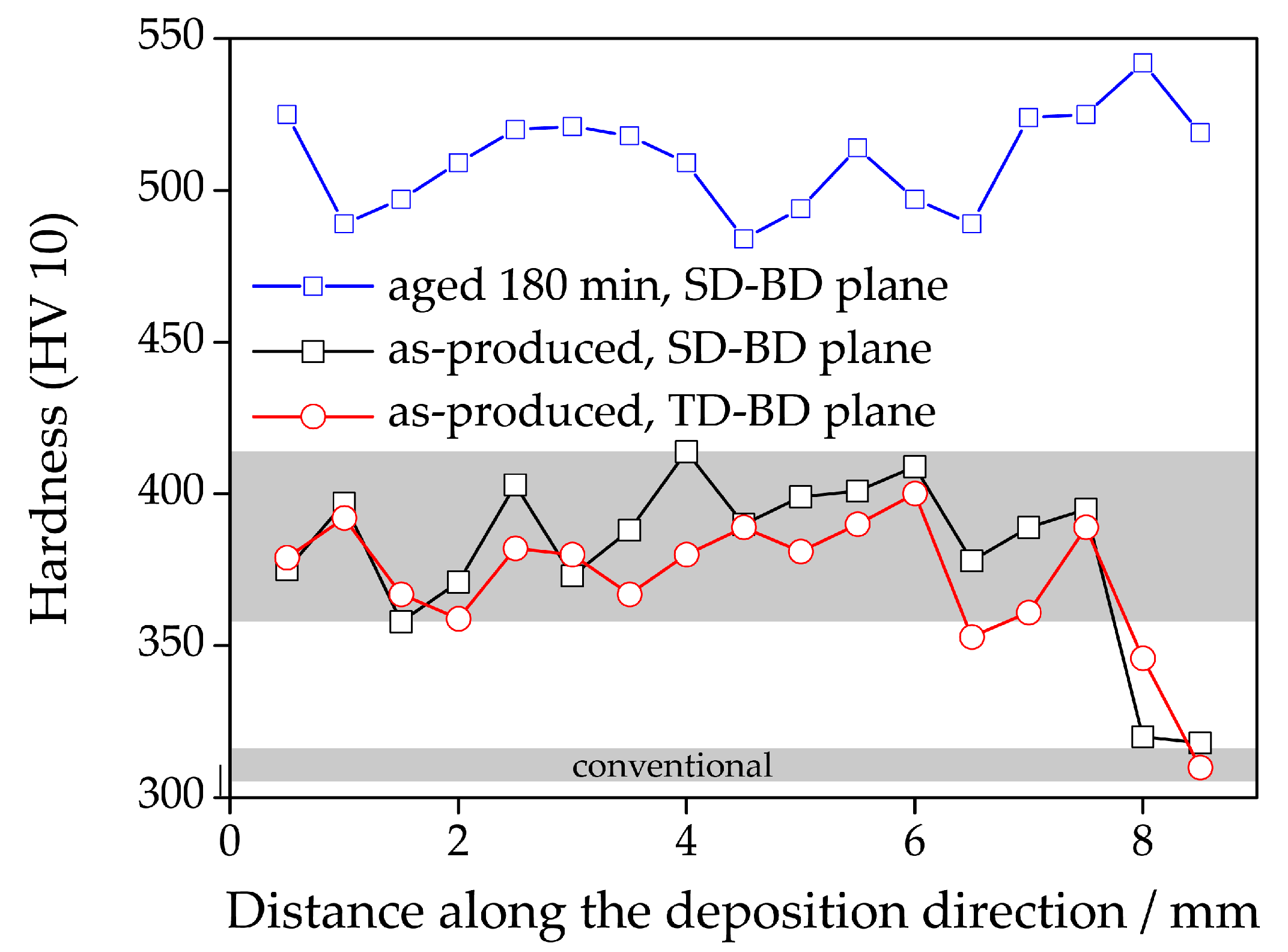
3.3. Hardness Drop in Topmost Layers of As-LMD-Produced Material
3.4. Microstructure after Short Term (5 min) Ageing
3.5. Microstructure in the Peak Aged (480 min) State
4. Discussion
- (i).
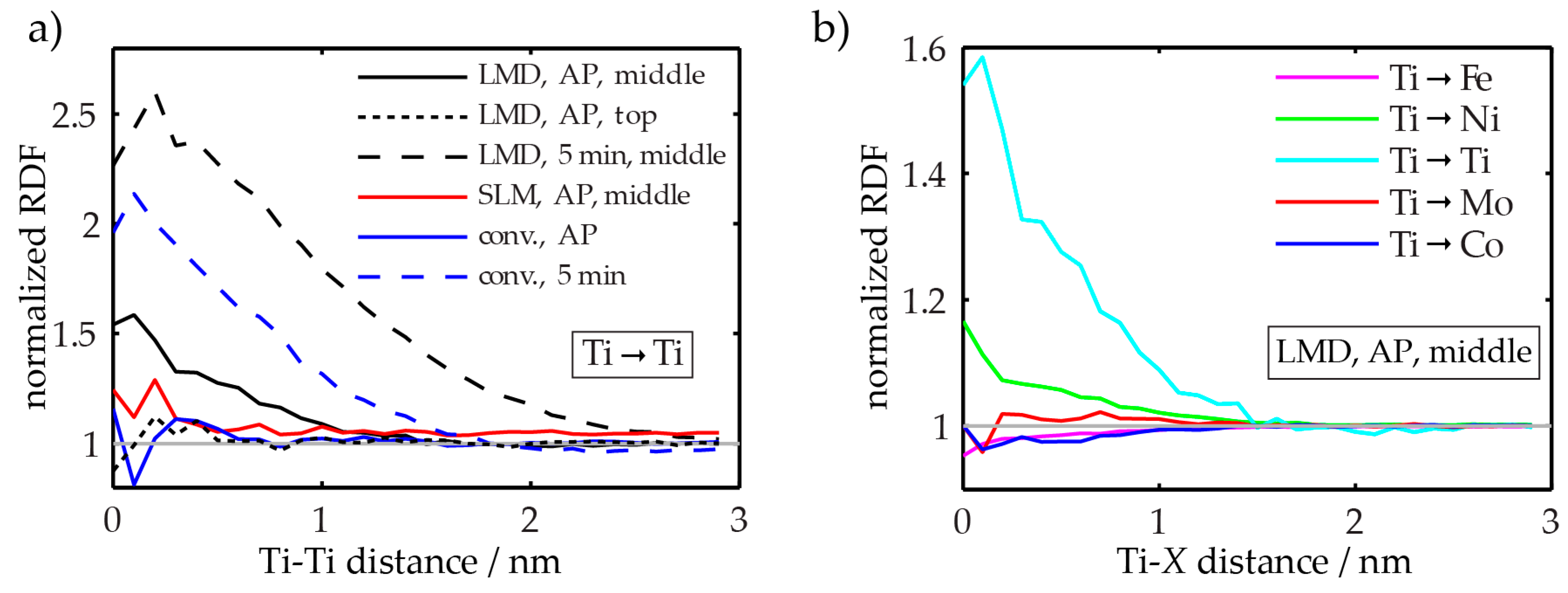
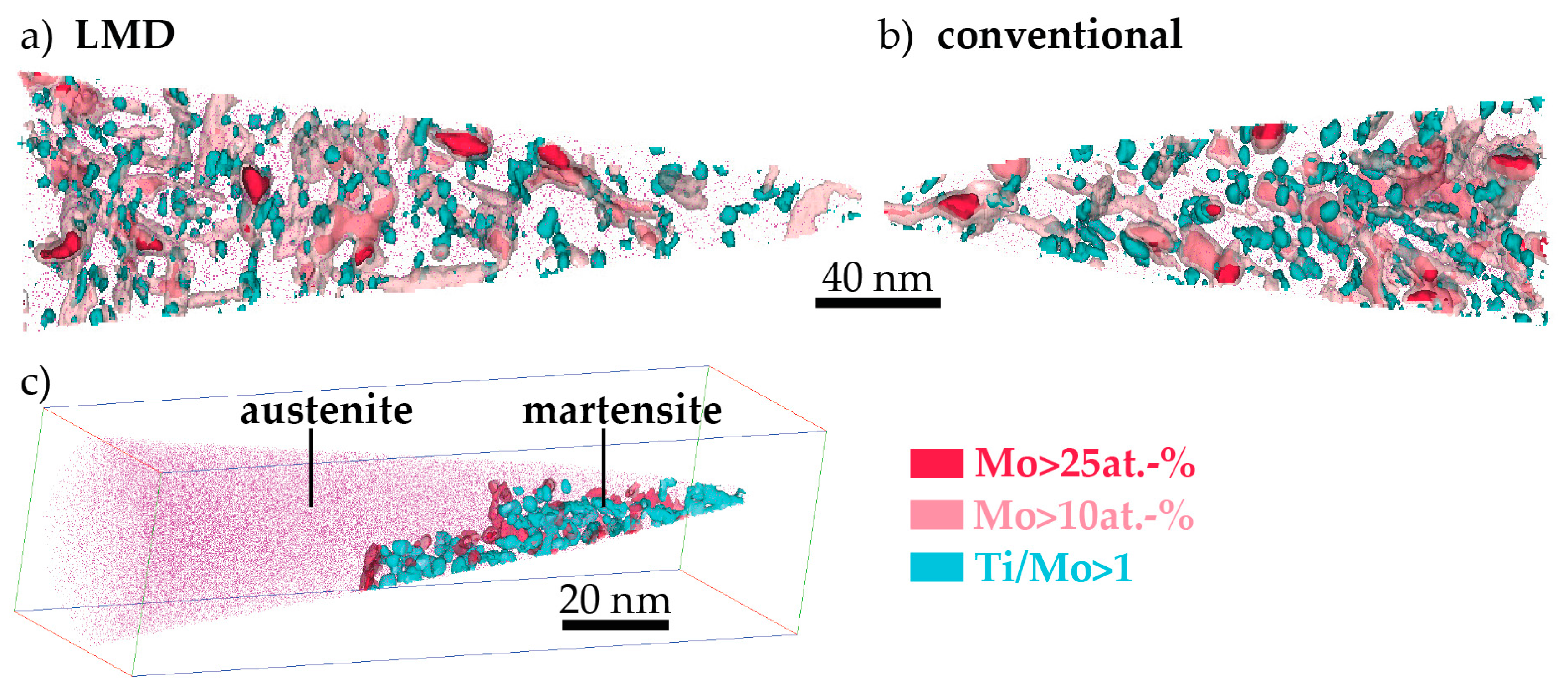
- The hardness of as-LMD-produced material is higher than both as-SLM-produced and conventional as-received material. The reason for this lies in the early stages of precipitation that are detected by APT in the (and only in the) LMD-P material. Apparently, already the very small clusters have a significant strengthening effect. In principle, the precipitation (clustering) could occur either during the cooling down just after deposition and solidification of material in the LMD-process or during the pulse-like re-heating (intrinsic heat treatment) upon deposition of adjacent tracks and overlying layers. Both of these effects differ between SLM and LMD. In SLM, the melt pool is smaller and the scanning speed is higher than in LMD (cf. Table 2), leading to both a slower cooling rate after deposition and a less pronounced reheating during the intrinsic heat treatment. However, the results of Figure 7 allow to separate the effects of cooling rate and intrinsic heat treatment. Since the hardness of LMD-P material is comparable to C-P and SLM-P material in the very top layers, clearly the clustering in the as-produced state is due to the intrinsic heat treatment (that does not apply or applies less strongly to the very top layers). If the cooling after deposition were the origin of the clustering, it could also be observed in the top layers (cf. also the discussion in reference [24]). The fact that the intrinsic heat treatment is strong enough to induce (early stages of) precipitation in the present maraging steel, a material that needs several hours to reach a peak aged stage, suggests that the intrinsic heat treatment might be exploited to design a maraging steel that is fully in-situ precipitation strengthened—i.e., that does not need an additional heat treatment after AM production. We are currently optimizing the LMD-process and designing a model maraging steel to achieve this goal. First results with a Fe-Ni-Al alloy show promising results, including a very high number density of precipitates in the as-LMD-P state (publication in preparation).
- (ii).
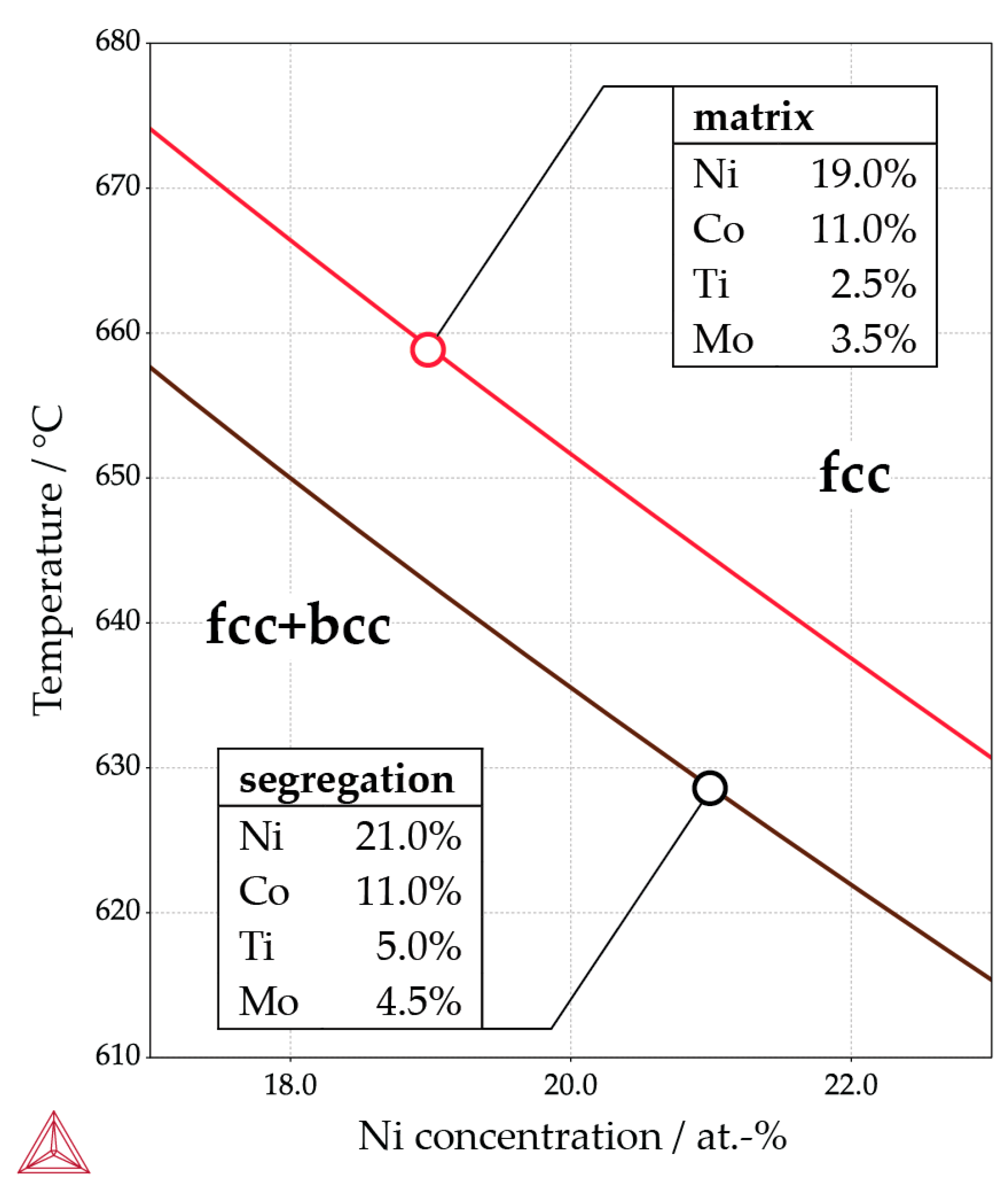
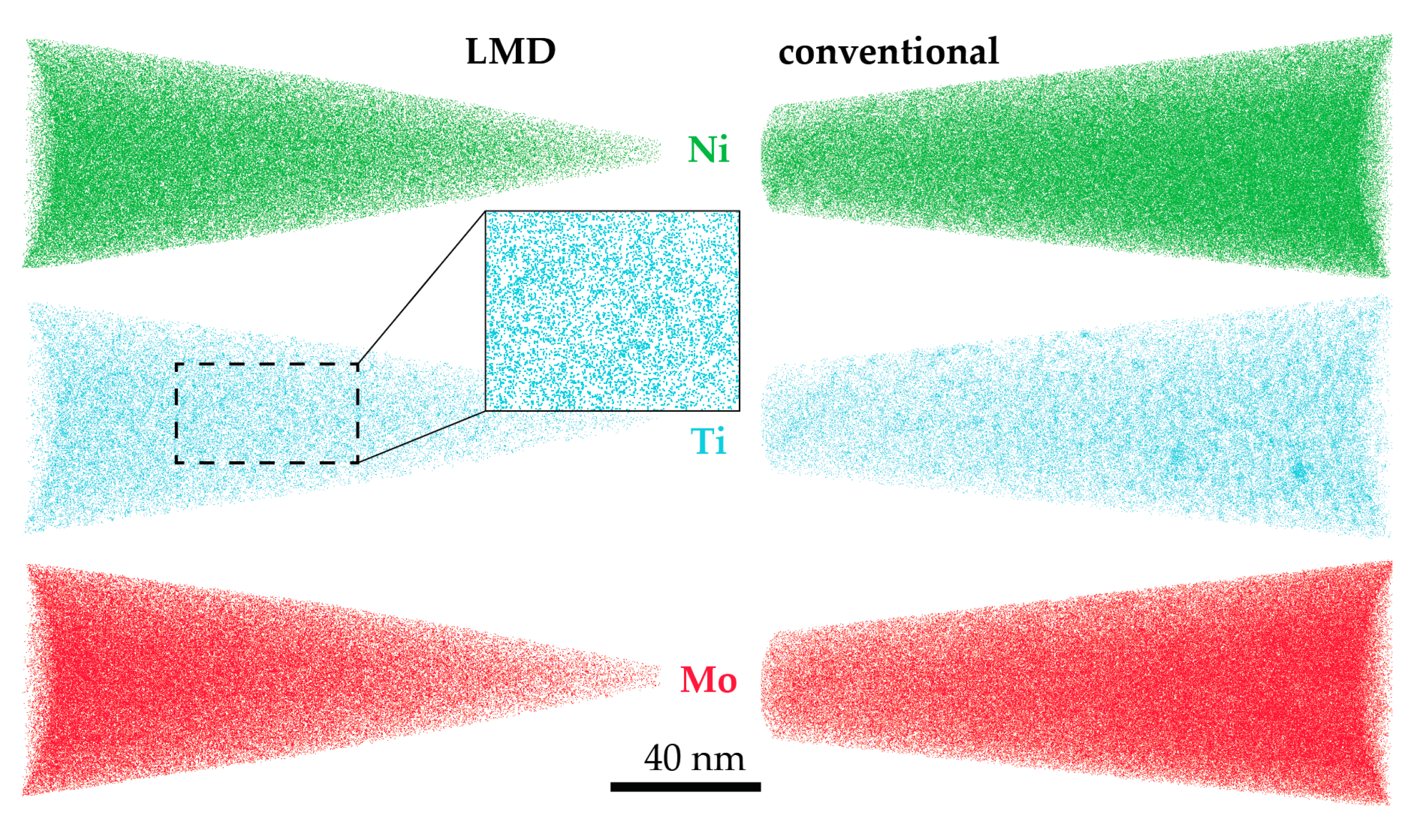
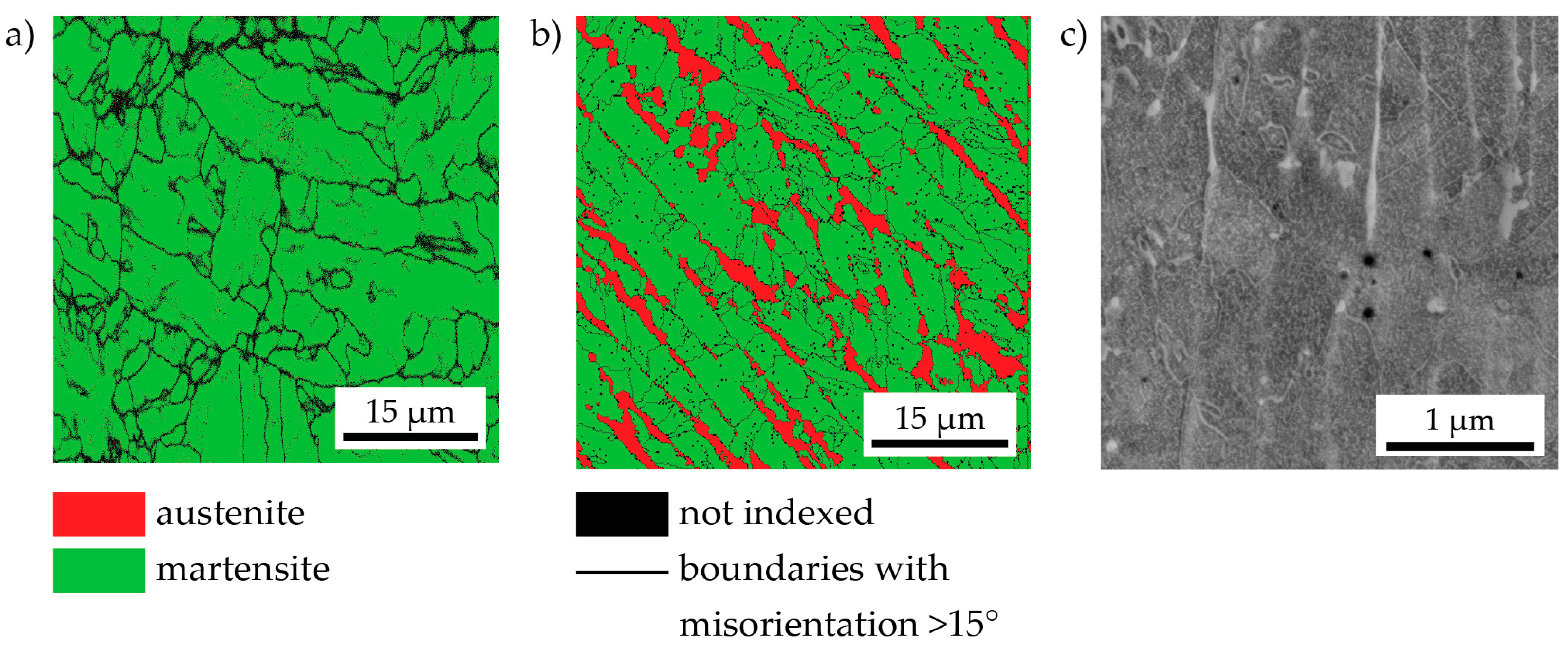
- The peak hardness is lower in both AM-produced materials compared to the C-P material. This is most likely due to the significant amount of comparatively soft retained (and reversed) austenite in the AM-produced materials (cf. Figure 3, Figure 4 and Figure 10). The austenite, in turn, is present because of the chemical inhomogeneity due to microsegregation during solidification. This microsegregation is present also in SLM-P material [13,20] that has been solidified at a higher rate than LMD-P material. Despite often being referred to as a process inducing rapid solidification, obviously neither LMD nor SLM enable effective trapping of solutes in the solidifying material in this particular alloy. Even though C-P material initially—i.e., after casting—surely also contained such inhomogeneity, these had been homogenized during the subsequent standard downstream thermomechanical processing such as hot rolling and annealing. Due to the near-net shape nature of AM processes this is not a viable option for AM-P material. Potentially, a prolonged solution annealing before ageing might remove the segregation and hence the retained austenite in AM-produced material, yet, it would also add an undesired additional processing step and additional cost to the AM production process. Note that the interdendritic spacing and thus also the width of the austenite regions is smaller in SLM-P material than in LMD-P material. Also the volume fraction of retained and reversed austenite—namely, 5.8% and 9.4% in the as-produced and peak aged states, respectively [13]—could be smaller, but this is not certain given the errors in the determination of the austenite content. The presence of reversed austenite in (over-)aged maraging steels is well documented (see e.g., [6,25,26]). Even though austenite lowers the strength of the material, it may be a desired microstructure constituent because it allows tailoring the ductility and toughness [27,28]. A slightly overaged condition may therefore be ideal for certain applications [29]. The exact influence of the fraction of retained and reversed austenite on the hardness of the materials is beyond the scope of this study due to the difficulty in separating the effects of grain size (prior austenite grain size, martensite block size, and morphology) and crystallographic texture from the austenite fraction.
- (iii).
- The kinetics of precipitation is not noticeably different between C-P and AM-P material. It could have been expected that the presence of a high density of lattice defects in the AM-P material, originating due to the high residual stress imposed in the AM processes, leads to a quicker nucleation and growth of precipitates in the AM-produced materials or to a different morphology. This is, however, not the case. After 480 min of ageing, all differences in the nanostructure that have been present in the as-produced state are of no significance any more, as evidenced by the very similar composition, distribution, and sizes of the observed three types of precipitates (cf. Figure 11 and Table 3). Another point to note is a certain inhomogeneity in the LMD-P material. This is visible in the slightly uneven distribution of austenite and in the high scatter of the hardness values compared to the C-P material. We performed sets of microhardness indents with decreased load across a melt pool but did not find systematic changes in hardness. Hence, the observed variation in the hardness values seems to be truly random. A homogenizing heat treatment might alleviate this effect, too.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sha, W.; Guo, Z. Maraging Steels: Modelling of Microstructure, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Kundig, K.J.A. Copper and copper alloys. In Handbook of Materials Selection; Myer, K., Ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 135–200. [Google Scholar]
- Vasudevan, V.; Kim, S.; Wayman, C. Precipitation reactions and strengthening behavior in 18 wt pct nickel maraging steels. Metall. Trans. A 1990, 21, 2655–2668. [Google Scholar] [CrossRef]
- Sha, W.; Cerezo, A.; Smith, G.D.W. Atom Probe Studies of Early Stages of Precipitation in Maraging Steels. Scr. Mater. 1992, 26, 517–522. [Google Scholar] [CrossRef]
- Tewari, R.; Mazumder, S.; Batra, I.S.; Dey, G.K.; Banerjee, S. Precipitation in 18 wt% Ni maraging steel of grade 350. Acta Mater. 2000, 48, 1187–1200. [Google Scholar] [CrossRef]
- Rao, M.N. Progress in understanding the metallurgy of 18% nickel maraging steels. Int. J. Mater. Res. 2006, 97, 1594–1607. [Google Scholar] [CrossRef]
- Lang, F.H.; Kenyon, N. Welding of Maraging Steels; Welding Research Council: Shaker Heights, OH, USA, 1971. [Google Scholar]
- Stanford, M.; Kibble, K.; Lindop, M.; Mynors, D.; Durnall, C. An investigation into fully melting a maraging steel using direct metal laser sintering (DMLS). Steel Res. Int. 2008, 2, 847–852. [Google Scholar]
- Cabeza, M.; Castro, G.; Merino, P.; Pena, G.; Román, M. Laser surface melting: A suitable technique to repair damaged surfaces made in 14 Ni (200 grade) maraging steel. Surf. Coat. Technol. 2012, 212, 159–168. [Google Scholar] [CrossRef]
- Grum, J.; Slabe, J.M. The State of Differently Heat-Treated 12% Ni Maraging Steel after Laser Remelting. Mater. Sci. Forum 2007, 537–538, 647–654. [Google Scholar] [CrossRef]
- Thijs, L.; Humbeeck, J. Van Investigation on the inclusions in maraging steel produced by Selective Laser Melting. In Innovative Developments in Virtual and Physical Prototyping; Bártolo, P.J., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 297–304. [Google Scholar]
- Yasa, E.; Kempen, K.; Kruth, J. Microstructure and mechanical properties of Maraging Steel 300 after selective laser melting. In Proceedings of the 21st International Solid Freeform Fabrication Symposium, Austin, TX, USA, 9–11 August 2010; pp. 383–396.
- Kempen, K.; Yasa, E.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Microstructure and mechanical properties of Selective Laser Melted 18Ni-300 steel. Phys. Procedia 2011, 12, 255–263. [Google Scholar] [CrossRef]
- Becker, T.; Dimitrov, D. The achievable mechanical properties of SLM produced Maraging Steel 300 components. Rapid Prototyp. J. 2016, 22, 487–494. [Google Scholar] [CrossRef]
- Casalino, G.; Campanelli, S.L.; Contuzzi, N.; Ludovico, A.D. Experimental investigation and statistical optimisation of the selective laser melting process of a maraging steel. Opt. Laser Technol. 2015, 65, 151–158. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.N.; Tuissi, A.; Vedani, M. Aging behavior and mechanical performance of 18-Ni 300 steel processed by selective laser melting. Metals 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- LBC Engineering “Leistungsspektrum Lasergenerieren”. Available online: http://www.lbc-engineering.de/de/lasergenerierung.php (accessed on 23 November 2016).
- Decker, R.F. Source Book on Maraging Steels; American Society for Metals: Metals Park, OH, USA, 1979. [Google Scholar]
- Decker, R.F.; Eash, J.T.; Goldman, A.J. 18% Nickel maraging steel. Trans. ASM 1962, 55, 58–76. [Google Scholar]
- Jägle, E.A.; Choi, P.; van Humbeeck, J.; Raabe, D. Precipitation and austenite reversion behavior of a maraging steel produced by selective laser melting. J. Mater. Res. 2014, 29, 2072–2079. [Google Scholar]
- Larson, D.; Prosa, T.; Kelly, T. Local Electrode Atom Probe Tomography—A User’s Guide; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Liu, C.; Zhao, Z.; Northwood, D.O.; Liu, Y. A new emperical formula for the calculation of ms temperatures in pure iron and super low carbon alloy steels. J. Mater. Process. Technol. 2001, 113, 556–562. [Google Scholar] [CrossRef]
- Springer, H.; Baron, C.; Szczepaniak, A.; Jägle, E.A.; Wilms, M.B.; Weisheit, A.; Raabe, D. Efficient additive manufacturing production of oxide- and nitride-dispersion-strengthened materials through atmospheric reactions in liquid metal deposition. Mater. Des. 2016, 111, 60–69. [Google Scholar] [CrossRef]
- Jägle, E.A.; Sheng, Z.; Wu, L.; Lu, L.; Risse, J.; Weisheit, A.; Raabe, D.; Wu, L.; Risse, J.; Weisheit, A.; et al. Precipitation Reactions in Age-Hardenable Alloys During Laser Additive Manufacturing. JOM 2016, 68, 943–949. [Google Scholar]
- Shekhter, A.; Aaronson, H.; Miller, M. Effect of aging and deformation on the microstructure and properties of Fe-Ni-Ti maraging steel. Metall. Mater. Trans. A 2004, 35, 973–983. [Google Scholar]
- Galindo-Nava, E.I.; Rainforth, W.M.; Rivera-Díaz-del-Castillo, P.E.J. Predicting microstructure and strength of maraging steels: Elemental optimisation. Acta Mater. 2016, 117, 270–285. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Dmitrieva, O.; Sander, B. Designing Ultrahigh Strength Steels with Good Ductility by Combining Transformation Induced Plasticity and Martensite Aging. Adv. Eng. Mater. 2009, 11, 547–555. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Dmitrieva, O.; Sander, B. Nanoprecipitate-hardened 1.5 GPa steels with unexpected high ductility. Scr. Mater. 2009, 60, 1141–1144. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Sethumadhavan, V. Effects of austenite reversion during overageing on the mechanical properties of 18 Ni (350) maraging steel. Mater. Sci. Eng. A 2005, 398, 367–372. [Google Scholar] [CrossRef]

| Alloying Element | C | Si | Mn | Mo | Ni | Al | Co | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt % | 0.0018 | 0.025 | 0.011 | 5.03 | 18.3 | 0.077 | 8.74 | 0.68 | Bal. |
| at % | 0.0087 | 0.052 | 0.012 | 3.03 | 17.95 | 0.165 | 8.57 | 0.82 | Bal. |
| LAM Process | Laser Power (W) | Scan Speed (mm/s) | Laser Focus Diameter (μm) | Layer Thickness (μm) | Hatch Spacing (μm) | Energy Density (J/mm3) | Inert Atmosphere |
|---|---|---|---|---|---|---|---|
| LMD | 800 | 10 | 1700 | 420 | 900 | 112.0 | Ar |
| SLM Data from [13] | 100 | 150 | 180 | 30 | 112 | 123.5 | N2 |
| Precipitate | Material | Fe | Ni | Co | Mo | Ti | Number Density |
|---|---|---|---|---|---|---|---|
| (at %) | (m−3) | ||||||
| (Fe,Ni,Co)3(Ti,Mo) | C-P | 10 | 60 | 6 | 4 | 20 | ~4 × 1023 |
| LMD-P | 15 | 61 | 2 | 3 | 20 | ~3 × 1023 | |
| SLM-P [20] | 12 | 60 | 4 | 5 | 18 | – | |
| (Fe,Ni,Co)3(Mo,Ti) | C-P | 21 | 50 | 5 | 19 | 5 | – |
| LMD-P | 22 | 52 | 2 | 22 | 2 | – | |
| SLM-P [20] | 22 | 52 | 5 | 16 | 5 | – | |
| (Fe,Ni,Co)7Mo6 | C-P | 40 | 17 | 4 | 39 | 0 | ~8 × 1022 |
| LMD-P | 38 | 20 | 2 | 40 | 0 | ~5 × 1022 | |
| SLM-P [20] | 37 | 17 | 4 | 38 | 0 | – | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jägle, E.A.; Sheng, Z.; Kürnsteiner, P.; Ocylok, S.; Weisheit, A.; Raabe, D. Comparison of Maraging Steel Micro- and Nanostructure Produced Conventionally and by Laser Additive Manufacturing. Materials 2017, 10, 8. https://doi.org/10.3390/ma10010008
Jägle EA, Sheng Z, Kürnsteiner P, Ocylok S, Weisheit A, Raabe D. Comparison of Maraging Steel Micro- and Nanostructure Produced Conventionally and by Laser Additive Manufacturing. Materials. 2017; 10(1):8. https://doi.org/10.3390/ma10010008
Chicago/Turabian StyleJägle, Eric A., Zhendong Sheng, Philipp Kürnsteiner, Sörn Ocylok, Andreas Weisheit, and Dierk Raabe. 2017. "Comparison of Maraging Steel Micro- and Nanostructure Produced Conventionally and by Laser Additive Manufacturing" Materials 10, no. 1: 8. https://doi.org/10.3390/ma10010008