Facile Synthesis of ZnO Nanoparticles on Nitrogen-Doped Carbon Nanotubes as High-Performance Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Preparation of ZnO/NCNT and ZnO/CNT
2.3. Material Characterizations
2.4. Electrochemical Measurements
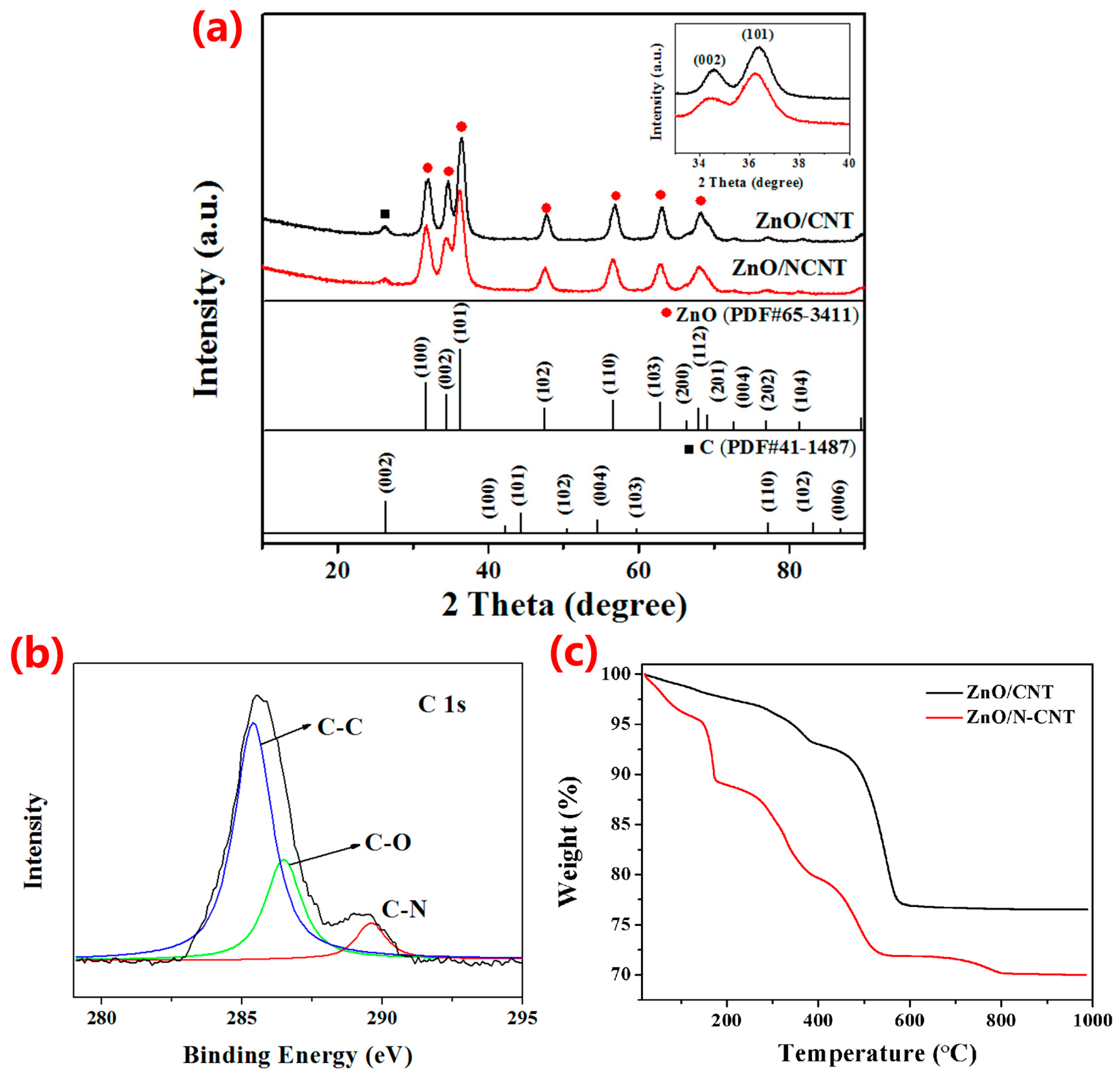
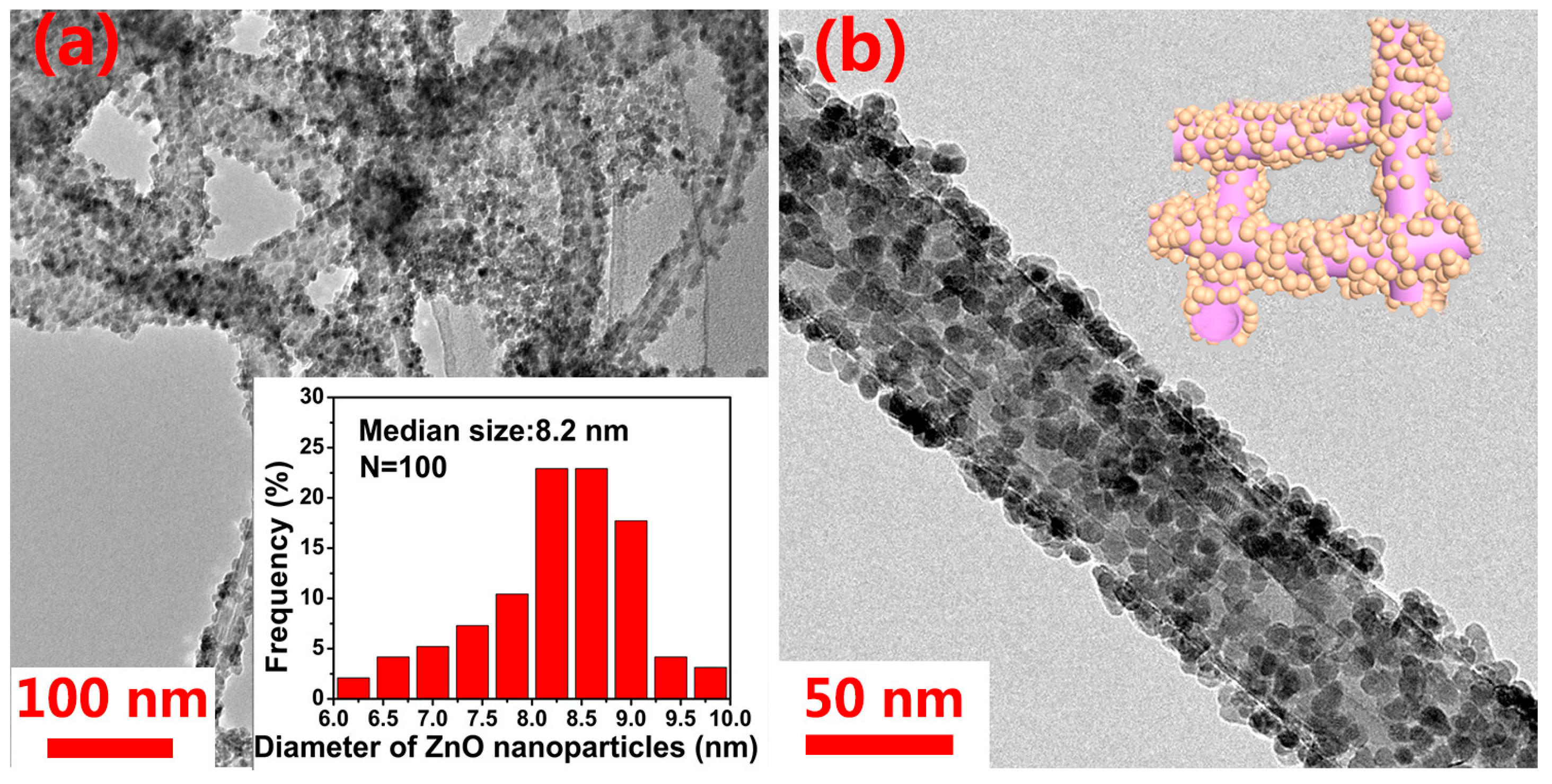
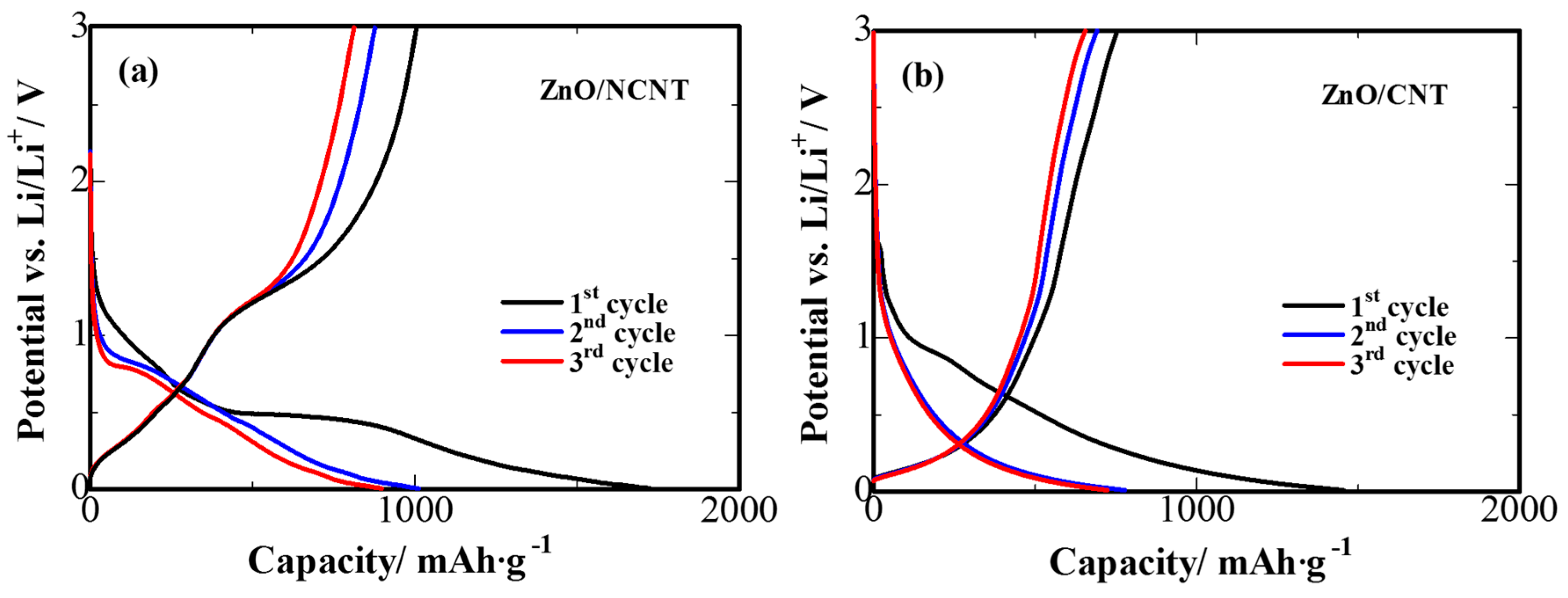
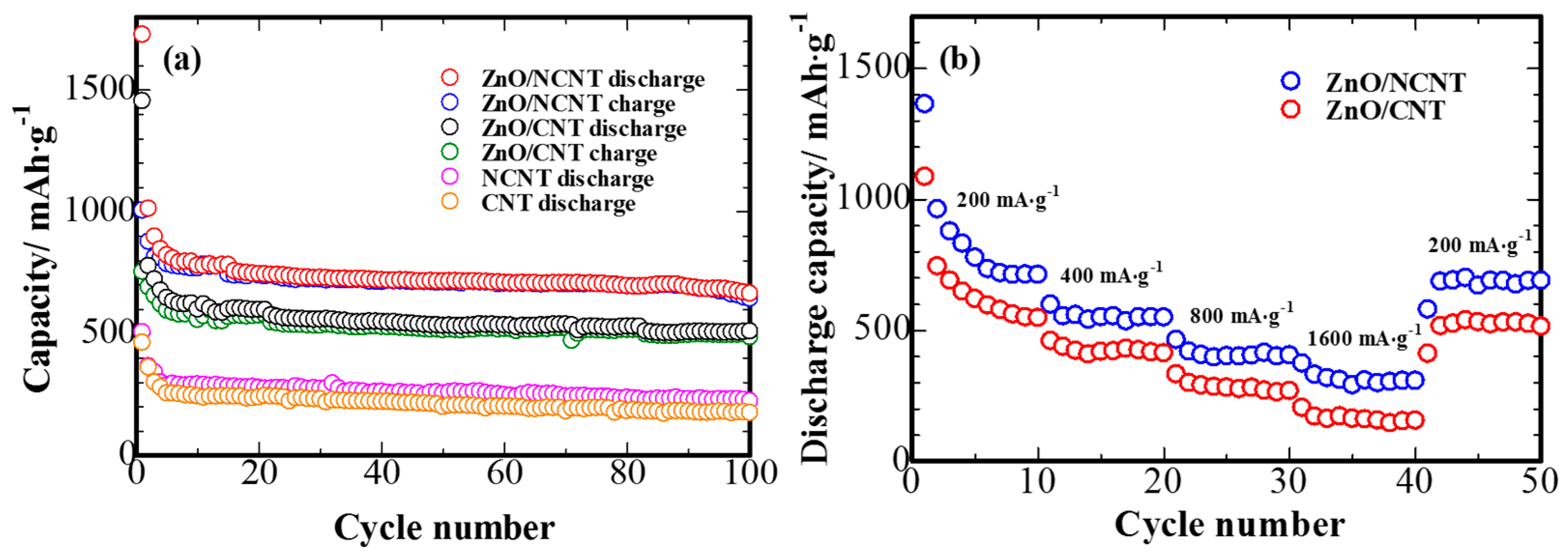
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Babaa, M.R.; Konarov, A.; Ding, C.; Chen, P. Effect of graphene on sulfur/polyacrylonitrile nanocomposite cathode in high performance lithium/sulfur batteries. J. Electrochem. Soc. 2013, 160, A1194–A1198. [Google Scholar] [CrossRef]
- Kundu, M.; Karunakaran, G.; Kumari, S.; Van Minh, N.; Kolesnikov, E.; Gorshenkov, M.V.; Kuznetsov, D. One-pot ultrasonic spray pyrolysis mediated hollow Mg0.25Cu0.25Zn0.5Fe2O4/NiFe2O4 nanocomposites: A promising anode material for high-performance lithium-ion battery. J. Alloys Compd. 2017, 725, 665–672. [Google Scholar] [CrossRef]
- Kundu, M.; Karunakaran, G.; Kolesnikov, E.; Dmitry, A.; Gorshenkov, M.V.; Kuznetsov, D. Hollow (Co0.62Fe1.38)FeO4/NiCo2O4 nanoboxes with porous shell synthesized via chemical precipitation: A novel form as a high performance lithium ion battery anode. Microporous Mesoporous Mater. 2017, 247, 9–15. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, Y.; Zhang, C. Facile synthesis of carbon-coated Fe3O4 core—Shell nanoparticles as anode materials for lithium-ion batteries. J. Nanopart. Res. 2015, 17, 370. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.M.; Ali, S.; Ahmad, N.; Ali, N.; Abbas, S. Structure and electrochemical performance of ZnO/CNT composite as anode material for lithium-ion batteries. J. Mater. Sci. 2013, 48, 5429–5436. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Li, W.J.; Fu, Z.W. Electrochemical reactivity of nanocomposite ZnO-Se for lithium-ion batteries. Electrochim. Acta 2012, 59, 435–440. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Tu, J.P.; Huang, X.H.; Yuan, Y.F.; Chen, X.T.; Mao, F. Electrochemical performances of Ni-coated ZnO as an anode material for lithium-ion batteries. J. Solid State Chem. 2007, 154, A65–A69. [Google Scholar] [CrossRef]
- Huang, X.H.; Xia, X.H.; Yuan, Y.F.; Zhou, F. Porous ZnO nanosheets grown on copper substrates as anodes for lithium ion batteries. Electrochim. Acta 2011, 56, 4960–4965. [Google Scholar] [CrossRef]
- Wang, J.; Du, N.; Zhang, H.; Yu, J.; Yang, D. Layer-by-layer assembly synthesis of ZnO/SnO2 composite nanowire arrays as high-performance anode for lithium-ion batteries. Mater. Res. Bull. 2011, 46, 2378–2384. [Google Scholar] [CrossRef]
- Laurenti, M.; Garino, N.; Porro, S.; Fontana, M.; Gerbaldi, C. Zinc oxide nanostructures by chemical vapour deposition as anodes for Li-ion batteries. J. Alloys Compd. 2015, 640, 321–326. [Google Scholar] [CrossRef]
- Arora, P.; White, R.E.; Doyle, M. Capacity fade mechanisms and side reactions in lithium-ion batteries. J. Electrochem. Soc. 1998, 145, 3647–3667. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ding, R.; Jiang, J.; Hu, Y.; Ji, X.; Chi, Q.; Zhu, Z.; Huang, X. Carbon/ZnO nanorod array electrode with significantly improved lithium storage capability. J. Phys. Chem. C 2009, 113, 5336–5339. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Liu, J.; Li, Z.; Wu, G.; Wu, M. Facile synthesis of ZnO/mesoporous carbon nanocomposites as high-performance anode for lithium-ion battery. Chem. Eng. J. 2015, 271, 173–179. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, C.Y.; Chen, Y.F.; Lin, J.S. Synthesis of ZnO@Graphene composites as anode materials for lithium ion batteries. Electrochim. Acta 2013, 111, 359–365. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Z.; Chen, C.; Wang, J.; Fu, X.; Fan, C.; Qian, G. Preparation of carbon-encapsulated ZnO tetrahedron as an anode material for ultralong cycle life performance lithium-ion batteries. Electrochim. Acta 2014, 146, 52–59. [Google Scholar] [CrossRef]
- Guler, M.O.; Cetinkaya, T.; Tocoglu, U.; Akbulut, H. Electrochemical performance of MWCNT reinforced ZnO anodes for Li-ion batteries. Microelectron. Eng. 2014, 118, 54–60. [Google Scholar] [CrossRef]
- Sui, J.; Zhang, C.; Hong, D.; Li, J.; Cheng, Q.; Li, Z.; Cai, W. Facile synthesis of MWCNT-ZnFe2O4 nanocomposites as anode materials for lithium ion batteries. J. Mater. Chem. 2012, 22, 13674–13681. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Dong, A.; Yang, D. Novel Fe3O4-CNTs nanocomposite for Li-ion batteries with enhanced electrochemical performance. Electrochim. Acta 2014, 144, 235–242. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, K.; Kim, K.; Kim, S. Pore-structure-optimized cnt-carbon nanofibers from starch for rechargeable lithium batteries. Materials 2016, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, H.; Wu, J.; Chu, X.; He, Y.B.; Han, C.; Miao, C.; Wang, S.; Li, B.; Kang, F. Fe3O4 nanoparticles encapsulated in electrospun porous carbon fibers with a compact shell as high-performance anode for lithium ion batteries. Carbon 2015, 87, 347–356. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Désilets, S.; Abou-Rachid, H.; Jaidann, M.; Lussier, L.S. Structural and morphological control of aligned nitrogen-doped carbon nanotubes. Carbon 2010, 48, 1498–1507. [Google Scholar] [CrossRef]
- Mi, R.; Liu, H.; Wang, H.; Wong, K.W.; Mei, J.; Chen, Y.; Lau, W.M.; Yan, H. Effects of nitrogen-doped carbon nanotubes on the discharge performance of Li-air batteries. Carbon 2014, 67, 744–752. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, F.; Zhang, Y.; Zhang, C.; Mentbayeva, A.; Umirov, N.; Xie, H.; Bakenov, Z. A free-standing sulfur/nitrogen-doped carbon nanotube electrode for high-performance lithium/sulfur batteries. Nanoscale Res. Lett. 2015, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bakenova, Z.; Zhang, Y.; Peng, H.; Xie, H.; Bakenov, Z. High performance sulfur/nitrogen-doped graphene cathode for lithium/sulfur batteries. Ionics 2015, 21, 1925–1930. [Google Scholar] [CrossRef]
- Tao, H.C.; Yang, X.L.; Zhang, L.L.; Ni, S.B. One-step synthesis of nickel sulfide/N-doped graphene composite as anode materials for lithium ion batteries. J. Electroanal. Chem. 2015, 739, 36–42. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Zhang, C.; Wang, G.; Zhao, Y.; Yin, F.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhao, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Bakenov, Z. Simple one-pot synthesis of hexagonal ZnO nanoplates as anode material for lithium-ion batteries. J. Nanomater. 2016, 2016, 4675960. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, S. Photodegradation of methyl orange by photocatalyst of CNTs/P-TiO(2) under uv and visible-light irradiation. J. Hazard. Mater. 2011, 185, 77. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Guo, S.; Lu, G.; Fu, Y.; Liu, J.; Wei, H.; Yan, X.; Wang, Y.; Guo, Z. Carbon coating and Zn2+ doping of magnetite nanorods for enhanced electrochemical energy storage. Electrochim. Acta 2014, 148, 118–126. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, X.; Yu, H.; Chen, Y.; Gao, P.; Li, C.; Zhu, C. Growth of ultrathin MoS2 nanosheets with expanded spacing of (002) plane on carbon nanotubes for high-performance sodium-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 21880. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wang, Q.; Shi, Z.; Ma, C.; Ding, Y.; Huo, N.; Zhang, J.; Yang, S. Porous hierarchical nitrogen-doped carbon coated ZnFe2O4 composites as high performance anode materials for lithium ion batteries. Electrochim. Acta 2015, 180, 622–628. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, B.; Yang, J.Q.; Huang, X.X.; Wen, G. Boron and nitrogen doped carbon nanotubes/Fe3O4 composite architectures with microwave absorption property. Ceram. Int. 2015, 41, 8163–8170. [Google Scholar] [CrossRef]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Cai, D.; Li, D.; Wang, S.; Zhu, X.; Yang, W.; Zhang, S.; Wang, H. High rate capability of TiO2/nitrogen-doped graphene nanocomposite as an anode material for lithium-ion batteries. J. Alloys Compd. 2013, 561, 54–58. [Google Scholar] [CrossRef]
- Dai, J.; Wang, M.; Song, M.; Li, P.; Zhang, C.; Xie, A.; Shen, Y. A novel synthesis of ZnO/N-doped reduced graphene oxide composite as superior anode material for lithium-ion batteries. Scr. Mater. 2016, 112, 67–70. [Google Scholar] [CrossRef]
- Yan, J.; Wang, G.; Wang, H.; Zhang, Z.; Ruan, X.; Zhao, W.; Yun, J.; Xu, M. Preparation and electrochemical performance of bramble-like ZnO array as anode materials for lithium-ion batteries. J. Nanopart. Res. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y.; Fan, N.; Guo, C.; Tang, B. One-step synthesis of ZnO@C nanospheres and their enhanced performance for lithium-ion batteries. Mater. Lett. 2014, 119, 16–19. [Google Scholar] [CrossRef]
- Cai, D.; Wang, S.; Lian, P.; Zhu, X.; Li, D.; Yang, W.; Wang, H. Superhigh capacity and rate capability of high-level nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Electrochim. Acta 2013, 90, 492–497. [Google Scholar] [CrossRef]
- Du, M.; Xu, C.; Sun, J.; Gao, L. One step synthesis of Fe2O3/nitrogen-doped graphene composite as anode materials for lithium ion batteries. Electrochim. Acta 2012, 80, 302–307. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, Z.; Yang, S.; Zhao, Y.; Feng, Y.; Bakenov, Z.; Zhang, C.; Yin, F. Facile Synthesis of ZnO Nanoparticles on Nitrogen-Doped Carbon Nanotubes as High-Performance Anode Material for Lithium-Ion Batteries. Materials 2017, 10, 1102. https://doi.org/10.3390/ma10101102
Li H, Liu Z, Yang S, Zhao Y, Feng Y, Bakenov Z, Zhang C, Yin F. Facile Synthesis of ZnO Nanoparticles on Nitrogen-Doped Carbon Nanotubes as High-Performance Anode Material for Lithium-Ion Batteries. Materials. 2017; 10(10):1102. https://doi.org/10.3390/ma10101102
Chicago/Turabian StyleLi, Haipeng, Zhengjun Liu, Shuang Yang, Yan Zhao, Yuting Feng, Zhumabay Bakenov, Chengwei Zhang, and Fuxing Yin. 2017. "Facile Synthesis of ZnO Nanoparticles on Nitrogen-Doped Carbon Nanotubes as High-Performance Anode Material for Lithium-Ion Batteries" Materials 10, no. 10: 1102. https://doi.org/10.3390/ma10101102




