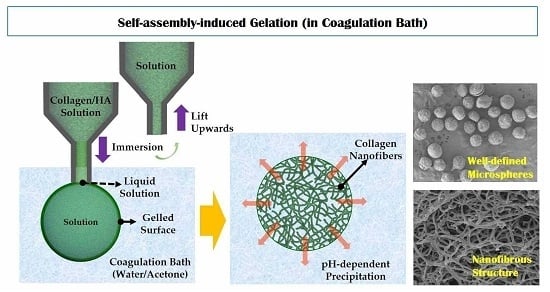
Novel Self-Assembly-Induced Gelation for Nanofibrous Collagen/Hydroxyapatite Composite Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Collagen/HA Composite Solutions Preparation
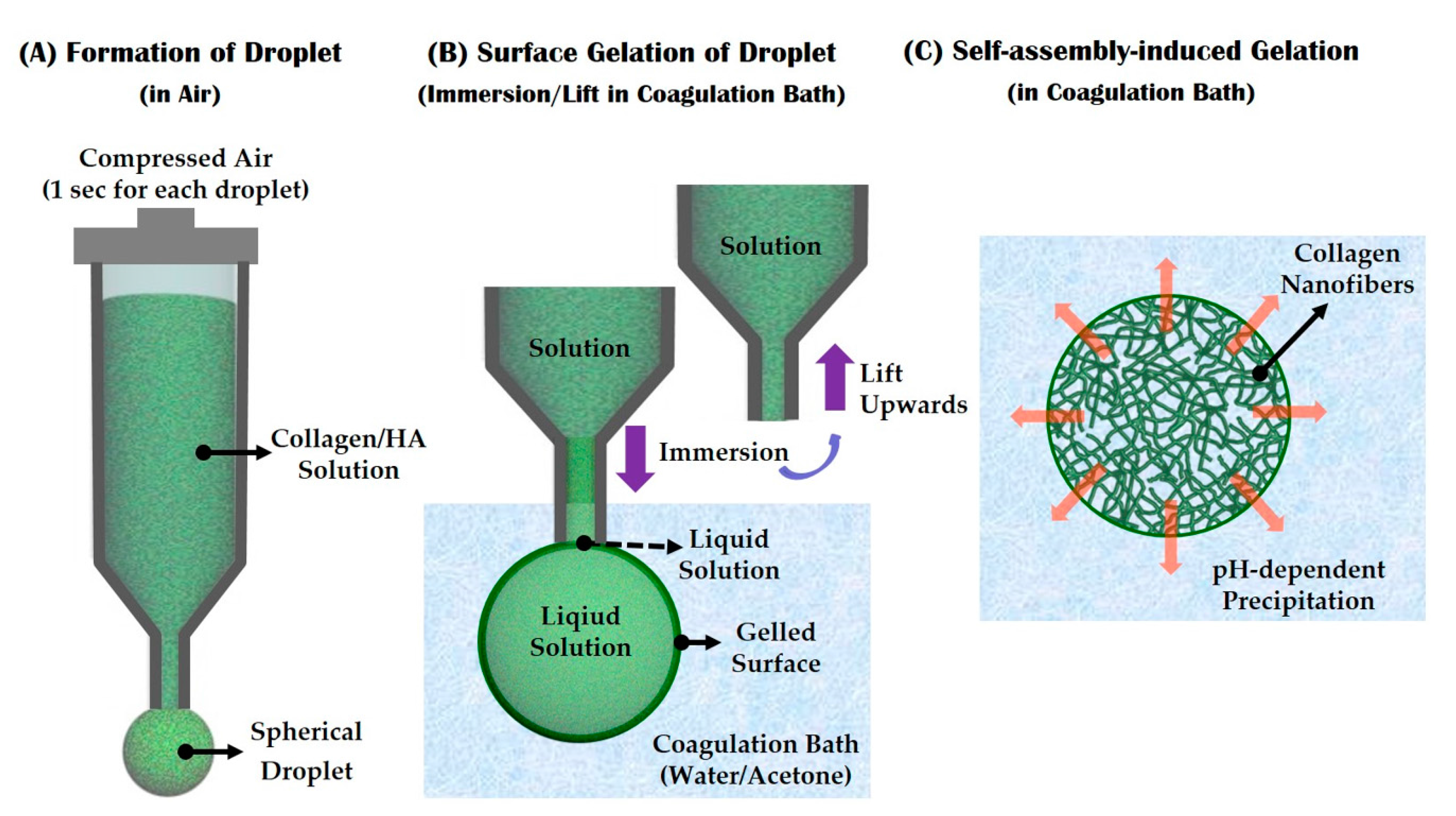
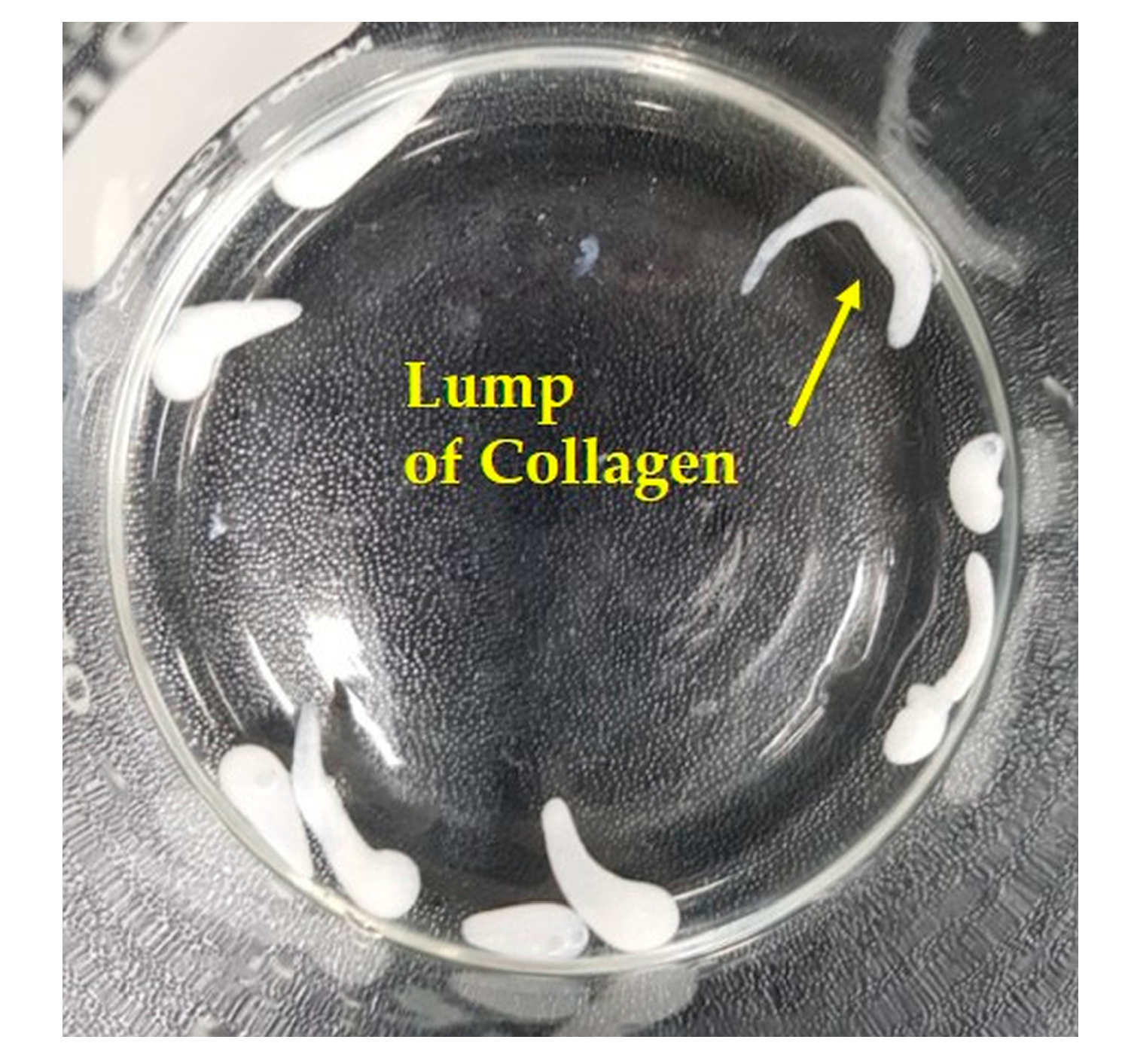
2.3. Self-Assembly-Induced Gelation
2.4. Cross-Linking and Freeze Drying
2.5. Morphology, Microstructure, and Nanostructure Characterization
2.6. Chemical Composition and Crystalline Phase Analyses
2.7. TGA Analysis
2.8. In Vitro Apatite-Forming Ability Evaluation
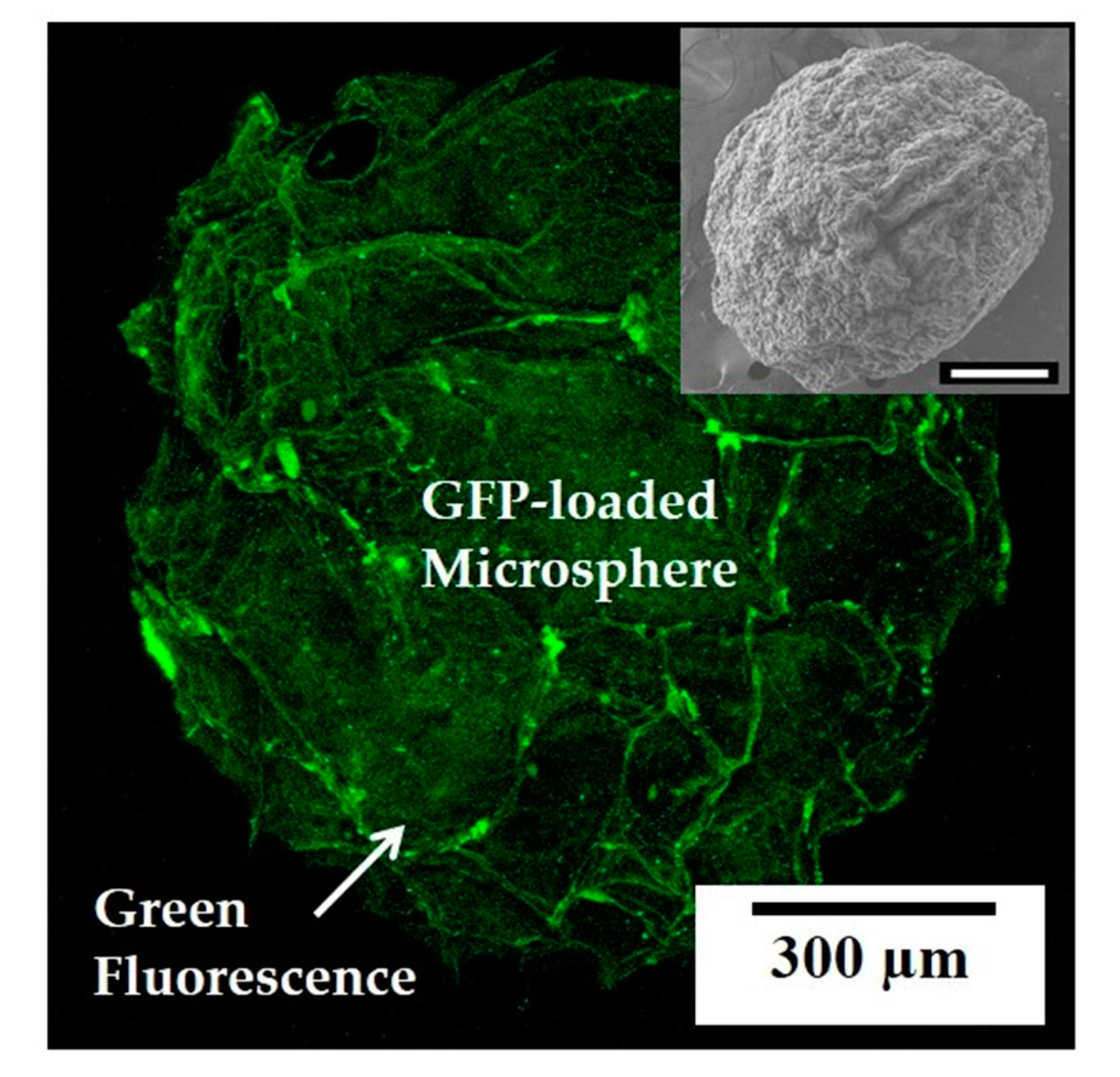
2.9. Bioactive Molecule-Loaded Microsphere Synthesis and Evaluation
3. Results and Discussion
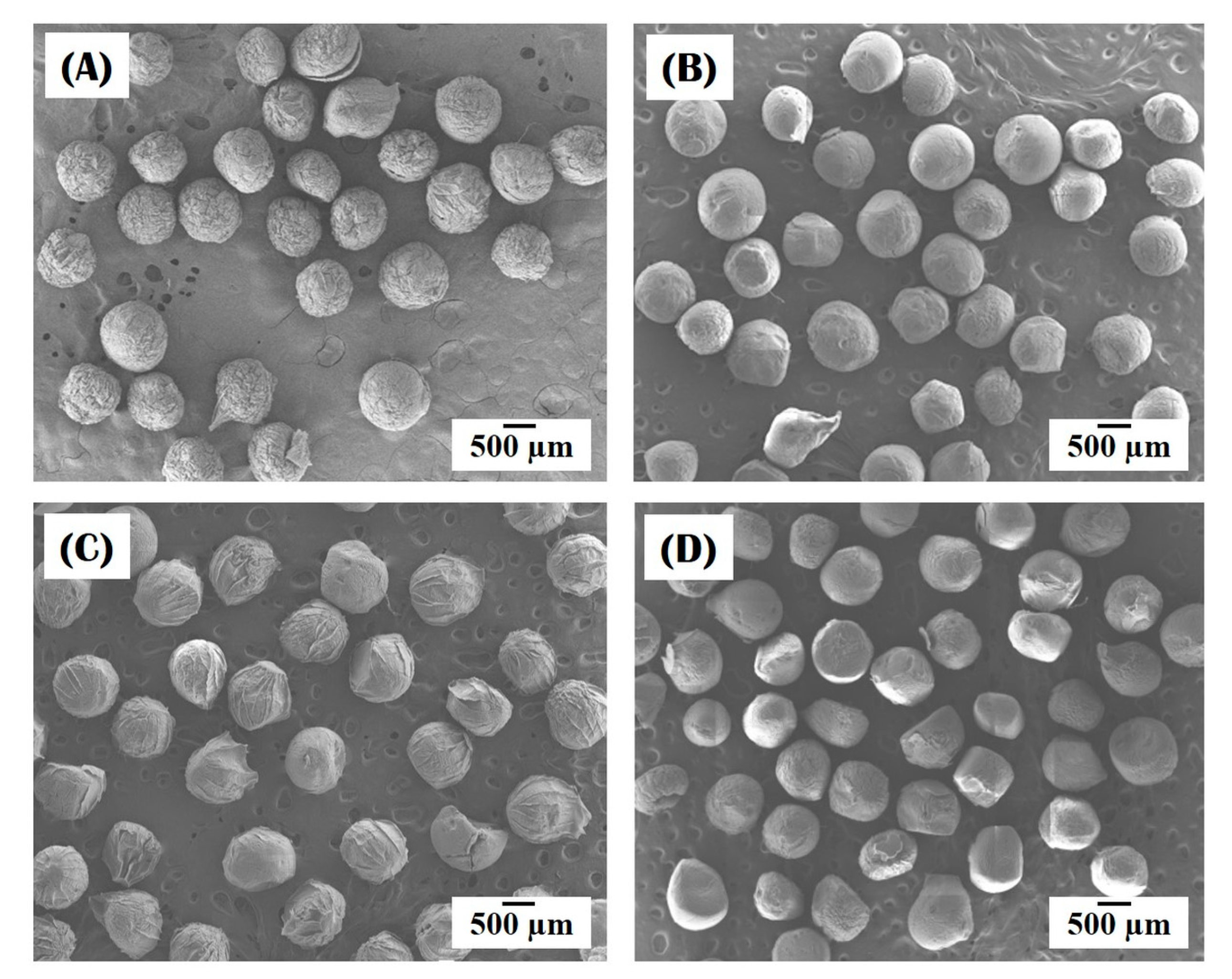
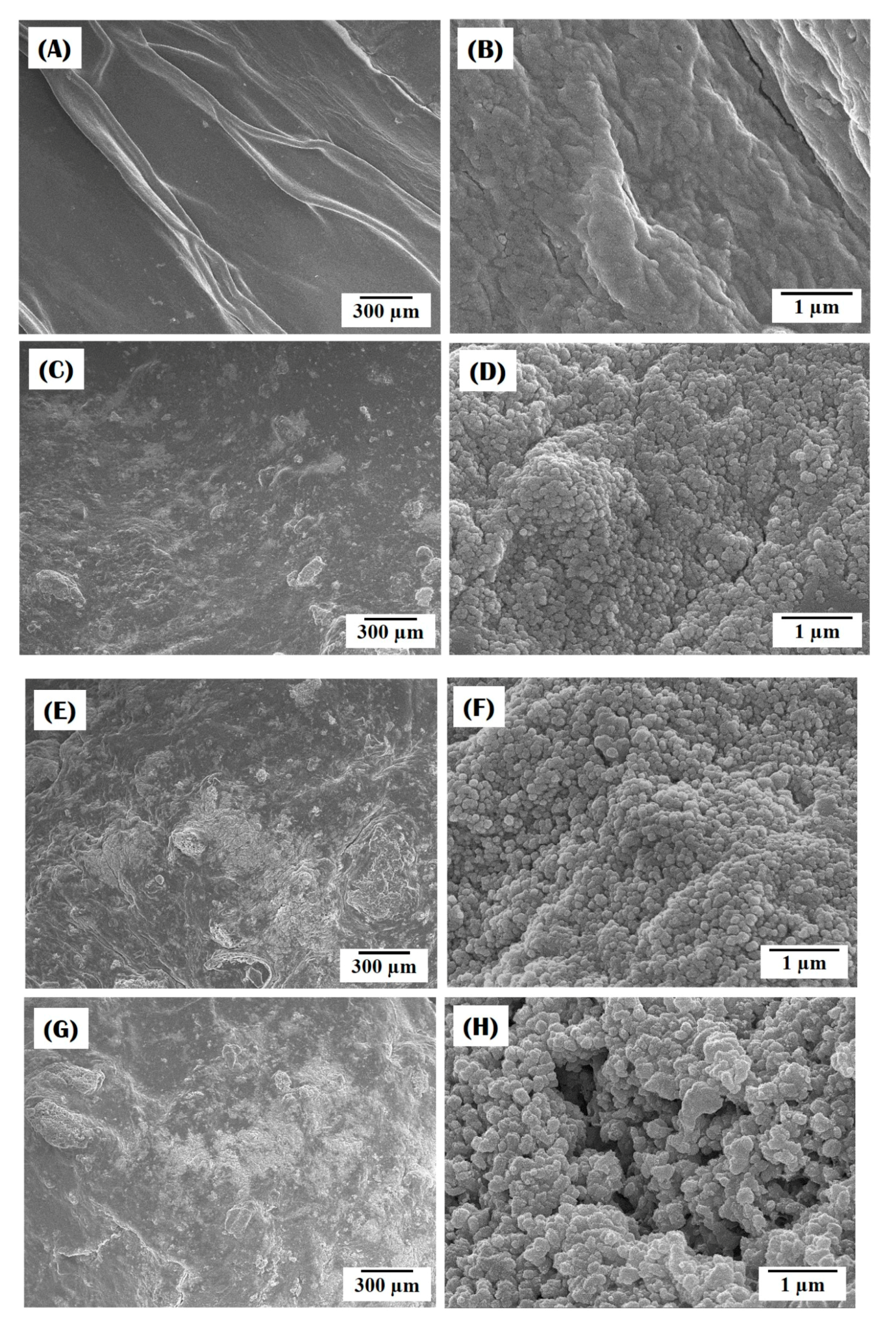
3.1. Morphology of Collagen/HA Composite Microspheres
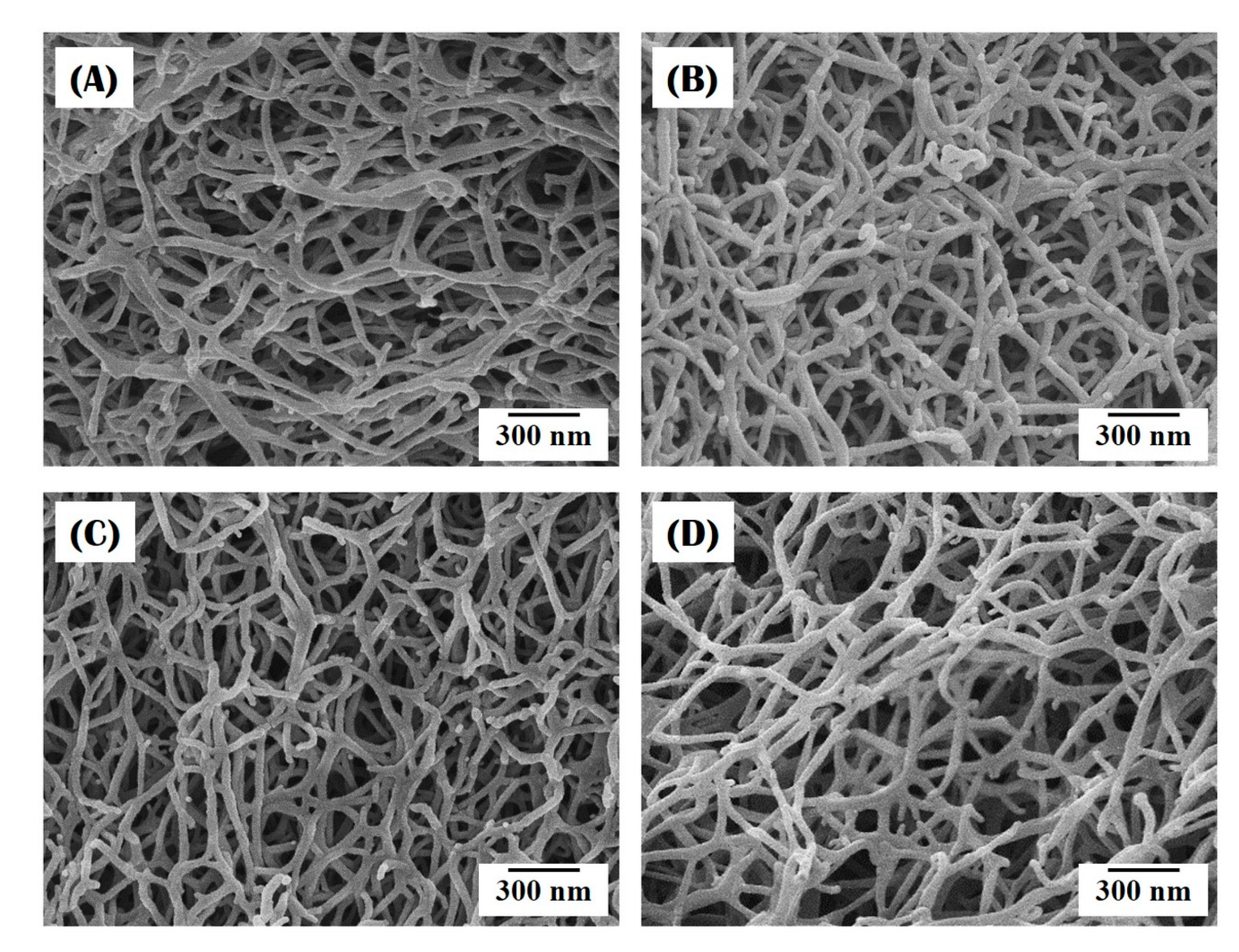
3.2. Nanofibrous Structure of Collagen/HA Composite Microspheres
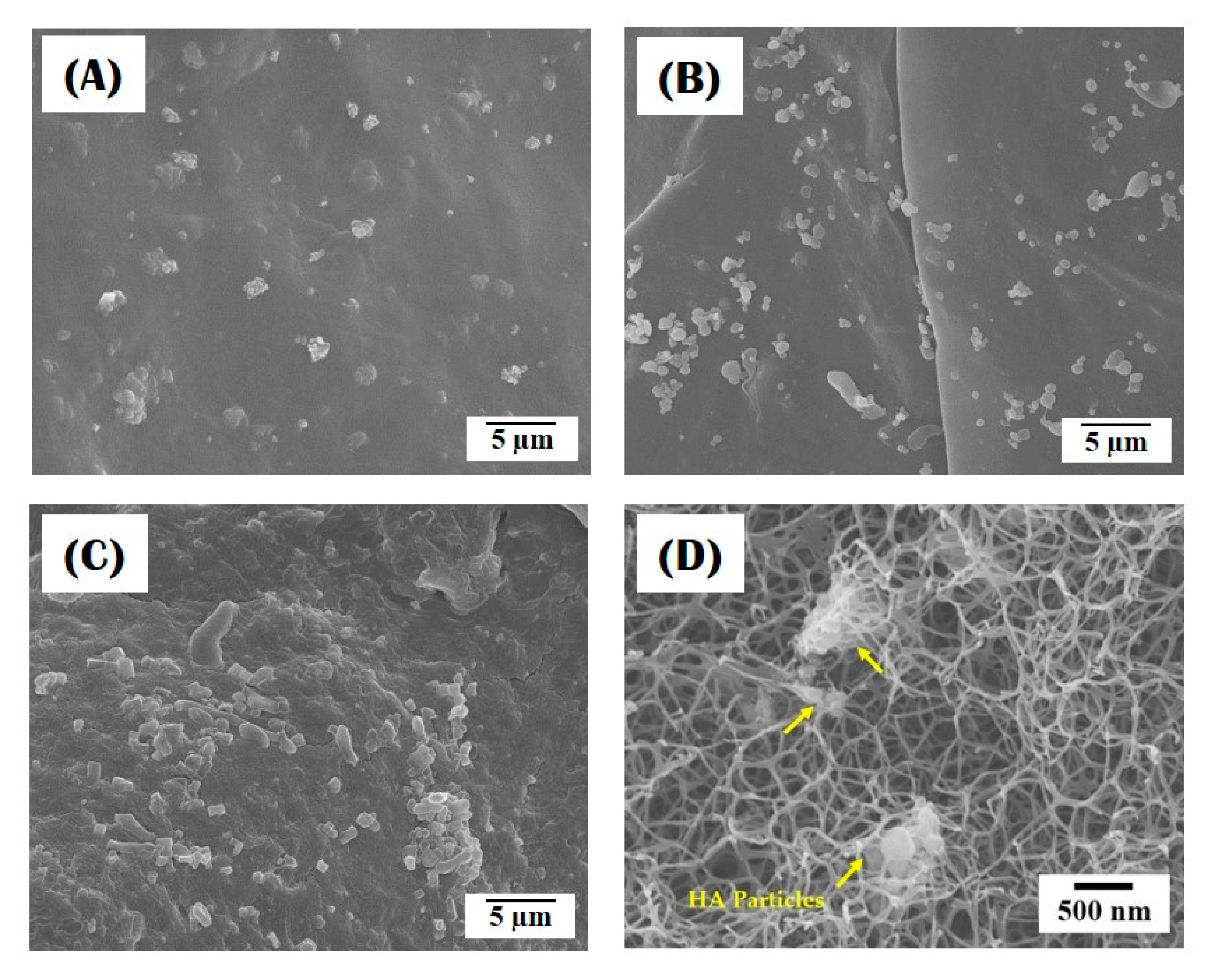
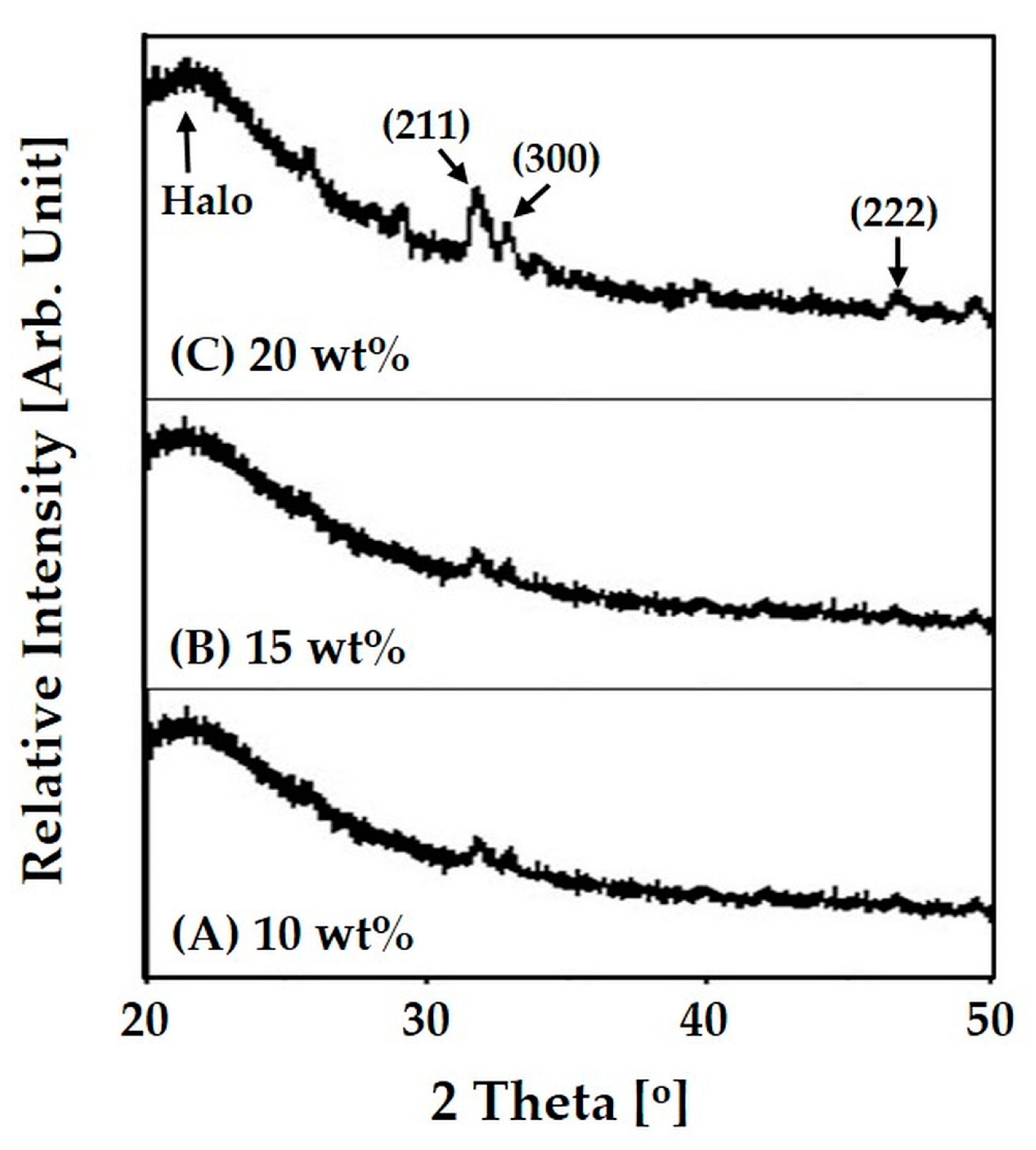
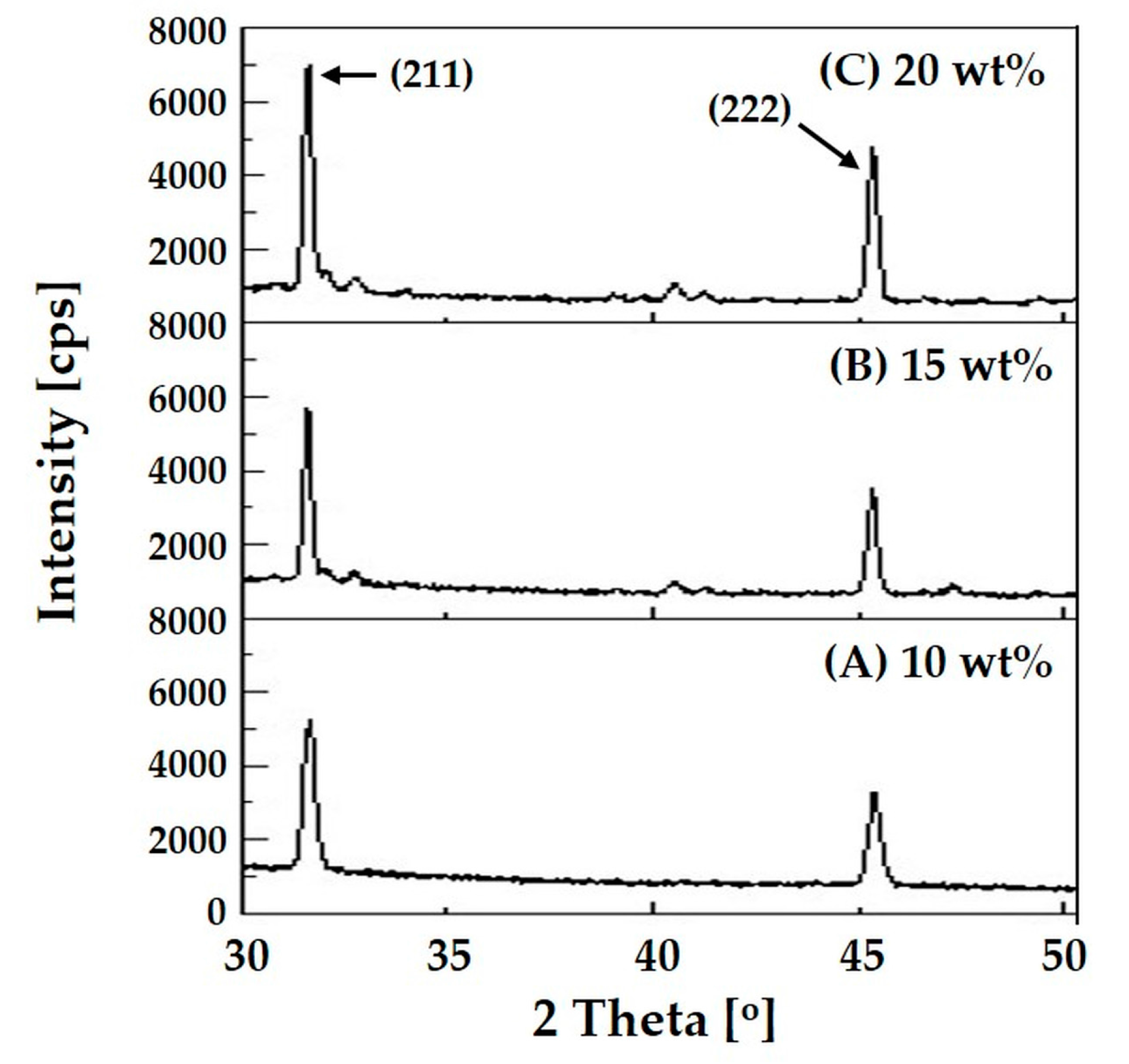
3.3. Incorporation of HA Particles into Composite Microspheres
3.4. In Vitro Apatite-Forming Ability of Composite Microspheres
3.5. Utility of Self-Assembly-Induced Gelation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Cui, F.Z.; Zhang, W.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 2000, 50, 518–527. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Chen, X.; Zhao, N. Biomimetic formation of hydroxyapatite/collagen matrix composite. Adv. Eng. Mater. 2006, 8, 97–100. [Google Scholar] [CrossRef]
- Honda, Y.; Kamakura, S.; Sasaki, K.; Suzuki, O. Formation of bone-like apatite enhanced by hydrolysis of octacalcium phosphate crystals deposited in collagen matrix. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80B, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, G.; Castano-Izquierdo, H.; Zhu, Y.; Mao, C. Biomineralization of natural collagenous nanofibrous membranes and their potential use in bone tissue engineering. J. Biomed. Nanotechnol. 2015, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, K.H.; Shin, S.Y.; Rhyu, I.C.; Lee, Y.M.; Park, Y.J.; Chung, C.P.; Lee, S.J. Enhanced bone formation by transforming growth factor-beta1-releasing collagen/chitosan microgranules. J. Biomed. Mater. Res. A 2006, 76, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.C.M.; So, K.-F.; Chan, B.P. Fabrication of nano-fibrous collagen microspheres for protein delivery and effects of photochemical crosslinking on release kinetics. J. Control. Release 2008, 129, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kumasaka, N.; Kawashima, T.; Kaji, H.; Nishizawa, M.; Abe, T. Preparation and characterization of collagen microspheres for sustained release of VEGF. J. Mater. Sci. Mater. Med. 2010, 21, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Leeuwenburgh, S.C.G.; Li, Y.; Jansen, J.A. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng. Part B Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Rössler, B.; Kreuter, J.; Scherer, D. Collagen microparticles: Preparation and properties. J. Microencapsul. 1995, 12, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.Y.; Chueh, S.C.; Wang, Y.J. Microspheres of hydroxyapatite/reconstituted collagen as supports for osteoblast cell growth. Biomaterials 1999, 20, 1931–1936. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Müller, W.; Kreuter, J. Microparticles derived from marine sponge collagen (SCMPs): Preparation, characterization and suitability for dermal delivery of all-trans retinol. Eur. J. Pharm. Biopharm. 2002, 54, 125–133. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Hong, X.; Liu, Z.; Yuan, W. Porous microsphere and its applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar]
- Matsuhashi, A.; Nam, K.; Kimura, T.; Kishida, A. Fabrication of fibrillized collagen microspheres with the microstructure resembling an extracellular matrix. Soft Matter 2015, 11, 2844–2851. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Knowles, J.C.; Day, R.M. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 2008, 4, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Thapar, N.; Burns, A.J.; Mordan, N.; Knowles, J.C.; Forbes, A.; Day, R.M. Microporous collagen spheres produced via thermally induced phase separation for tissue regeneration. Acta Biomater. 2010, 6, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.Y.; An, Y.H.; Jo, I.H.; Koh, Y.M.; Kim, H.E. Synthesis of nanofibrous gelatin/silica bioglass composite microspheres using emulsion coupled with thermally induced phase separation. Mater. Sci. Eng. C 2016, 62, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.B.; Huang, C.; Jiang, L.; Wang, S. Nanoporous microspheres: From controllable synthesis to healthcare applications. J. Mater. Chem. B 2013, 1, 2222–2235. [Google Scholar] [CrossRef]
- Meyer, M.; Baltzer, H.; Schwikal, K. Collagen fibres by thermoplastic and wet spinning. Mater. Sci. Eng. C 2010, 30, 1266–1271. [Google Scholar] [CrossRef]
- Caves, J.M.; Kumar, V.A.; Wen, J.; Cui, W.; Martinez, A.; Apkarian, R.; Coats, J.E.; Berland, K.; Chaikof, E.L. Fibrillogenesis in continuously spun synthetic collagen fiber. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Achilli, M.; Mantovani, D. Tailoring mechanical properties of collagen-based scaffolds for vascular tissue engineering: The effects of pH, temperature and ionic strength on gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef]
- Siriwardane, M.L.; DeRosa, K.; Collins, G.; Pfister, B.J. Controlled formation of cross-linked collagen fibers for neural tissue engineering applications. Biofabrication 2014, 6, 015012. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; Kim, J.W.; Koh, Y.H.; Kim, H.E. Novel self-assembly-induced 3D plotting for macro/nano-porous collagen scaffolds comprised of nanofibrous collagen filaments. Mater. Lett. 2015, 143, 265–268. [Google Scholar] [CrossRef]
- Rodrigues, C.V.M.; Serricella, P.; Linhares, A.B.R.; Guerdes, R.M.; Borojevic, R.; Rossi, M.A.; Duarte, M.E.L.; Farina, M. Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials 2003, 24, 4987–4997. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates as bioceramics: State of the art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Jung, H.D.; Jang, T.S.; Kim, S.; Kang, M.H.; Kim, H.E.; Koh, Y.H. Synthesis and evaluation of bone morphogenetic protein (BMP)-loaded hydroxyapatite microspheres for enhanced bone regeneration. Ceram. Int. 2016, 42, 7748–7756. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYang, H. Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules 2014, 15, 1185–1193. [Google Scholar] [CrossRef] [PubMed]


| Ion | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− |
|---|---|---|---|---|---|---|---|---|
| Concentrations (mM) | 142.0 | 5.0 | 1.5 | 2.5 | 148.8 | 4.2 | 1.0 | 0 |
| HA Content [wt %] | 0 | 10 | 15 | 20 |
|---|---|---|---|---|
| Diameter [µm] | 802 ± 40 | 798 ± 42 | 806 ± 38 | 809 ± 34 |
| Initial HA Content [wt %] | 10 | 15 | 20 |
| Final HA Content [wt %] | 10.4 | 13.3 | 17.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Kim, J.-W.; Jo, I.-H.; Koh, Y.-H.; Kim, H.-E. Novel Self-Assembly-Induced Gelation for Nanofibrous Collagen/Hydroxyapatite Composite Microspheres. Materials 2017, 10, 1110. https://doi.org/10.3390/ma10101110
Choi J-W, Kim J-W, Jo I-H, Koh Y-H, Kim H-E. Novel Self-Assembly-Induced Gelation for Nanofibrous Collagen/Hydroxyapatite Composite Microspheres. Materials. 2017; 10(10):1110. https://doi.org/10.3390/ma10101110
Chicago/Turabian StyleChoi, Jae-Won, Jong-Woo Kim, In-Hwan Jo, Young-Hag Koh, and Hyoun-Ee Kim. 2017. "Novel Self-Assembly-Induced Gelation for Nanofibrous Collagen/Hydroxyapatite Composite Microspheres" Materials 10, no. 10: 1110. https://doi.org/10.3390/ma10101110