New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
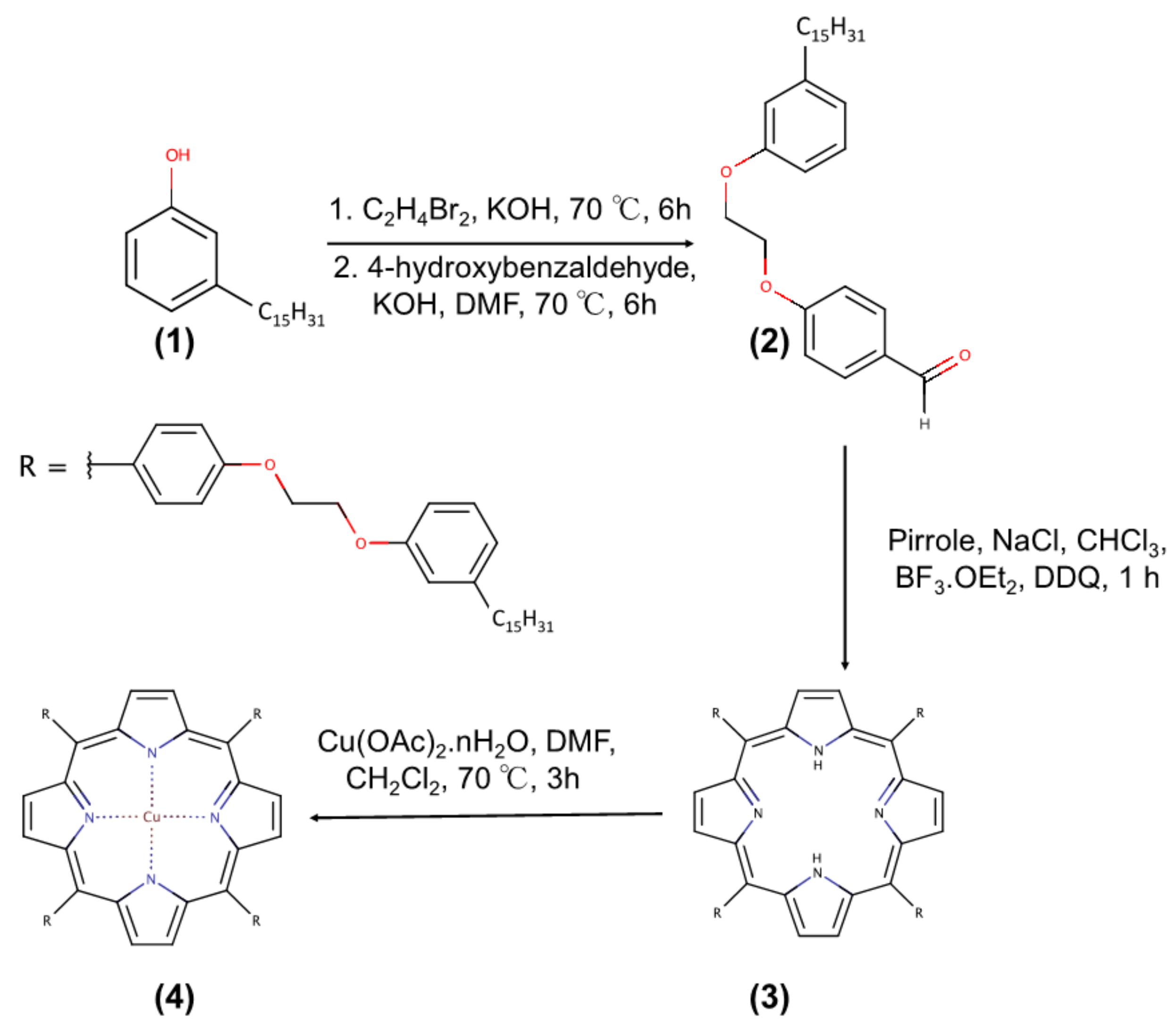
2.2. Synthesis of the Porphyrins H2Pp (3) and CuPp (4) from Cardanol
H2Pp: Yield 27%. Anal. Calcd. for C136H182N4O8: C, 81.64%; H, 9.17%; N, 2.80%. Found: C, 81.14%; H, 9.76%; N, 2.99%. MALDI-TOF MS m/z: 2000 [M]+; Molecular weight: 2001 amu; 1H-NMR (CDCl3, 300 MHz): δ, ppm: −2.70 (s, 2H, NH), 0.98 (t, 12H, CH3), 4.59 (dd, 16H, O–(CH2)2–O), 6.87–8.15 (m, 24H, o-Ph, m-Ph and p-Ph), 8.89 (s, 8H, β-Pyr); FT-IR (KBr), cm−1: 966 (δ N–H), 1286 (ν C–N), 2848 and 2918 (ν C–H Aliphatic), 3034 and 3068 (ν C–H Aromatic), 3325 (ν N–H); UV-Vis, CHCl3 (λmax, nm): 421; 519; 556; 594; 651.
CuPp: Yield 93%. Anal. Calcd. for C136H180N4O8Cu: C, 79.20; H, 8.80; N, 2.72. Found: C, 79.28; H, 9.75; N, 2.16%. MALDI-TOF MS m/z: 2062 [M]+; Molecular weight: 2062 amu; 1H-NMR (CDCl3, 300 MHz): δ, ppm: 0.88 (brs, 12H, CH3), 4.46 (brs, 16H, O–(CH2)2–O), 6.85 (brs, o-Ph, m-Ph and p-Ph); FT-IR (KBr), cm−1: 998 (N–M), 1287 (ν C–N), 2849 and 2917 (ν C–H Aliphatic), 3029 and 3067 (ν C–H Aromatic); UV-Vis, CHCl3 (λmax, nm): 419; 541; 578.
2.3. Preparation of the H2Pp-ZnO and CuPp–ZnO Photocatalysts
2.4. Sample Characterizations
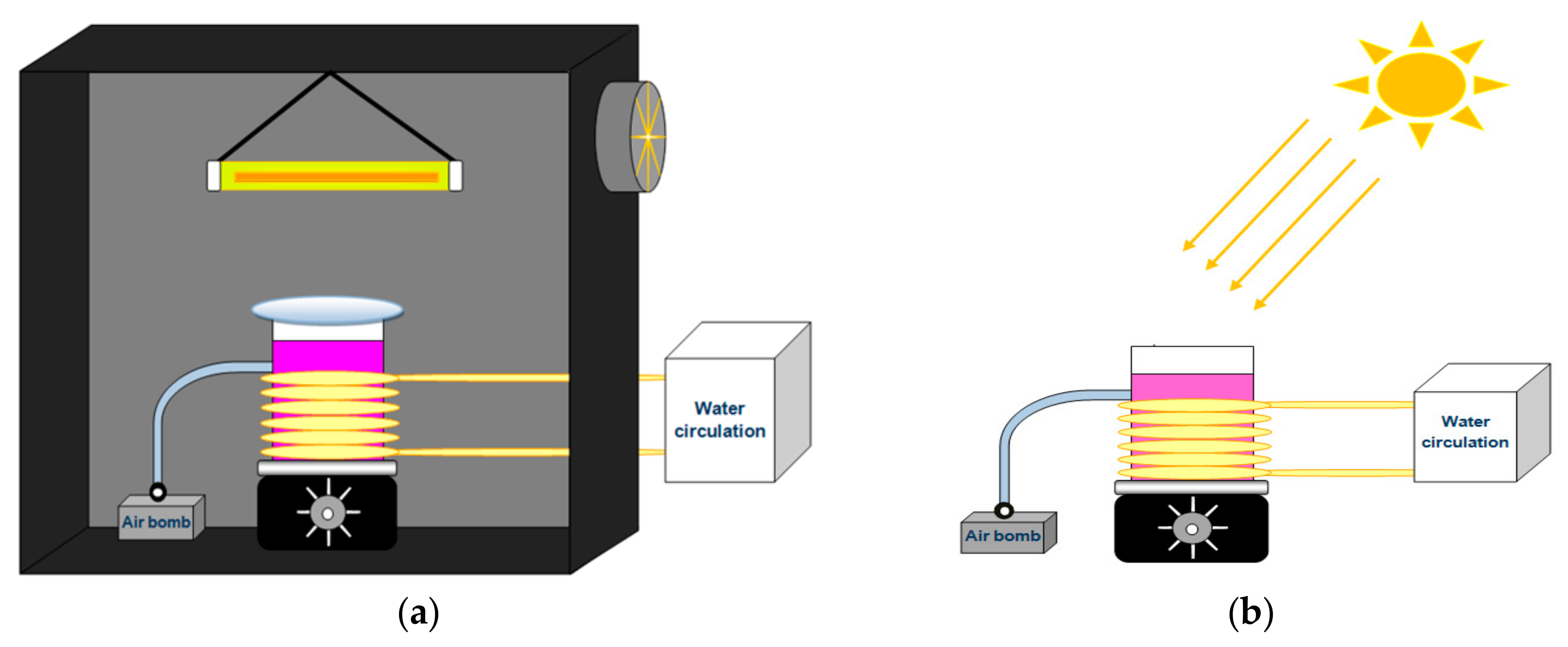
2.5. Photocatalytic Activity Measurements
3. Results and Discussion
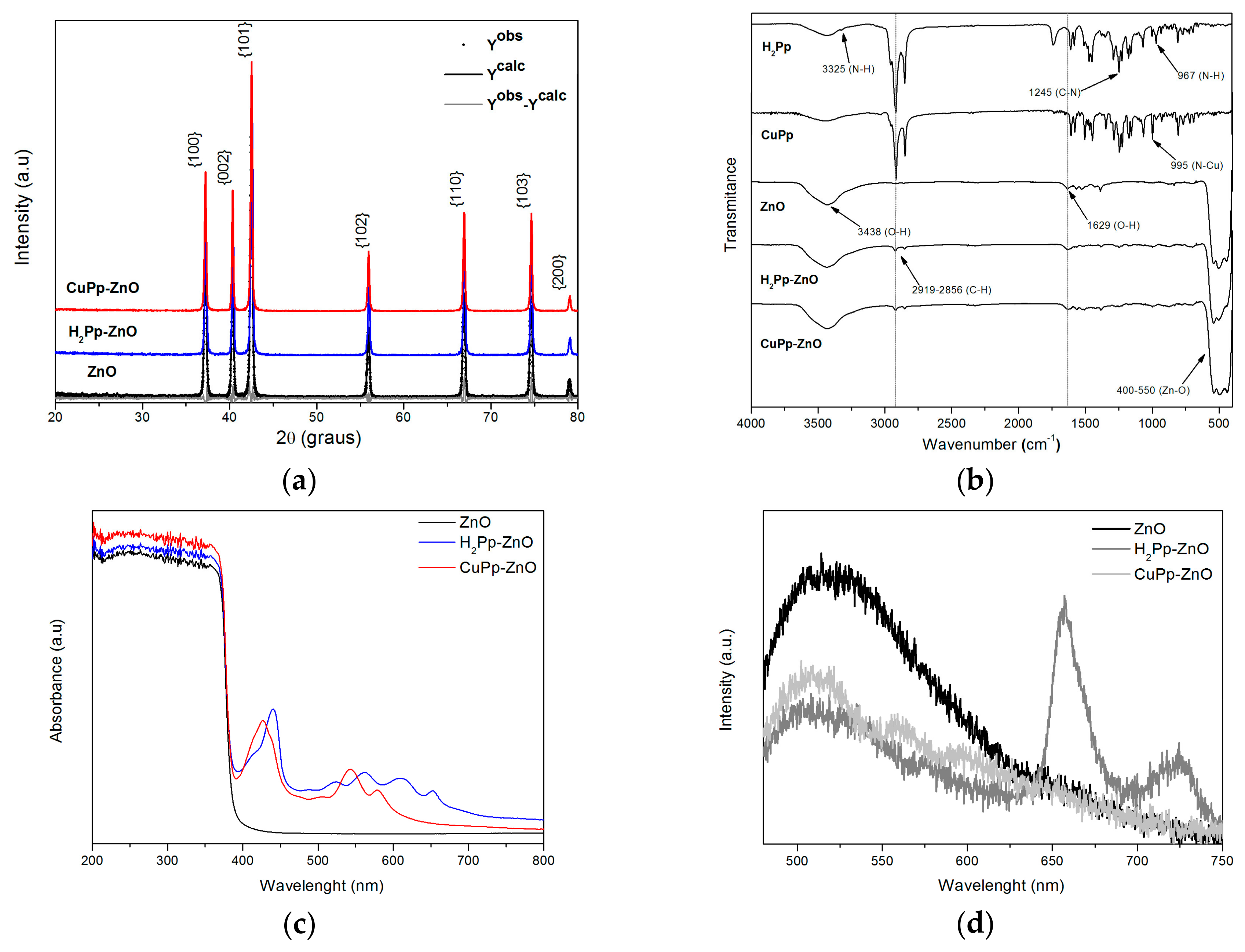
3.1. XRD Analysis, FT-IR Spectroscopy, UV-Vis DRS and Photoluminescent Properties
3.2. Morphology Analysis Based on TEM
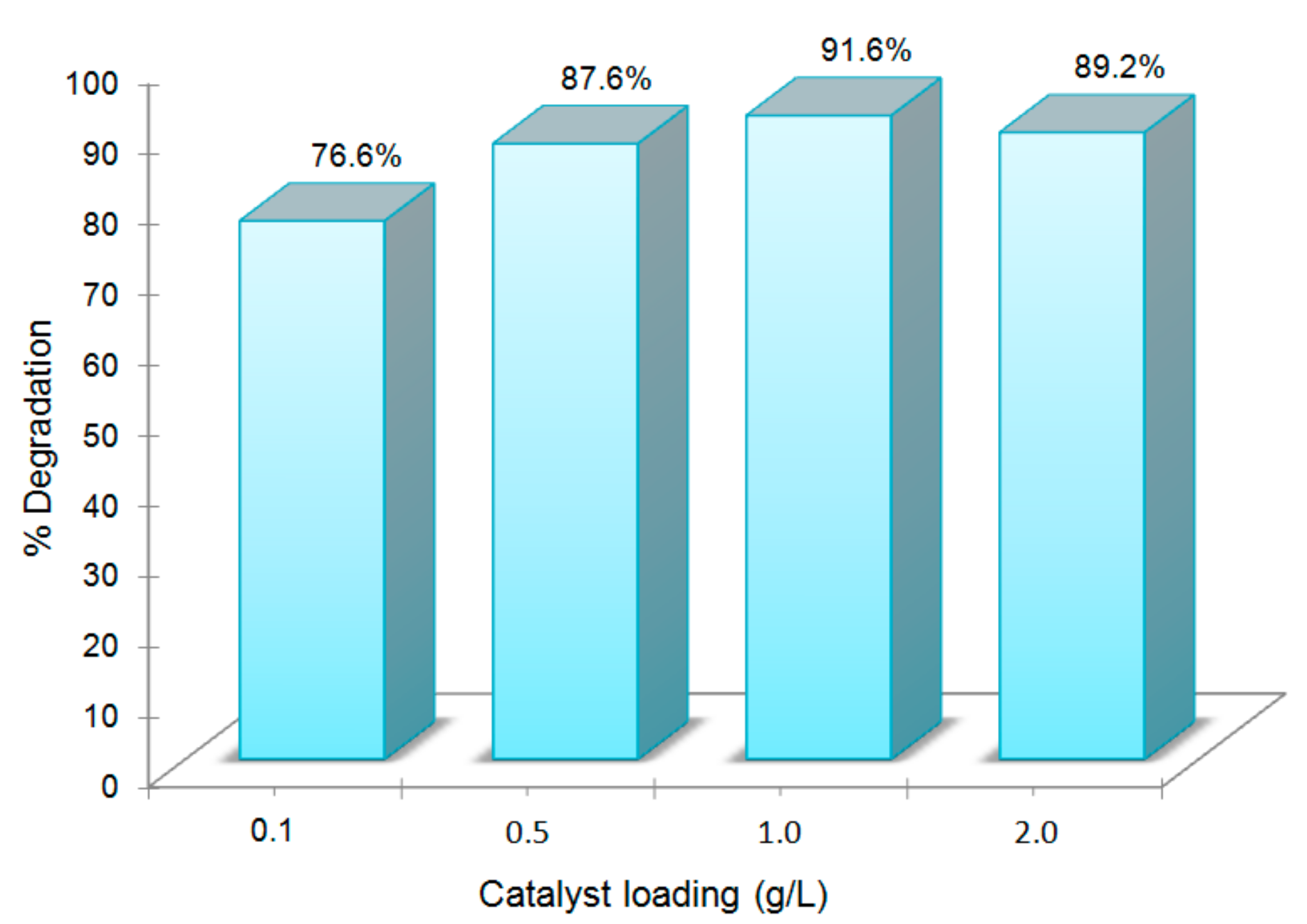
3.3. Effect of Catalyst Loading
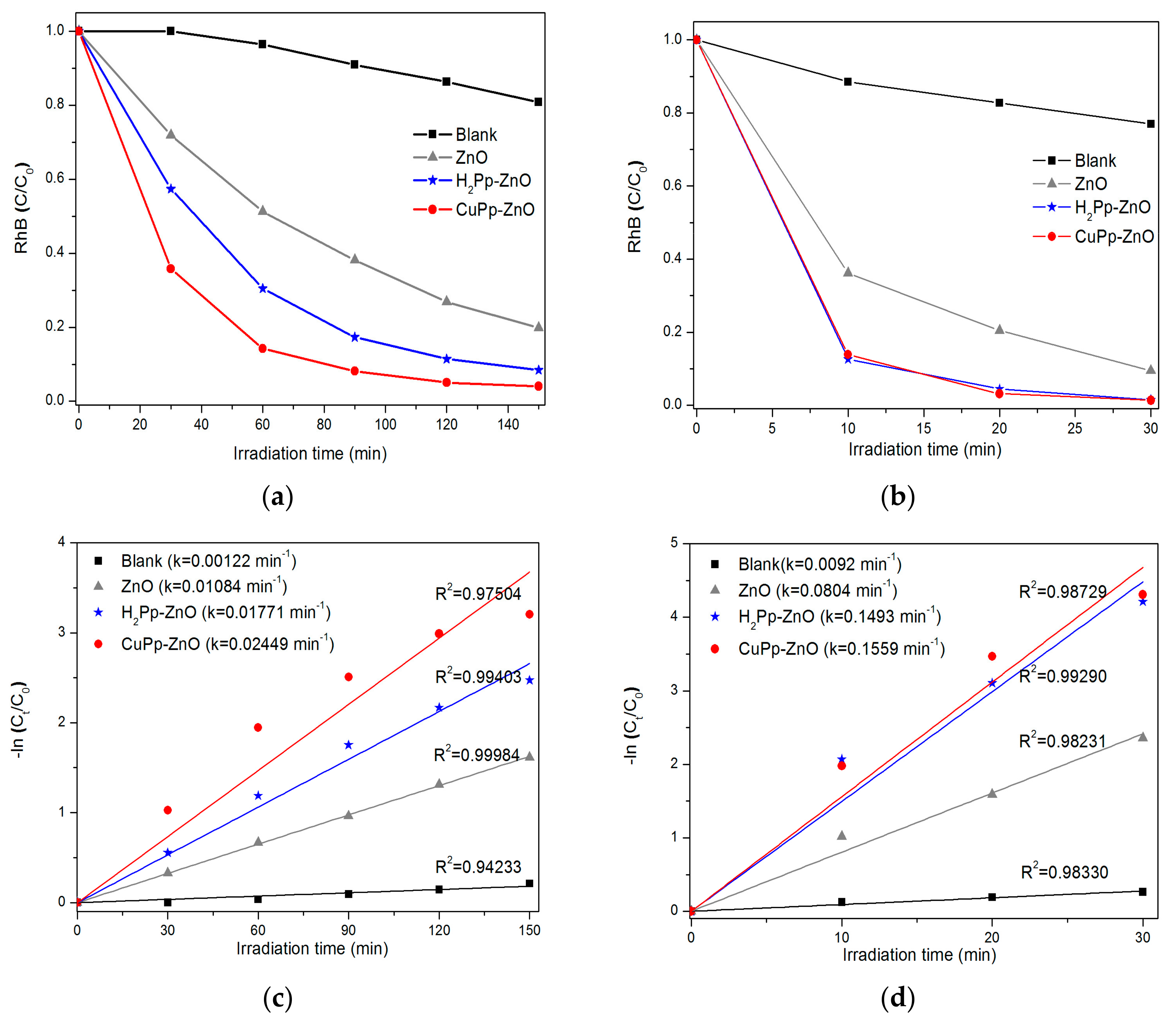
3.4. Evaluation of Photocatalytic Activity
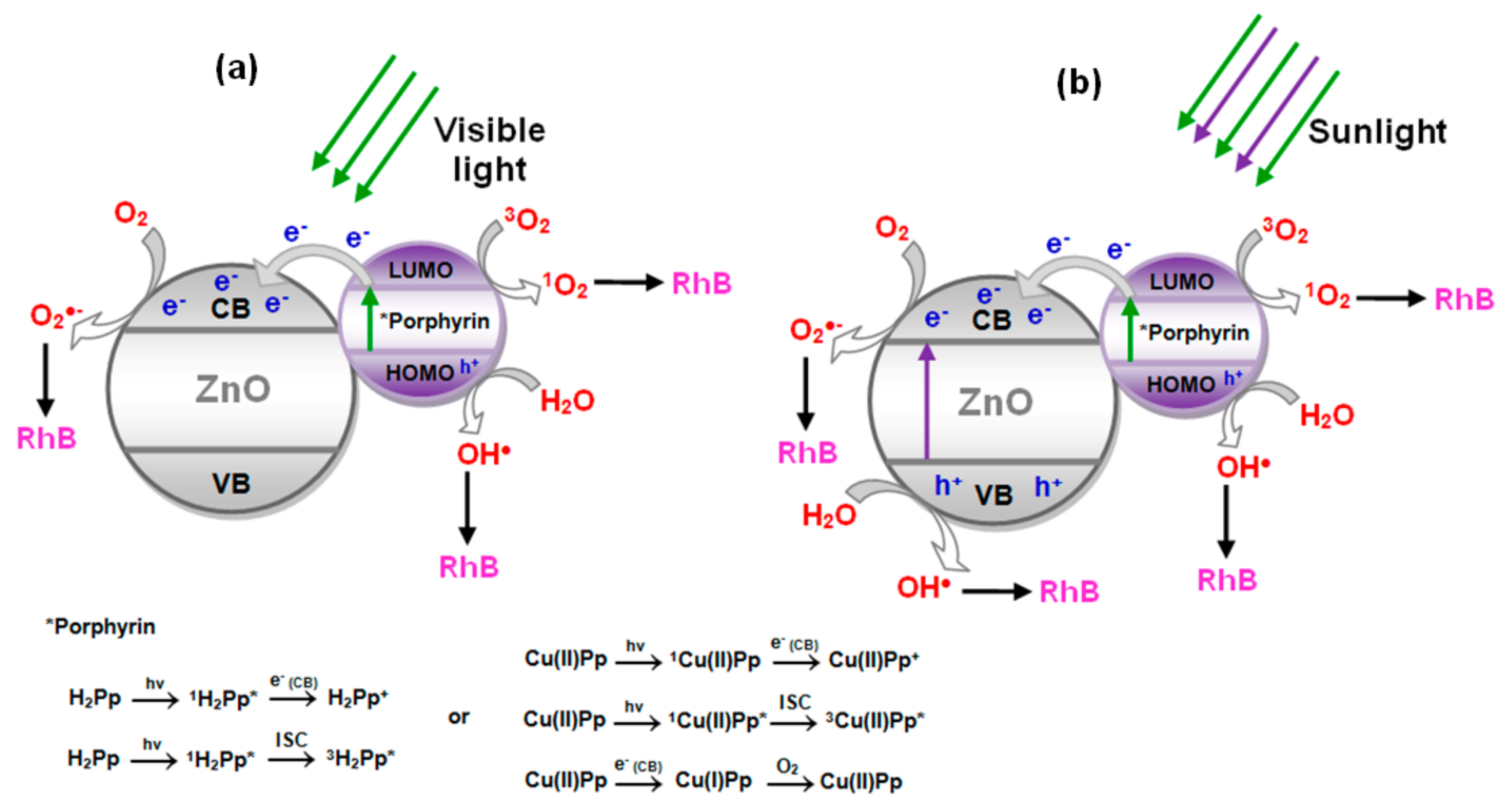
3.5. Proposed Photocatalytic Mechanism
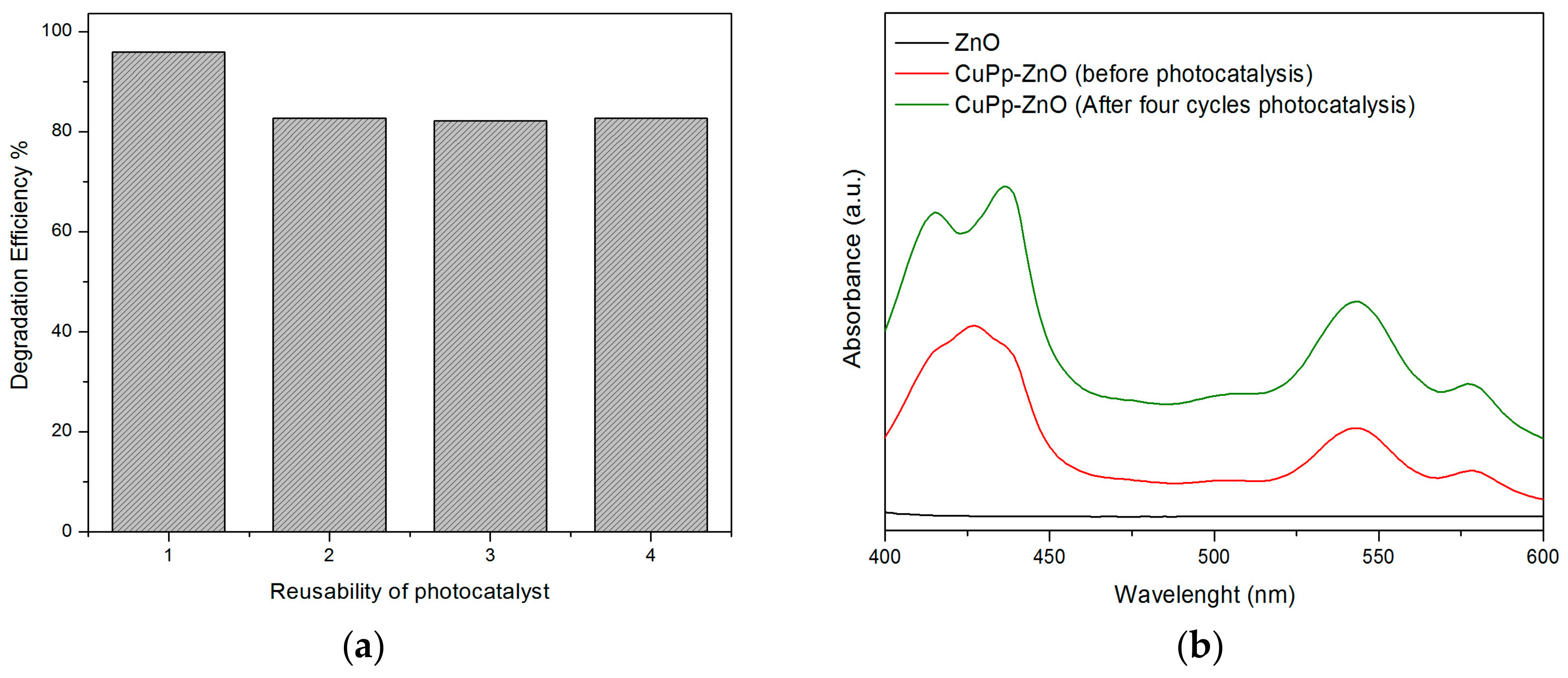
3.6. Reusability of Photocatalyst
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Jastrzębska, A.; Olszyna, A.; Baia, L. UV Light-Assisted Degradation of Methyl Orange, Methylene Blue, Phenol, Salicylic Acid, and Rhodamine B: Photolysis Versus Photocatalyis. Water Air Soil Pollut. 2017, 228, 41–53. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhan, Y.; Tang, W.; Cheng, X.; Liu, H. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B. Chem. Phys. Lett. 2016, 653, 101–107. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elkady, M.F.; El-Shazly, A.H.; Bamufleh, H.S. Formulation of Synthesized Zinc Oxide Nanopowder into Hybrid Beads for Dye Separation. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Pigeot-Rémy, S.; Dufour, F.; Herissan, A.; Ruaux, V.; Maugé, F.; Hazime, R.; Foronato, C.; Guillard, C.; Chaneac, C.; Durupthy, O.; et al. Bipyramidal anatase TiO2 nanoparticles, a highly efficient photocatalyst? Towards a better understanding of the reactivity. Appl. Catal. B Environ. 2017, 203, 324–334. [Google Scholar] [CrossRef]
- Yang, C.; Yu, J.; Li, Q.; Yu, Y. Facile synthesis of monodisperse porous ZnO nanospheres for organic pollutant degradation under simulated sunlight irradiation: The effect of operational parameters. Mater. Res. Bull. 2017, 87, 72–83. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Rahimi, R.; Yaghoubi-Berijani, M.; Zargari, S.; Rabbani, M.; Shariatinia, S. SnTCPP-modified ZnO nanorods prepared via a simple co-precipitation method: Application as a new photocatalyst for photodegradation and photoreduction processes. Res. Chem. Intermed. 2016, 42, 4697–4714. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, P.; Ao, Y.; Hou, J.; Qian, J. Effect of oxygen vacancy on enhanced photocatalytic activity of reduced ZnO nanorod arrays. Appl. Surf. Sci. 2015, 325, 112–116. [Google Scholar] [CrossRef]
- Kumar, S.G.; Koteswara Rao, K.S.R. Zinc oxide based photocatalysis: tailoring surface bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Hamrouni, A.; Moussa, N.; Parrino, F.; Di Paola, A.; Houas, A.; Palmisano, L. Sol–gel synthesis and photocatalytic activity of ZnO–SnO2 nanocomposites. J. Mol. Catal. A Chem. 2014, 390, 133–141. [Google Scholar] [CrossRef]
- Maiti, U.N.; Maiti, S.; Chattopadhyay, K.K. An ambient condition, one pot route for large scale production of ultrafine (<15 nm) ZnO nanowires from commercial zinc exhibiting excellent recyclable catalytic performance: Approach extendable to CuO nanostructures. CrystEngComm 2012, 14, 640–647. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Vijaya, J.J.; Kennedy, L.J. Effects of Morphology and Zr Doping on Structural, Optical, and Photocatalytic Properties of ZnO Nanostructures. Ind. Eng. Chem. Res. 2012, 51, 16333–16345. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Vijila, C.; Jose, R.; Uddin, A.; Ramakrishna, S. Metal oxide semiconducting interfacial layers for photovoltaic and photocatalytic applications. Mater. Renew. Sustain. Energy 2015, 4, 11–36. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar] [CrossRef]
- Sun, W.-J.; Li, J.; Mele, G.; Zhang, Z.; Zhang, F. Enhanced photocatalytic degradation of rhodamine B by surface modification of ZnO with copper (II) porphyrin under both UV-vis and visible light irradiation. J. Mol. Catal. A Chem. 2013, 366, 84–91. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Kang, S.; Mu, J. Preparation and enhanced visible light-driven catalytic activity of ZnO microrods sensitized by porphyrin heteroaggregate. Appl. Surf. Sci. 2010, 256, 6705–6709. [Google Scholar] [CrossRef]
- Rabbani, M.; Heidari-Golafzani, M.; Rahimi, R. Synthesis of TCPP/ZnFe2O4@ZnO nanohollow sphere composite for degradation of methylene blue and 4-nitrophenol under visible light. Mater. Chem. Phys. 2016, 179, 35–41. [Google Scholar] [CrossRef]
- Verma, S.; Ghosh, H.N. Exciton Energy and Charge Transfer in Porphyrin Aggregate/Semiconductor (TiO2) Composites. J. Phys. Chem. Lett. 2012, 3, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mei, Q.; Hua, Q.; Tian, R.; Weng, J.; Shi, Y.; Huang, W. Synthesis, characterization, energy transfer and photophysical properties of ethynyl bridge linked porphyrin–naphthalimide pentamer and its metal complexes. J. Mol. Struct. 2015, 1094, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Yu, M.; Wang, C.; Li, J. The highly efficient and stable Cu, Co, Zn-porphyrin-TiO2 photocatalysts with heterojunction by using fashioned one-step method. Dyes Pigments 2017, 136, 648–656. [Google Scholar] [CrossRef]
- Su, X.-Q.; Li, J.; Zhang, Z.-Q.; Yu, M.-M.; Yuan, L. Cu(II) porphyrins modified TiO2 photocatalysts: Accumulated patterns of Cu(II) porphyrin molecules on the surface of TiO2 and influence on photocatalytic activity. J. Alloys Compd. 2015, 626, 252–259. [Google Scholar] [CrossRef]
- Liu, X.; Yu, M.; Zhang, Z.; Zhao, X.; Li, J. Solvothermal preparation of copper(II) porphyrin sensitized mesoporous TiO2 composites: Enhanced photocatalytic activity and stability in degradation of 4-nitrophenol. Res. Chem. Intermed. 2016, 42, 5197–5208. [Google Scholar] [CrossRef]
- Sarkar, S.; Makhal, A.; Bora, T.; Lakhsman, K.; Singha, A.; Dutta, J.; Pal, S.K. Hematoporphyrin—ZnO Nanohybrids: Twin Applications in Efficient Visible-Light Photocatalysis and Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2012, 4, 7027–7035. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Sardar, S.; Alarousu, E.; Sun, J.; Seddigi, Z.S.; Ahmed, S.A.; Danish, E.Y.; Mohammed, O.F.; Pal, S.K. Impact of Metal Ions in Porphyrin-Based Applied Materials for Visible-Light Photocatalysis: Key Information from Ultrafast Electronic Spectroscopy. Chem. Eur. J. 2014, 20, 10475–10483. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; ArunaKumari, M.L.; Anitha, B.G.; Shyamala, R.; Poornima, G. Photocatalytic evaluation of Hemin (chloro(protoporhyinato)iron(III)) anchored ZnO hetero-aggregate system under UV/solar light irradiation: A surface modification method. Surf. Interfaces 2016, 1–3, 52–58. [Google Scholar] [CrossRef]
- Mota, J.P.F.; Ribeiro, V.G.P.; da Silva, F.L.F.; Costa Junior, A.E.; Oliveira, D.R.; Kotzebue, L.R.V.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. Developing eco-friendly methods for purification of compounds derived from hydrogenated cardanol. Sep. Sci. Technol. 2016, 51, 2473–2483. [Google Scholar] [CrossRef]
- Attanasi, O.A.; Del Sole, R.; Filippone, P.; Mazzetto, S.E.; Mele, G.; Vasapollo, G. Synthesis of novel lipophilic porphyrin-cardanol derivatives. J. Porphyr. Phthalocyanines 2004, 8, 1276–1284. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Lomonaco, D.; Mele, G.; Mazzetto, S.E. Cashew Nutshell Liquid (CNSL): From an Agro-industrial Waste to a Sustainable Alternative to Petrochemical Resources. In Cashew Nut Shell Liquid, 1st ed.; Anilkumar, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 19–38. ISBN 978-3-319-47455-7. [Google Scholar]
- Mele, G.; Vasapollo, G. Fine Chemicals and New Hybrid Materials from Cardanol. Mini-Rev. Organ. Chem. 2008, 5, 243–253. [Google Scholar] [CrossRef]
- Mele, G.; Lomonaco, D.; Mazzetto, S.E. Cardanol-Based Heterocycles: Synthesis and Applications. In Cashew Nut Shell Liquid, 1st ed.; Anilkumar, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 39–56. ISBN 978-3-319-47455-7. [Google Scholar]
- Lomonaco, D.; Santiago, G.M.P.; Ferreira, Y.S.; Arriaga, A.M.C.; Mazzetto, S.E.; Mele, G.; Vasapollo, G. Study of technical CNSL and its main components as new green larvicides. Green Chem. 2009, 11, 31–33. [Google Scholar] [CrossRef]
- Clemente, C.S.; Ribeiro, V.G.P.; Sousa, J.E.A.; Maia, F.J.N.; Barreto, A.C.H.; Andrade, N.F.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; et al. Porphyrin synthesized from cashew nut shell liquid as part of a novel superparamagnetic fluorescence nanosystem. J. Nanopart. Res. 2013, 15, 1739–1749. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [PubMed]
- Sandrino, B.; Clemente, C.S.; Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Pavinatto, F.J.; Mazzetto, S.E.; Lima-Neto, P.; Correia, A.N.; Pessoa, C.A.; Wohnrath, K. Amphiphilic porphyrin-cardanol derivatives in Langmuir and Langmuir–Blodgett films applied for sensing. Colloids Surf A Physicochem. Eng. Asp. 2013, 425, 68–75. [Google Scholar] [CrossRef]
- Mota, J.P.F.; Costa Júnior, A.E.; Ribeiro, V.G.P.; Sampaio, S.G.; Lima, N.M.A.; Silva, F.L.F.; Clemente, C.S.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. Synthesis, Characterization and Dielectric Properties of New 5-(4-Hydroxyphenyl)-10,15,20-tri-4-[2-(3-pentadecyl phenoxy)ethoxy.phenyl porphyrin and Their Ni, Co and Cu Complexes. J. Braz. Chem. Soc. 2017, 28, 1063–1073. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Hariharan, R.; Suganthi, A.; Ashokkumar, M.; Rajarajan, M.; Pitchumani, K. Synergistic photodynamic action of ZnO nanomaterials encapsulated meso-tetra(4-sulfonatophenyl) porphyrin. Powder Technol. 2013, 237, 497–505. [Google Scholar] [CrossRef]
- Di Mauro, A.; Smecca, E.; D’Urso, A.; Guglielmo, G.; Condorelli, G.G.; Fragalà, M.E. Tetra-anionic porphyrin loading onto ZnO nanoneedles: A hybrid covalent/non covalent approach. Mater. Chem. Phys. 2014, 143, 977–982. [Google Scholar] [CrossRef]
- Valicsek, Z.; Horváth, O. Application of the electronic spectra of porphyrins for analytical purposes: The effects of metal ions and structural distortions. Microchem. J. 2013, 107, 47–62. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Akir, S.; Barras, A.; Coffinier, Y.; Bououdina, M.; Boukherroub, R.; Omrani, A.D. Eco-friendly synthesis of ZnO nanoparticles with different morphologies and their visible light photocatalytic performance for the degradation of Rhodamine B. Ceram. Int. 2016, 42, 10259–10265. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Zoltan, T.; Rosales, M.C.; Yadarola, C. Reactive oxygen species quantification and their correlation with the photocatalytic activity of TiO2 (anatase and rutile) sensitized with asymmetric porphyrins. J. Environ. Chem. Eng. 2016, 4, 3967–3980. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S. Comparison and mechanism of photocatalytic activities of N-ZnO and N-ZrO2 for the degradation of rhodamine 6G. Environ. Sci. Pollut. Res. 2016, 23, 10177–10188. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Malekshoar, G.; Dhir, A.; Ray, A.K. Enhanced photocatalytic degradation of atenolol using graphene TiO2 composite. J. Photochem. Photobiol. A Chem. 2017, 332, 182–187. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Mele, G.; Yang, G.-M.; Zhang, F.-X.; Palmisano, L.; Vasapollo, G. Efficient degradation of 4-nitrophenol by using functionalized porphyrin-TiO2 photocatalysts under visible irradiation. Appl. Catal. B Environ. 2007, 76, 218–226. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Sun, W.-J.; Jiang, M.; Zhang, F.-X. Preparation, characterization, and photocatalytic properties of composite materials of copper(II) porphyrin/TiO2. J. Mater. Sci. 2014, 49, 5519–5528. [Google Scholar] [CrossRef]
- Wang, C.; Yang, G.-M.; Li, J.; Mele, G.; S1ota, R.; Broda, M.A.; Duan, M.-Y.; Vasapollo, G.; Zhang, X.; Zhang, F-X. Novel meso-substituted porphyrins: Synthesis, characterization and photocatalytic activity of their TiO2-based composites. Dyes Pigments 2009, 80, 321–328. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Takriff, M.S.; Kadhum, A.A.H.; Mohamad, A.B.; Benamor, A.; Mohammad, A.W. Solar photocatalytic degradation of 2-chlorophenol with ZnO nanoparticles: Optimisation with D-optimal design and study of intermediate mechanisms. Environ. Sci. Pollut. Res. 2016, 24, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. B 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Pichat, P. (Ed.) Photocatalysis and Water Purification: From Fundamentals to Recent Applications, 1st ed.; Wiley: Berlin, Germany, 2013; pp. 80–82. ISBN 9783527645404. [Google Scholar]
- Vargas, E.; Vargas, R.; Núnez, O. A TiO2 surface modified with copper(II) phthalocyanine-tetrasulfonicacid tetrasodium salt as a catalyst during photoinduced dichlorvos mineralization by visible solar light. Appl. Catal. B Environ. 2014, 156–157, 8–14. [Google Scholar] [CrossRef]
- La, D.D.; Rananaware, A.; Salimimarand, M.; Bhosale, S.V. Well-dispersed assembled porphyrin nanorods on graphene for the enhanced photocatalytic performance. ChemistrySelect 2016, 1, 4430–4434. [Google Scholar] [CrossRef]
- Mele, G.; Garcìa-Lòpez, E.; Palmisano, L.; Dyrda, G.; Słota, R. Photocatalytic degradation of 4-nitrophenol in aqueous suspension by using polycrystalline TiO2 impregnated with lanthanide double-decker phthalocyanine complexes. J. Phys. Chem. C 2007, 111, 6581–6588. [Google Scholar] [CrossRef]
- Sardar, S.; Sarkar, S.; Myint, M.T.Z.; Al-Harthi, S.; Dutta, J.; Pal, S.K. Role of central metal ions in hematoporphyrin functionalized titania in solar energy conversion dynamics. Phys. Chem. Chem. Phys. 2013, 15, 18562–18570. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, V.G.P.; Marcelo, A.M.P.; Da Silva, K.T.; Da Silva, F.L.F.; Mota, J.P.F.; Do Nascimento, J.P.C.; Sombra, A.S.B.; Clemente, C.D.S.; Mele, G.; Carbone, L.; et al. New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation. Materials 2017, 10, 1114. https://doi.org/10.3390/ma10101114
Ribeiro VGP, Marcelo AMP, Da Silva KT, Da Silva FLF, Mota JPF, Do Nascimento JPC, Sombra ASB, Clemente CDS, Mele G, Carbone L, et al. New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation. Materials. 2017; 10(10):1114. https://doi.org/10.3390/ma10101114
Chicago/Turabian StyleRibeiro, Viviane Gomes Pereira, Ana Maria Pereira Marcelo, Kássia Teixeira Da Silva, Fernando Luiz Firmino Da Silva, João Paulo Ferreira Mota, João Paulo Costa Do Nascimento, Antonio Sérgio Bezerra Sombra, Claudenilson Da Silva Clemente, Giuseppe Mele, Luigi Carbone, and et al. 2017. "New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation" Materials 10, no. 10: 1114. https://doi.org/10.3390/ma10101114




