Adsorption Mechanisms of Dodecylbenzene Sulfonic Acid by Corn Straw and Poplar Leaf Biochars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Method
2.2.1. Biochar Characterization
2.2.2. Batch Adsorption Experiments
2.2.3. Data Analysis
3. Results and Discussion
3.1. Characteristics of Biochars
3.2. Adsorption Kinetics and Isotherm
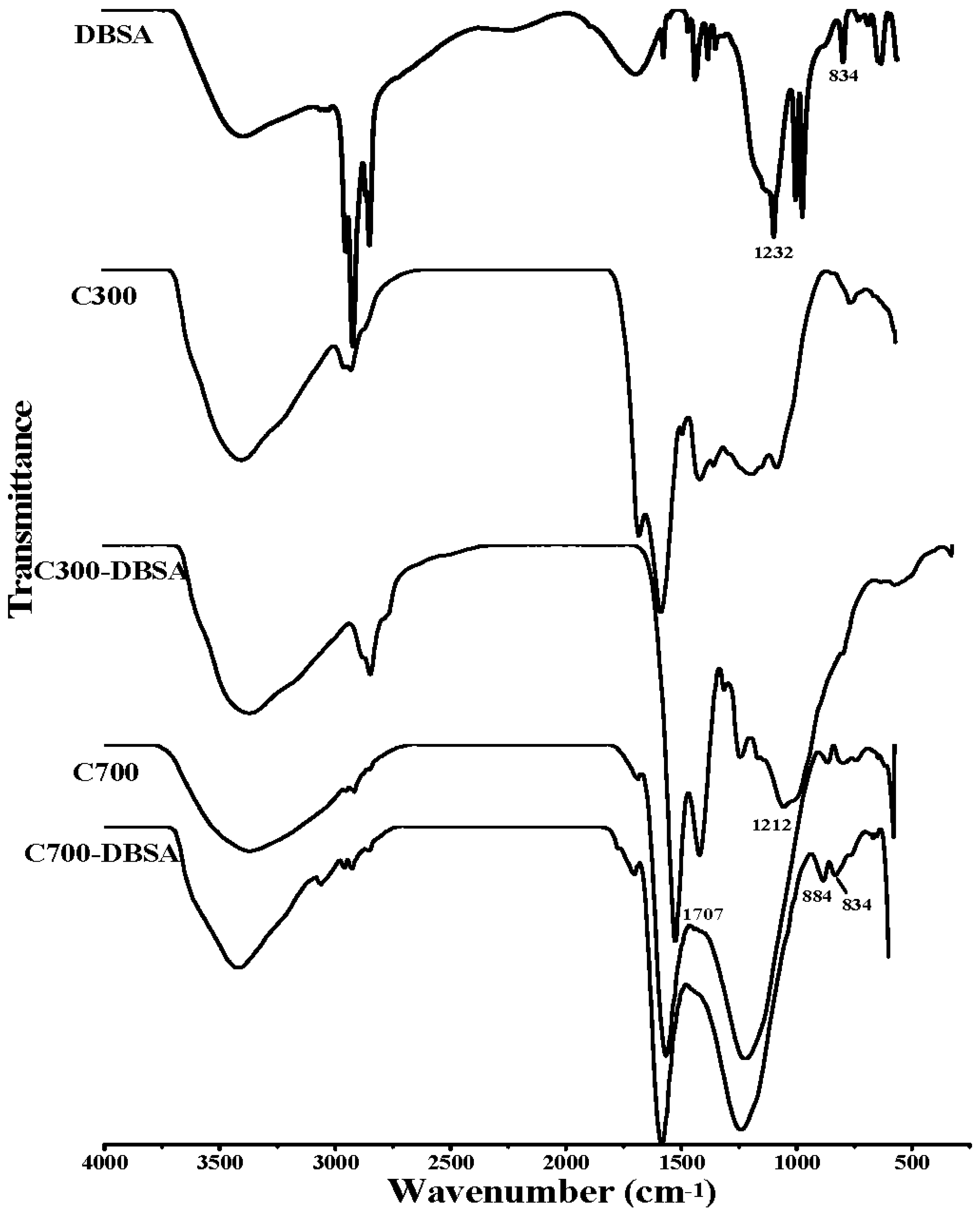
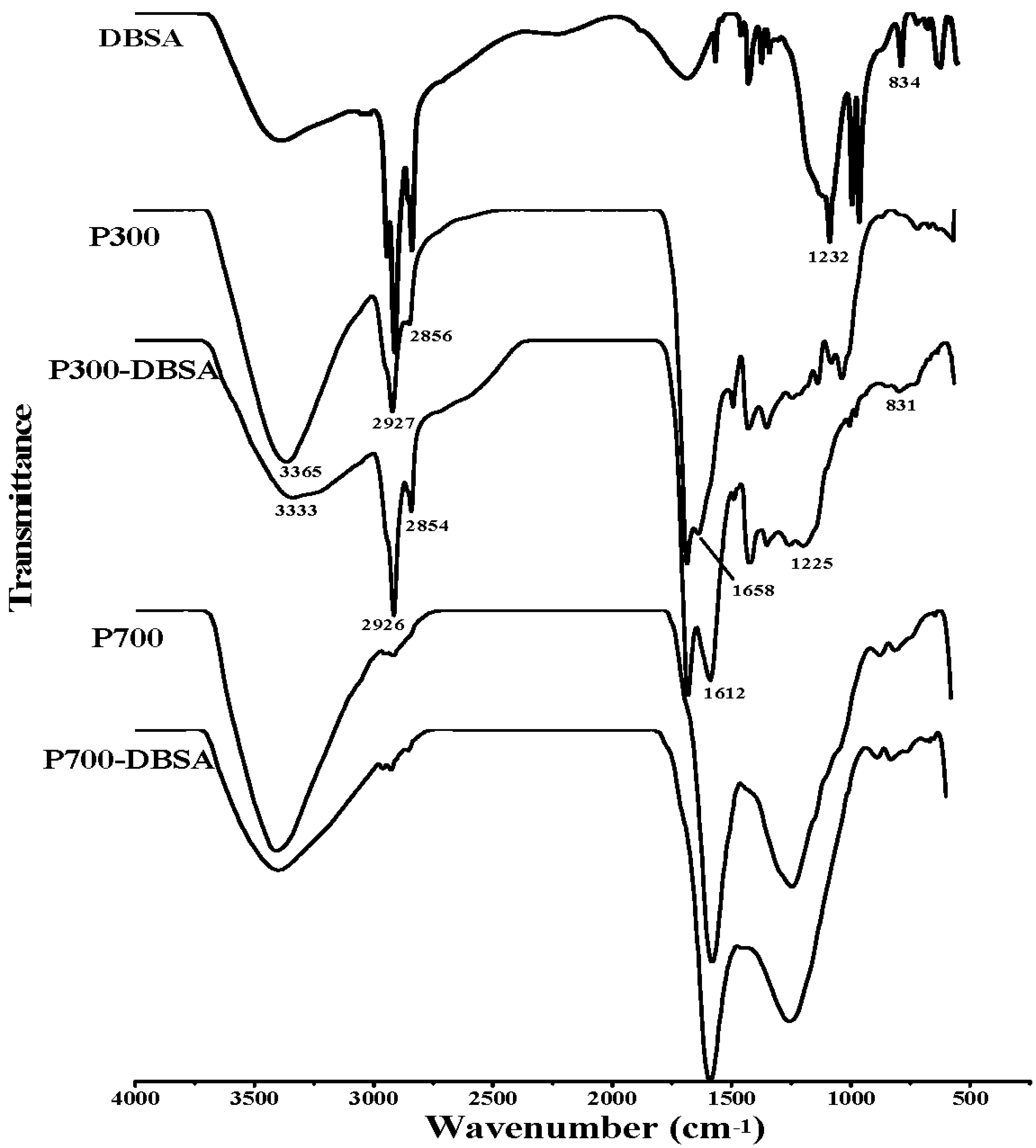
3.3. Adsorption Mechanisms
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Westall, J.C.; Chen, H.; Zhang, W.J.; Brownawell, B.J. Sorption of linear alkylbenzenesulfonates on sediment materials. Environ. Sci. Technol. 1999, 33, 3110–3118. [Google Scholar] [CrossRef]
- Tan, X.L.; Fang, M.; Chen, C.L.; Yu, S.M.; Wang, X.K. Counterion effects of nickel and sodium dodecylbenzene sulfonate adsorption to multiwalled carbon nanotubes in aqueous solution. Carbon 2008, 46, 1741–1750. [Google Scholar] [CrossRef]
- Fernández-Ramos, C.; Ballesteros, O.; Zafra-Gómez, A.; Blanc-García, R.; Navalón, A.; Crovetto, S.I.; Oliver-Rodríguez, B.; García-Delgado, R.A.; Vílchez, J.L. Sorption and desorption of alcohol sulfate surfactants in an agricultural soil. Environ. Toxicol. Chem. 2014, 33, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Ravindran, H.; Gupta, R.B.; Fasina, O.; Tu, M.; Fernando, S.D. Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour. Technol. 2010, 101, 8389–8395. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.L.; Ma, L.Q.; Li, Y.C. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Peterson, S.C.; Jackson, M.A. Simplifying pyrolysis: Using gasification to produce corn stover and wheat straw biochar for sorptive and horticultural media. Ind. Crops Prod. 2014, 53, 228–235. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K. Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: A spectroscopic investigation. Sci. Total Environ. 2015, 502, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Chen, Z.M. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Liu, R.L. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.W.; Li, Y.; Sun, T.H. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244–245, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Lu, J.; Wu, J.; Lu, Z.Y.; Wilson, P.C.; Shen, Y. Adsorption of fluoroquinolone antibiotics by wastewater sludge biochar: Role of the sludge source. Water Air Soil Pollut. 2013, 224, 1370. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.S.; Sanchez-Martin, M.J.; Sanchez-Camazano, M. A comparative study of adsorption of an anionic and a non-ionic surfactant by soils based on physicochemical and mineralogical properties of soils. Chemosphere 2005, 61, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sarmiento, D.C.; Pinzón-Bello, J.A. Adsorption of sodium dodecylbenzene sulfonate on organophilic bentonites. Appl. Clay Sci. 2001, 18, 173–181. [Google Scholar] [CrossRef]
- Cotoruelo, L.M.; Marqués, M.D.; Rodríguez-Mirasol, J.; Rodríguez, J.J.; Cordero, T. Lignin-based activated carbons for adsorption of sodium dodecylbenzene sulfonate: Equilibrium and kinetic studies. J. Colloid Interface Sci. 2009, 332, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sastry, N.Y.; Séquaris, J.M.; Schwuger, M.J. Adsorption of polyacrylic acid and sodium dodecylbenzenesulfonate on kaolinite. J. Colloid Interface Sci. 1995, 3171, 224–233. [Google Scholar] [CrossRef]
- Zhao, N.; Lv, Y.Z.; Song, G.X.; Zhang, J. Sorption behavior of dodecylbenzene sulfonic acid on humic acids from Mollisol and Alluvial soils. Environ. Earth Sci. 2016, 75, 1–8. [Google Scholar] [CrossRef]
- Ando, N.; Kuwabara, Y.; Kodama, T.; Mori, Y.H. Surface tensions of aqueous solutions of lithium dodecyl sulfate, sodium oleate, and dodecylbenzene sulfonic acid in contact with methane under hydrate-forming conditions. Fluid Phase Equilib. 2012, 314, 146–151. [Google Scholar] [CrossRef]
- Jiang, J.H.; Zhang, L.; Wang, X.Y.; Holm, N.; Rajagopalan, K.; Chen, F.L.; Ma, S.G. Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Zhao, N.; Lv, Y.Z.; Yang, X.X. A new 3D conceptual structures modeling of biochars by molecular mechanics and molecular dynamic simulation. J. Soil Sediments 2017, 17, 641–655. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Chen, G.C.; Chen, L.G.; Chen, Y.X.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Johnson, E.J.; Chefetz, B. Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: The role of polarity and accessibility. Environ. Sci. Technol. 2005, 39, 6138–6146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, N.; Jin, X.; Zhou, D.; Li, H.; Wu, M.; Pan, B. The role of ash content on bisphenol a sorption to biochars derived from different agricultural wastes. Chemosphere 2017, 171, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin-alginic acid composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef]
- Wang, M.S.; Liao, L.B.; Zhang, X.L.; Li, Z.H. Adsorption of low concentration humic acid from water by palygorskite. Appl. Clay Sci. 2012, 67–68, 164–168. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhou, Y.M.; Cao, X.D. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ. Sci. Pollut. Res. 2015, 22, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Arampatzidou, A.C.; Deliyanni, E.A. Comparison of activation media and pyrolysis temperature for activated carbons development by pyrolysis of potato peels for effective adsorption of endocrine disruptor bisphenol-A. J. Colloid Interface Sci. 2016, 466, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.P.; Lee, C.K.; Juang, L.C.; Han, Y.L. Sorption of organic compounds, oxyanions, and heavy metal ions on surfactant modified titanate nanotubes. Ind. Eng. Chem. Res. 2013, 52, 9843–9850. [Google Scholar] [CrossRef]
- Yan, Q.G.; Wan, C.X.; Liu, J.; Gao, J.S.; Yu, F.; Zhang, J.L.; Cai, Z.Y. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15, 1631–1640. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 1997, 61, 221–227. [Google Scholar] [CrossRef]
- D’Orazio, V.; Loffredo, E.; Brunetti, G.; Senesi, N. Triallate adsorption onto humic acids of different origin and nature. Chemosphere 1999, 39, 183–198. [Google Scholar] [CrossRef]
- Russell, L.; Stokes, A.R.; Macdonald, H.; Muscolo, A.; Nardi, S. Stomatal responses to humic substances and auxin are sensitive to inhibitors of phospholipase A2. Plant Soil 2006, 283, 175–185. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Baes, A.U.; Bloom, P.R. Diffuse reflectance and transmission Fourier Transform Infrare (DRIFT) spectroscopy of humic and fulvic acids. Soil Sci. Soc. Am. J. 1989, 53, 695–700. [Google Scholar] [CrossRef]
- Zhang, J.J.; Hu, F.; Li, H.X.; Gao, Q.; Song, X.Y.; Ke, X.K.; Wang, L.C. Effects of earthworm activity on humus composition and humic acid characteristics of soil in a maize residue amended rice-wheat rotation agroecosystem. Appl. Soil Ecol. 2001, 51, 1–8. [Google Scholar] [CrossRef]
- Senesi, N. Binding mechanisms of pesticides to soil humic substances. Sci. Total Environ. 1992, 123–124, 63–76. [Google Scholar] [CrossRef]
- Fang, G.; Zhu, C.; Dionysiou, D.D.; Gao, J.; Zhou, D. Mechanism of hydroxyl radical generation from biochar suspensions: Implications to diethyl phthalate degradation. Bioresour. Technol. 2015, 176, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Testini, C. Physico-chemical investigations of interaction mechanisms between s-triazineherbicides and soil humic acids. Geoderma 1982, 28, 129–146. [Google Scholar] [CrossRef]
- Mattson, J.S.; Mark, H.B.; Malbin, M.D.; Weber, W.J.; Critten, J.C. Surface chemistry of active carbon: Specific adsorption of phenol. J. Colloid Interface Sci. 1969, 3, 116–130. [Google Scholar] [CrossRef]
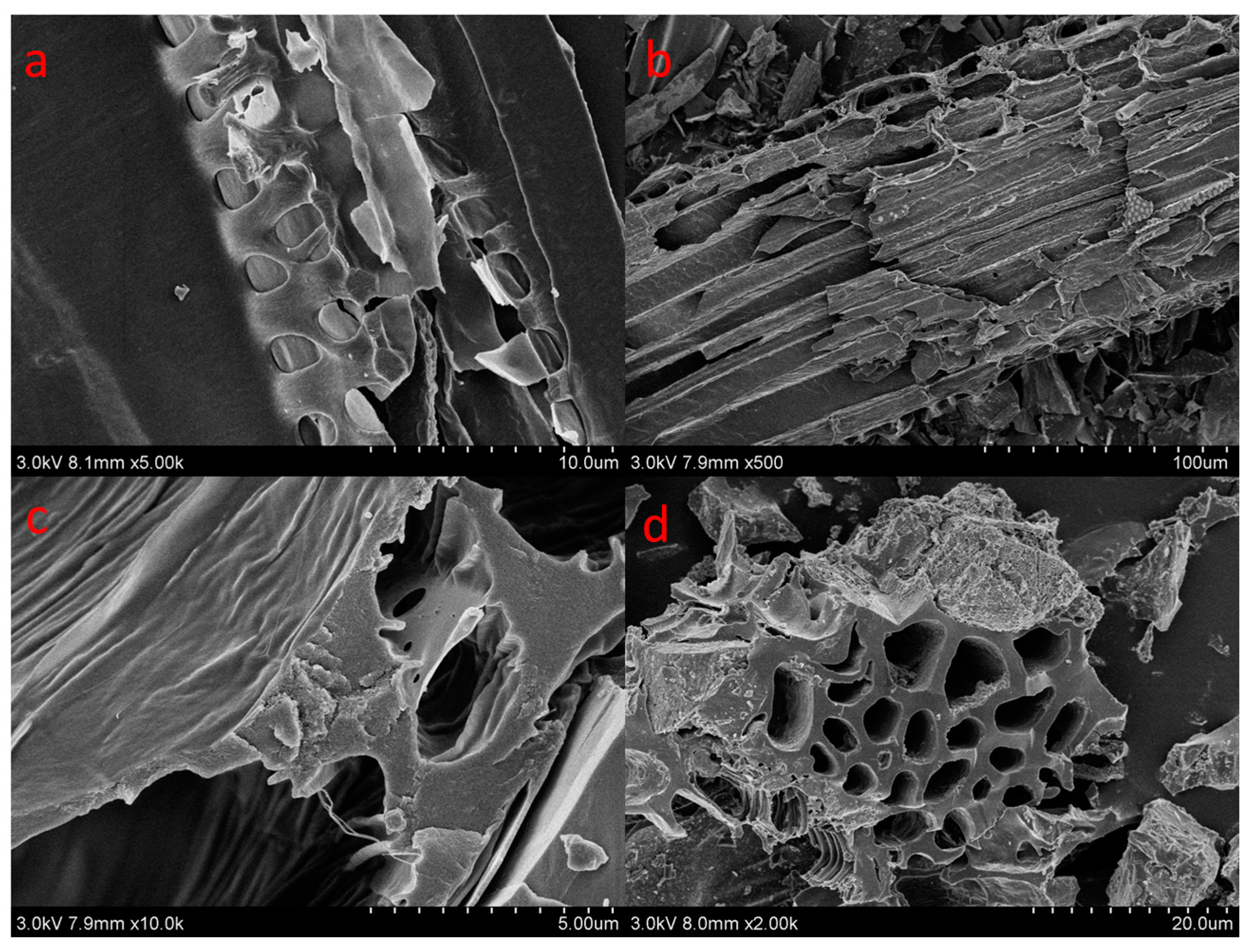


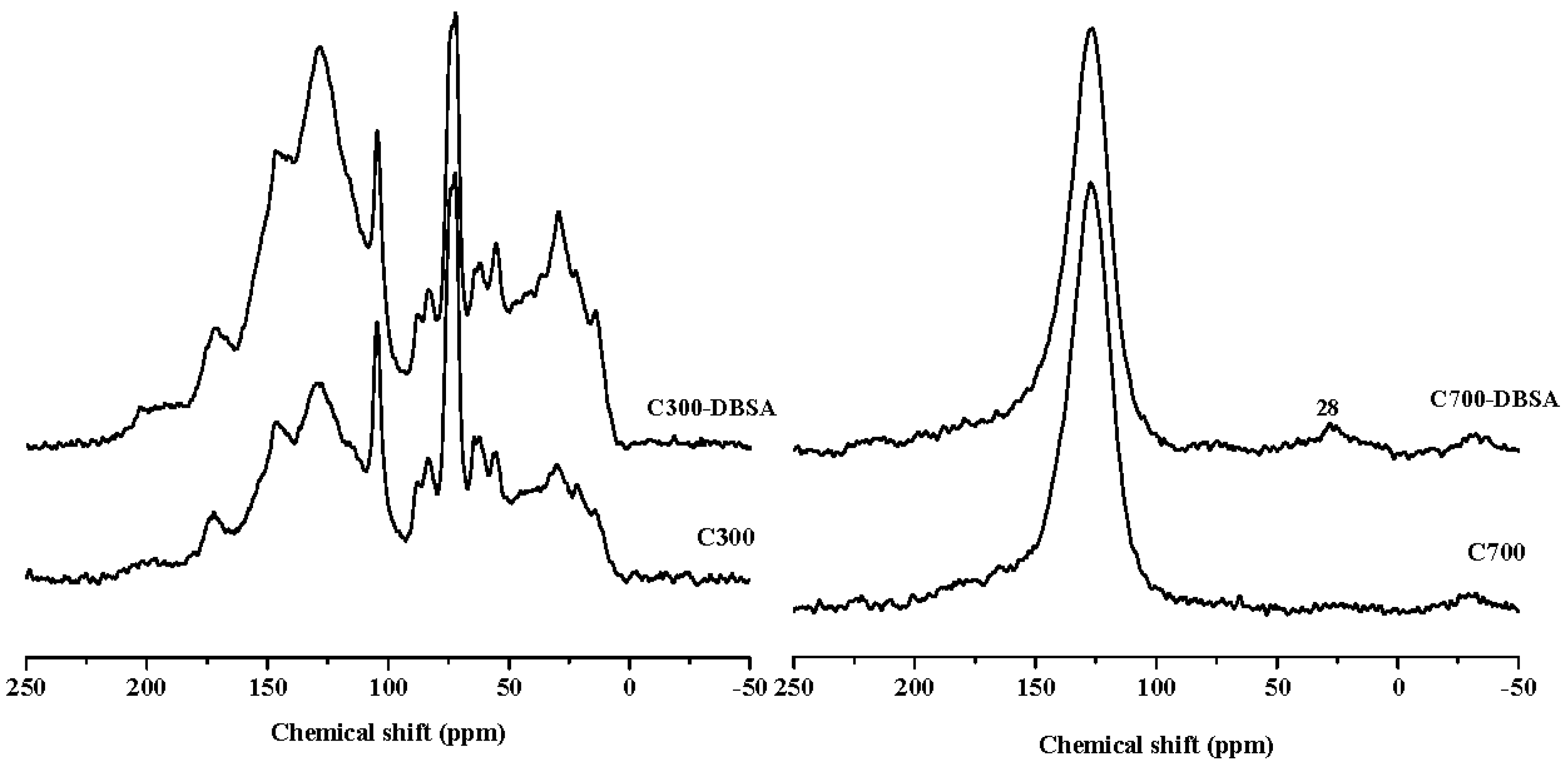
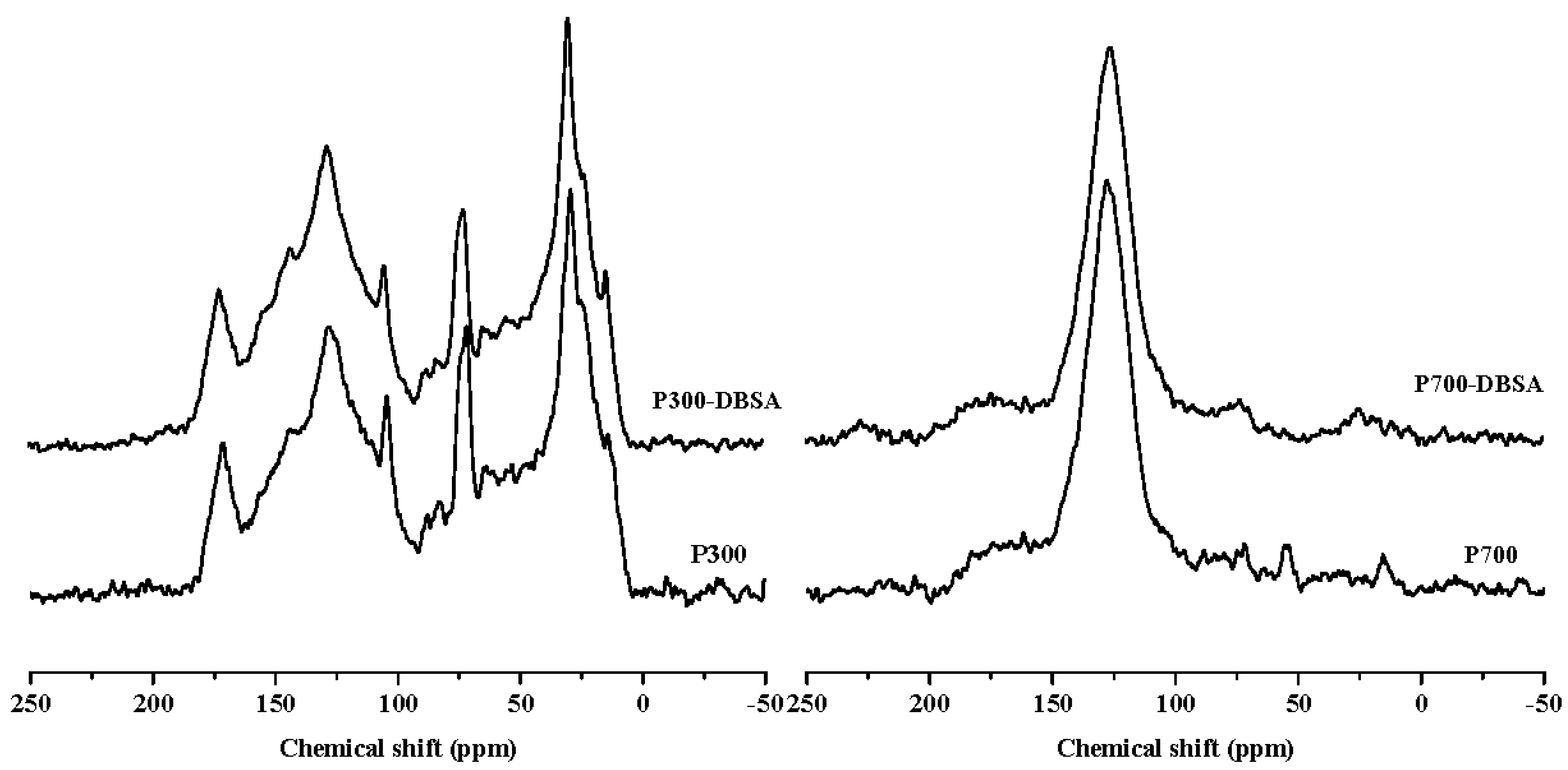
| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| C300 | 4.52 | 0.0042 | 8.33 |
| C700 | 375.89 | 0.2302 | 3.98 |
| P300 | 1.29 | 0.0023 | 12.27 |
| P700 | 105.88 | 0.0721 | 5.53 |
| Samples | C (%) | H (%) | N (%) | O (%) | H/C | O/C | C/N | (O + N)/C | Ash (%) |
|---|---|---|---|---|---|---|---|---|---|
| C300 | 61.67 | 5.41 | 1.88 | 31.04 | 1.05 | 0.43 | 38.27 | 0.46 | 0.44 |
| C700 | 83.76 | 1.50 | 1.76 | 10.42 | 0.21 | 0.11 | 55.52 | 0.12 | 2.56 |
| P300 | 59.04 | 5.87 | 5.33 | 27.37 | 1.19 | 0.40 | 12.92 | 0.47 | 2.39 |
| P700 | 69.98 | 2.19 | 3.75 | 18.14 | 0.38 | 0.22 | 21.77 | 0.27 | 5.94 |
| Sample | Pseudo-First Order Kinetic qt = qe[1 − exp(−K1t)] | Pseudo-Second Order Kinetic 1/qt = 1/K2 × qe2 + t/qe = 1/v0 + t/qe | |||||
|---|---|---|---|---|---|---|---|
| K1 (h−1) | qe (mg/g) | R2 | K2 (g/(mg·h)) | qe (mg/g) | v0 (mg/(g·h)) | R2 | |
| C300 | 0.148 ± 0.001 | 34.81 ± 0.035 | 0.975 | 0.006 ± 0.000 | 38.17 ± 0.053 | 8.636 ± 0.054 | 0.992 |
| C700 | 1.754 ± 0.012 | 35.87 ± 0.060 | 0.996 | 0.167 ± 0.004 | 36.21 ± 0.070 | 219.0 ± 3.931 | 0.999 |
| P300 | 0.675 ± 0.016 | 36.01 ± 0.048 | 0.967 | 0.026 ± 0.001 | 37.94 ± 0.074 | 37.43 ± 0.900 | 0.999 |
| P700 | 1.108 ± 0.021 | 34.92 ± 0.095 | 0.989 | 0.062 ± 0.002 | 35.88 ± 0.126 | 79.82 ± 2.537 | 0.999 |
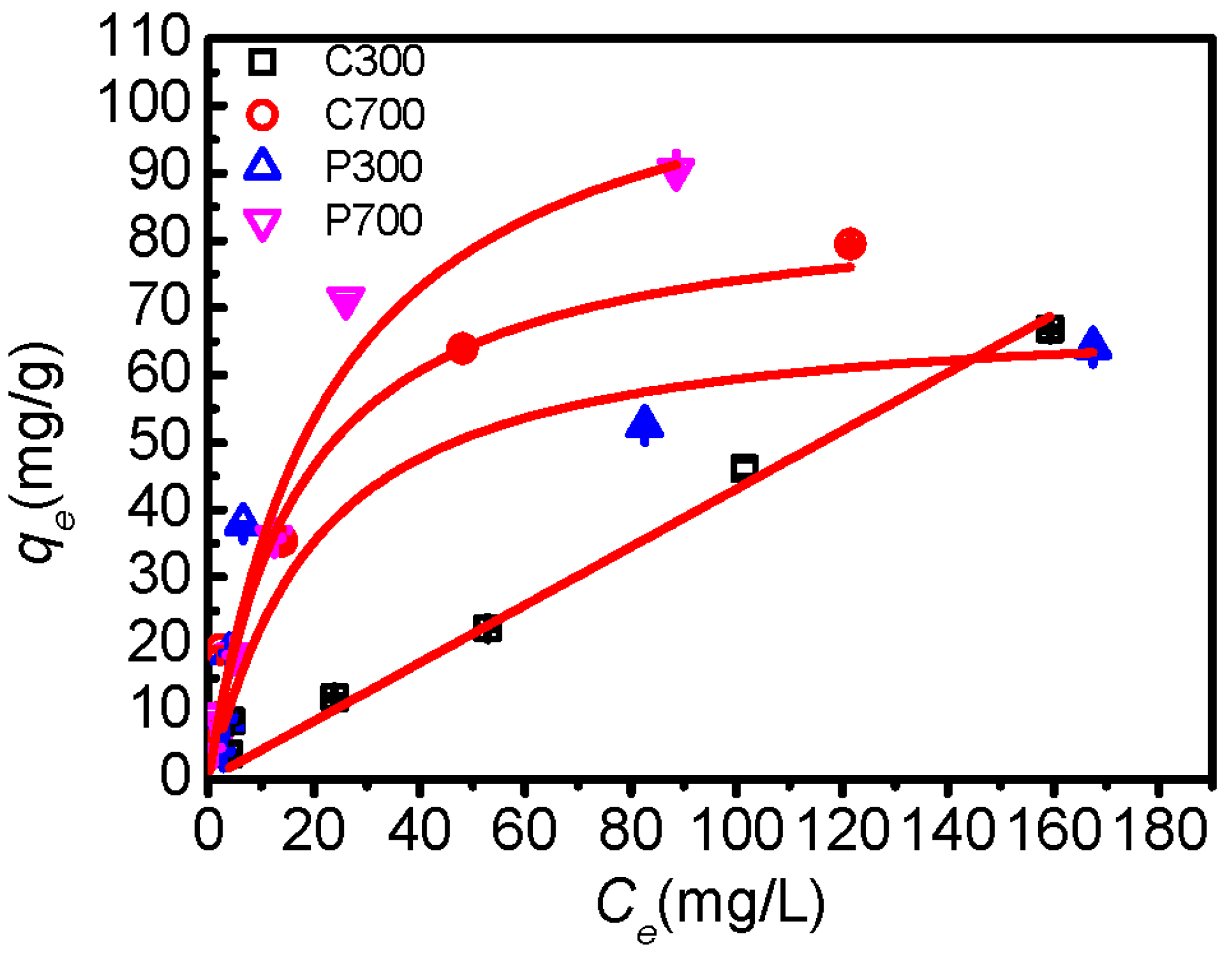
| Sample | Linear qe = KPCe | Langmuir qe = KqmaxCe/(1 + KCe) | Freundlich qe = KFCe n | |||||
|---|---|---|---|---|---|---|---|---|
| KP (L/g) | R2 | K (L/g) | qmax (mg/g) | R2 | KF (mg/g (L/mg) n) | n | R2 | |
| C300 | 0.431 ± 0.006 | 0.980 | 7.667 ± 0.376 | 74.82 ± 0.371 | 0.530 | 2.026 ± 0.036 | 0.654 ± 0.002 | 0.914 |
| C700 | 0.769 ± 0.069 | 0.588 | 57.50 ± 19.33 | 86.22 ± 0.433 | 0.981 | 9.427 ± 0.466 | 0.468 ± 0.013 | 0.948 |
| P300 | 0.442 ± 0.007 | 0.347 | 46.33 ± 4.779 | 71.33 ± 1.652 | 0.983 | 6.448 ± 0.231 | 0.489 ± 0.011 | 0.745 |
| P700 | 1.199 ± 0.029 | 0.592 | 43.50 ± 4.770 | 155.9 ± 23.37 | 0.981 | 5.047 ± 0.456 | 0.675 ± 0.037 | 0.954 |
| Index | K2 | Surface Area | Pore Volume | Average Pore Size | C | H | N | O | C/ H | O/ C | C/ N | ||
| Surface area | 0.99 ** | ||||||||||||
| Pore volume | 0.99 ** | 1.00 ** | |||||||||||
| Average pore size | −0.75 | −0.80 | −0.81 | ||||||||||
| C | 0.97 * | 0.99 * | 0.99 * | −0.89 | |||||||||
| H | −0.85 | −0.85 | −0.87 | 0.93 | −0.92 | ||||||||
| N | −0.42 | −0.53 | −0.53 | 0.70 | −0.57 | 0.42 | |||||||
| O | −0.96 * | −0.93 | −0.94 | 0.80 | −0.95 * | 0.95 | 0.30 | ||||||
| C/H | −0.85 | −0.86 | −0.87 | 0.94 | −0.93 | 1.00 ** | 0.45 | 0.94 | |||||
| O/C | −0.94 | −0.93 | −0.94 | 0.85 | −0.96 * | 0.97 * | 0.35 | 1.00 ** | 0.97 * | ||||
| C/N | 0.69 | 0.78 | 0.77 | −0.72 | 0.77 | −0.54 | −0.92 | −0.53 | −0.56 | −0.55 | |||
| (N + O)/C | −0.95 * | −0.96 * | −0.96 * | 0.89 | −0.99 * | 0.97 * | 0.46 | 0.98 * | 0.97 * | 0.99 ** | −0.65 | ||
| Ash | 0.25 | 0.18 | 0.21 | −0.37 | 0.28 | −0.60 | 0.37 | −0.52 | −0.58 | −0.53 | −0.35 | ||
| Aliphatic | −0.81 | −0.84 | −0.85 | 0.99 ** | −0.92 | 0.96 * | 0.65 | 0.86 | 0.97 * | 0.90 | −0.70 | ||
| Heteroaliphatic | −0.89 | −0.83 | −0.84 | 0.64 | −0.84 | 0.87 | 0.03 | 0.96 * | 0.86 | 0.94 | −0.30 | ||
| Acetal | −0.95 | −0.90 | −0.90 | 0.59 | −0.87 | 0.80 | 0.11 | 0.95 | 0.79 | 0.92 | −0.43 | ||
| Aromatic | 0.92 | 0.92 | 0.93 | −0.91 | 0.96 * | −0.99 ** | −0.43 | −0.98 * | −0.99 * | −0.99 ** | 0.60 | ||
| Carboxyl | 0.23 | 0.13 | 0.15 | −0.15 | 0.18 | −0.46 | 0.59 | −0.47 | −0.43 | −0.46 | −0.49 | ||
| Carbonyl | 0.50 | 0.59 | 0.60 | −0.88 | 0.68 * | −0.65 | −0.94 | −0.47 | −0.68 | −0.54 | 0.84 | ||
| Aliphaticity | −0.92 | −0.91 | −0.93 | 0.91 | −0.96 * | 0.99 * | 0.42 | 0.98 | 0.99 * | 0.99 ** | −0.59 | ||
| Hydrophobic functional group | 0.90 | 0.84 | 0.85 | −0.61 | 0.83 | −0.84 | −0.02 | −0.96 * | −0.83 | −0.93 | 0.31 | ||
| Hydrophilic functional group | −0.90 | −0.84 | −0.85 | 0.61 | −0.83 | 0.84 | 0.02 | 0.96 * | 0.83 | 0.93 | −0.31 | ||
| Organic free radical concentration | 0.97 * | 0.98 * | 0.98 * | −0.71 | 0.94 | −0.74 | −0.56 | −0.86 | −0.75 | −0.84 | 0.82 | ||
| Index | (N + O)/ C | Ash | Aliphatic | Hetero Aliphatic | Acetal % | Aromatic | Carboxyl | Carbonyl | Aliphaticity | Hydrophobic Functional Group | Hydrophilic Functional Group | ||
| Surface area | |||||||||||||
| Pore volume | |||||||||||||
| Average pore size | |||||||||||||
| C | |||||||||||||
| H | |||||||||||||
| N | |||||||||||||
| O | |||||||||||||
| C/H | |||||||||||||
| O/C | |||||||||||||
| C/N | |||||||||||||
| (N + O)/C | |||||||||||||
| Ash | −0.44 | ||||||||||||
| Aliphatic | 0.93 | −0.42 | |||||||||||
| Heteroaliphatic | 0.90 | −0.64 | 0.72 | ||||||||||
| Acetal | 0.89 | 0.42 | 0.67 | 0.97 * | |||||||||
| Aromatic | −1.00 ** | 0.52 | −0.95 | −0.90 | −0.87 | ||||||||
| Carboxyl | −0.34 | 0.95 * | −0.22 | −0.65 | −0.47 | 0.41 | |||||||
| Carbonyl | −0.63 | −0.02 | 0.83 | −0.23 | −0.22 | 0.62 | −0.29 | ||||||
| Aliphaticity | 0.99 ** | −0.53 | 0.95 | 0.91 | 0.86 | −1.00 ** | −0.42 | −0.62 | |||||
| Hydrophobic functional group | −0.89 | 0.59 | −0.69 | −1.00 ** | −0.98 * | 0.89 | 0.63 | 0.20 | −0.89 | ||||
| Hydrophilic functional group | 0.89 | −0.59 | 0.69 | 1.00 ** | 0.98 * | −0.89 | −0.63 | −0.20 | 0.89 | −1.00 ** | |||
| Organic free radical concentration | −0.88 | 0.00 | −0.75 | −0.75 | −0.86 | 0.83 | −0.02 | 0.55 | −0.82 | 0.77 | −0.77 | ||
| Samples | Aliphatic % | Heteroaliphatic % | Acetal % | Aromatic % | Carboxyl % | Carbonyl % | Aliphaticity % | Hydrophobic Functional Group % | Hydrophilic Functional Group % |
|---|---|---|---|---|---|---|---|---|---|
| δ0–60 | δ60–90 | δ90–110 | δ110–165 | δ165–190 | δ190–230 | ||||
| C300 | 22.91 | 23.98 | 10.18 | 35.98 | 4.81 | 2.13 | 61.33 | 69.07 | 30.93 |
| C300-DBSA | 23.75 | 17.24 | 8.78 | 42.22 | 5.61 | 2.39 | 54.10 | 74.75 | 25.25 |
| C700 | 0.10 | 1.52 | 4.28 | 85.83 | 5.93 | 2.35 | 6.45 | 90.21 | 9.79 |
| C700-DBSA | 4.29 | 1.12 | 3.22 | 82.07 | 5.74 | 3.55 | 9.51 | 89.58 | 10.42 |
| P300 | 36.66 | 14.41 | 7.74 | 34.25 | 6.30 | 0.64 | 63.20 | 78.65 | 21.35 |
| P300-DBSA | 35.19 | 12.80 | 7.32 | 36.40 | 7.14 | 1.14 | 60.31 | 78.91 | 21.09 |
| P700 | 7.87 | 6.96 | 7.07 | 69.03 | 7.17 | 1.90 | 24.08 | 83.97 | 16.03 |
| P700-DBSA | 6.29 | 6.40 | 7.75 | 68.80 | 7.37 | 3.38 | 22.90 | 82.84 | 17.16 |
| Samples | Organic Free Radical Concentration (Spins/g × 1014) | Line Width (Gauss) |
|---|---|---|
| C300 | 0.244 | 4.7 |
| C300-DBSA | 0.297 | 4.7 |
| C700 | 11.89 | 5.3 |
| C700-DBSA | 9.632 | 4.4 |
| P300 | 0.132 | 5.6 |
| P300-DBSA | 0.196 | 5.3 |
| P700 | 1.266 | 3.2 |
| P700-DBSA | 2.599 | 4.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Yang, X.; Zhang, J.; Zhu, L.; Lv, Y. Adsorption Mechanisms of Dodecylbenzene Sulfonic Acid by Corn Straw and Poplar Leaf Biochars. Materials 2017, 10, 1119. https://doi.org/10.3390/ma10101119
Zhao N, Yang X, Zhang J, Zhu L, Lv Y. Adsorption Mechanisms of Dodecylbenzene Sulfonic Acid by Corn Straw and Poplar Leaf Biochars. Materials. 2017; 10(10):1119. https://doi.org/10.3390/ma10101119
Chicago/Turabian StyleZhao, Nan, Xixiang Yang, Jing Zhang, Ling Zhu, and Yizhong Lv. 2017. "Adsorption Mechanisms of Dodecylbenzene Sulfonic Acid by Corn Straw and Poplar Leaf Biochars" Materials 10, no. 10: 1119. https://doi.org/10.3390/ma10101119




