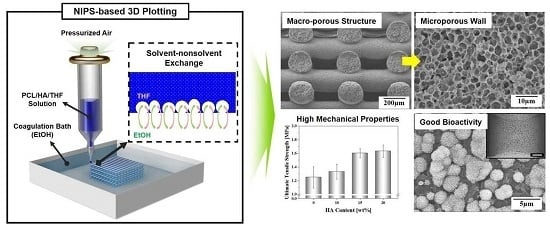
Production of Poly(ε-Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity
Abstract
:1. Introduction
2. Results and Discussion
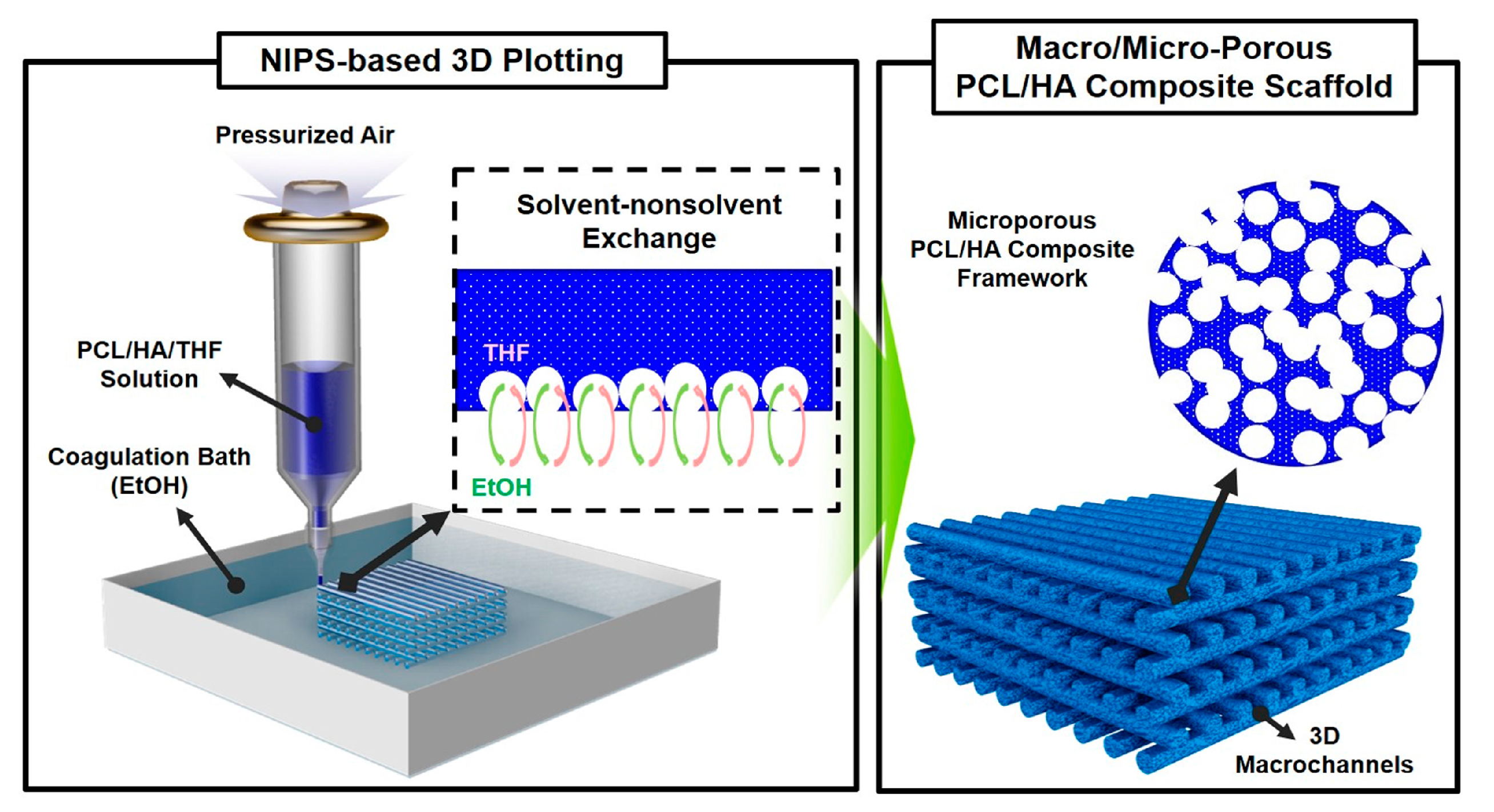
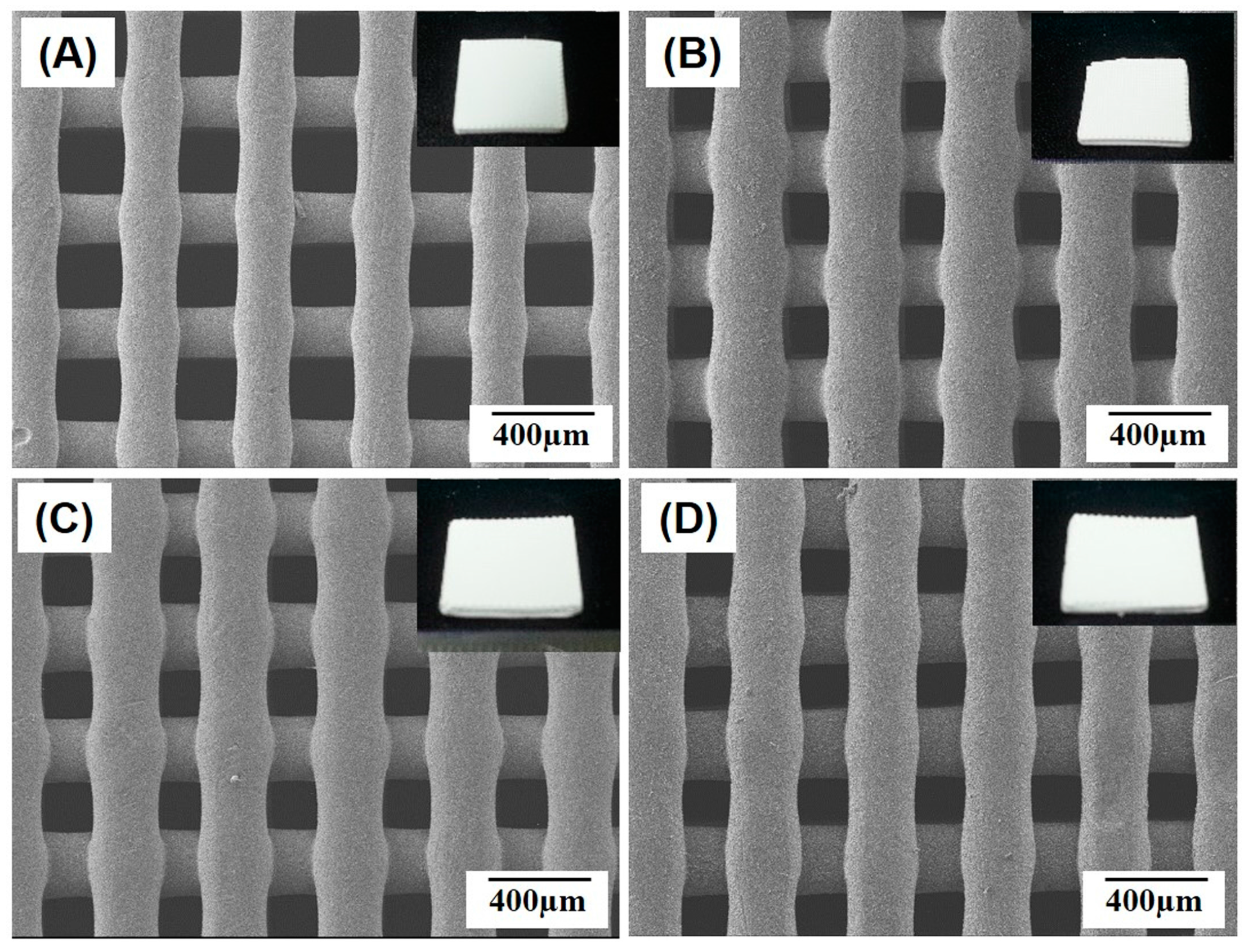
2.1. Macroporous Structure of Porous PCL/HA Composite Scaffolds
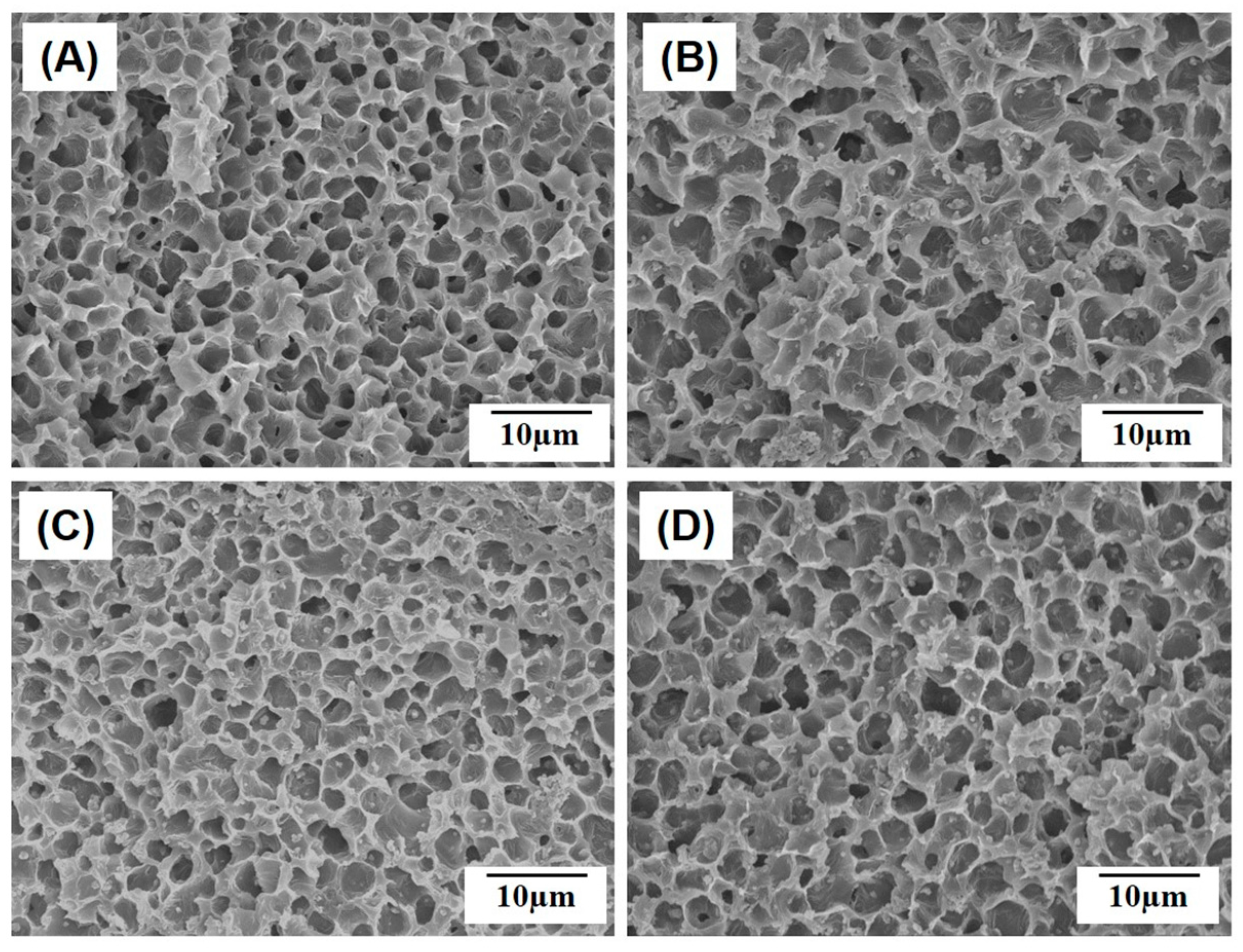
2.2. Microporous Structure of PCL/HA Composite Filaments
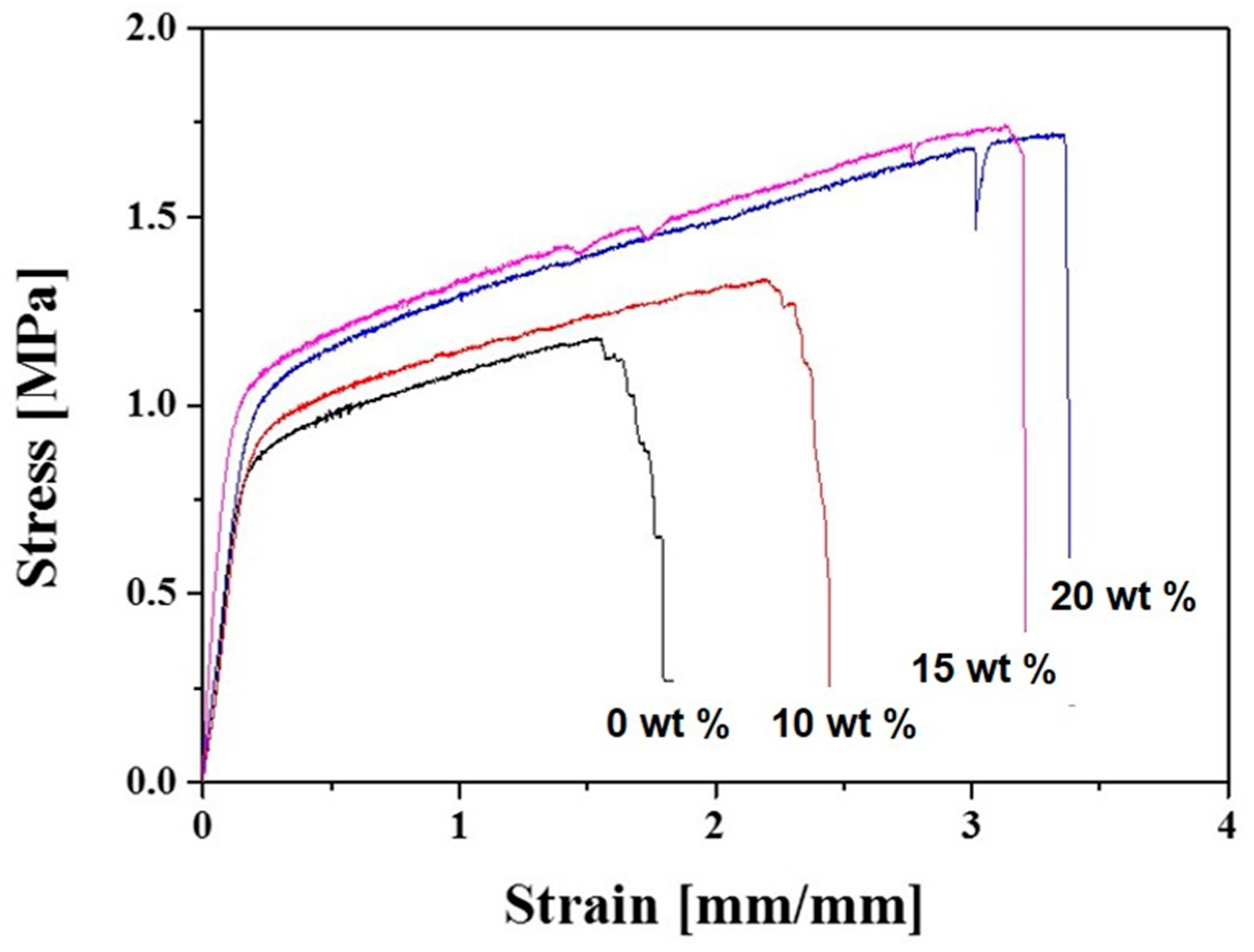
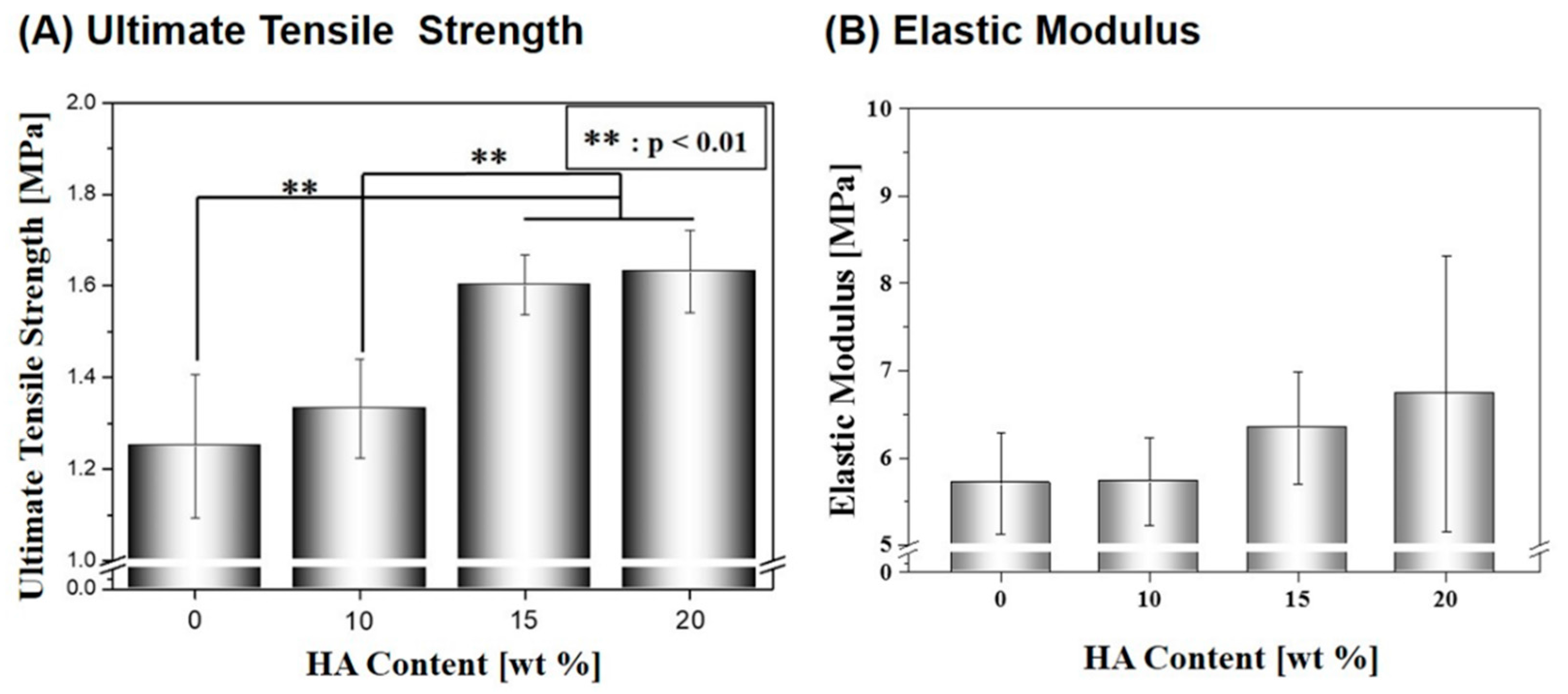
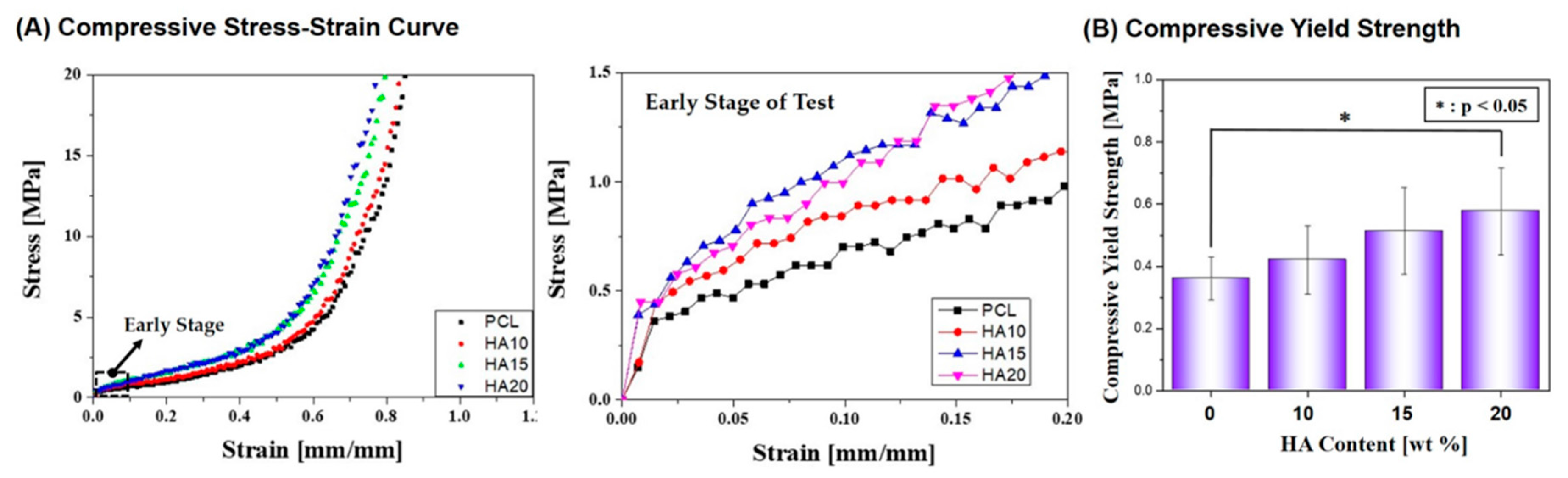
2.3. Mechanical Properties
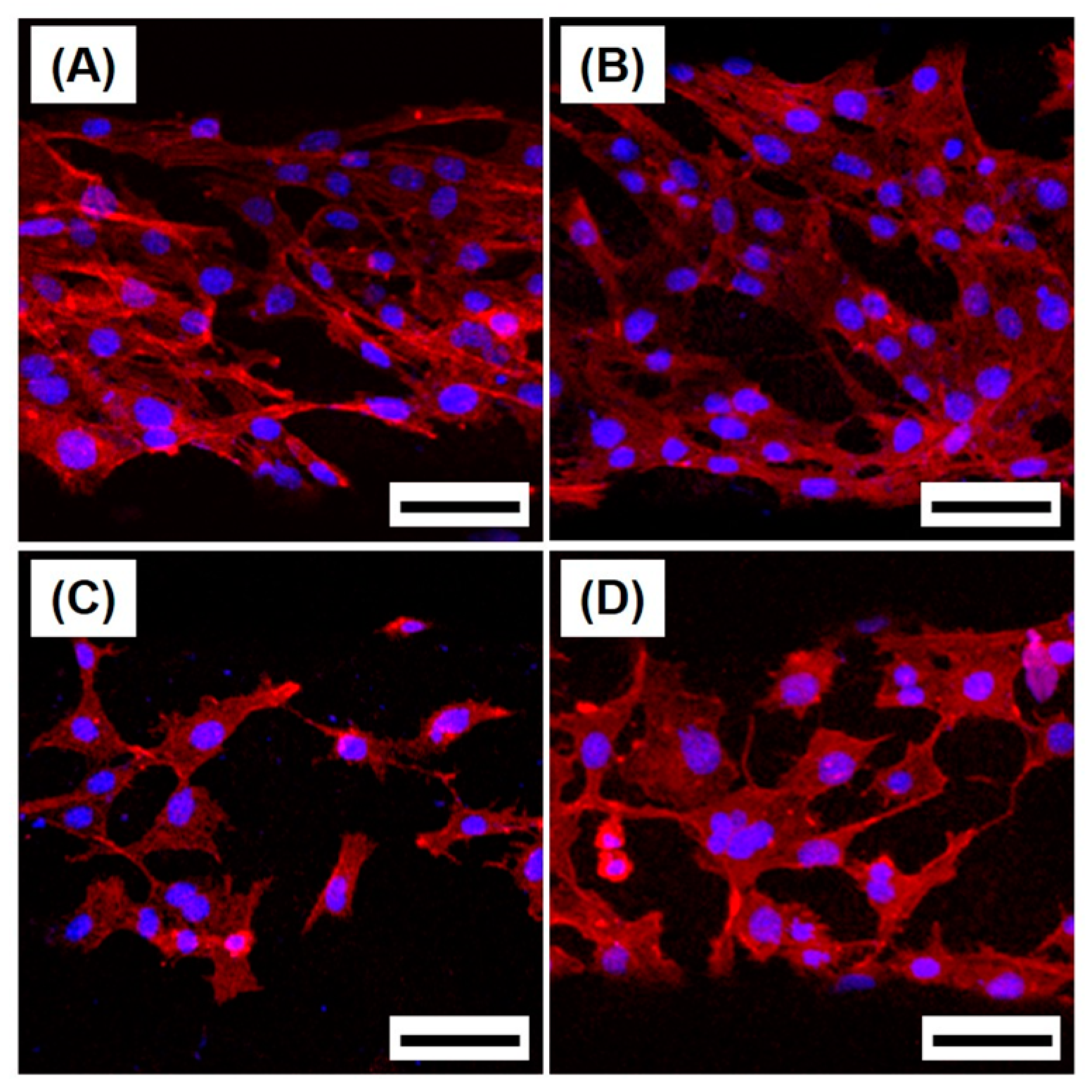
2.4. Cytocompatibility
2.5. In Vitro Apatite-Forming Ability
3. Materials and Methods
3.1. PCL/HA Composite Solutions Preparation
3.2. PCL/HA Composite Scaffolds Production Using 3D Plotting
3.3. Macro/Micro-Porous Structure Evaluation
3.4. TGA Analysis
3.5. Mechanical Properties Evaluation
3.6. In Vitro Cytocompatibility Evaluation
3.7. In Vitro Apatite-Forming Ability Evaluation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Akkineni, A.R.; Basu, B.; Gelinsky, M. Three-dimensional plotted hydroxyapatite scaffolds with predefined architecture: Comparison of stabilization by alginate cross-linking versus sintering. J. Biomater. Appl. 2016, 30, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandala, S.; Baruia, B.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basua, C. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Tapan, K.; Dash, V.; Konkimalla, B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Bowlin, G.L.; Jhurry, D. An assessment of biopolymer- and synthetic polymer-based scaffolds for bone and vascular tissue engineering. Polym. Int. 2013, 62, 523–533. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; Osman, N.A.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst. Eng. 2011, 34, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.H.; Jun, I.K.; Kim, H.E. Fabrication of poly(ε-caprolactone)/hydroxyapatite scaffold using rapid direct deposition. Mater. Lett. 2006, 60, 1184–1187. [Google Scholar] [CrossRef]
- Hong, S.J.; Jeong, I.; Noh, K.T.; Yu, H.S.; Lee, G.R.; Kim, H.W. Robotic dispensing of composite scaffolds and in vitro responses of bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2009, 20, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Dorj, B.; Won, J.E.; Kim, J.H.; Choi, S.J.; Shin, U.S.; Kim, H.W. Robocasting nanocomposite scaffolds of poly(caprolactone)/hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J. Biomed. Mater. Resour. A 2013, 101, 1670–1682. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, J.B.; Kim, Y.E.; Kim, J.E.; Lee, J.H.; Shin, J.W.; Kwon, I.K.; Kim, W.D. Fabrication of biomimetic PCL scaffold using rapid prototyping for bone tissue engineering. Macromol. Res. 2014, 22, 882–887. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Resour. A 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Dorj, B.; Park, J.H.; Kim, H.W. Robocasting chitosan/nanobioactive glass dual-pore structured scaffolds for bone engineering. Mater. Lett. 2012, 73, 119–122. [Google Scholar] [CrossRef]
- Shin, K.H.; Jo, I.H.; Kim, S.E.; Koh, Y.H.; Kim, H.E. Nonsolvent induced phase separation (NIPS)-based 3D plotting for 3-dimensionally macrochanneled poly(ε-caprolactone) scaffolds with highly porous frameworks. Mater. Lett. 2014, 122, 348–351. [Google Scholar] [CrossRef]
- Tamaddon, M.; Czernuszka, J.T. The need for hierarchical scaffolds in bone tissue engineering. Hard Tissue 2013, 2, 37. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Guillen, R.G.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Sotgiu, M.G.; Vinci, B.; Domenici, C.; Giusti, P. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(ε-caprolactone) blends for tissue engineering applications in the form of hollow fibers. J. Biomed. Mater. Resour. A 2008, 85, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; He, H.; Lee, L.J.; Ho, W.S.W. Synthesis and characterization of nanoporous polycaprolactone membranes via thermally- and nonsolvent-induced phase separations for biomedical device application. J. Membr. Sci. 2009, 343, 180–188. [Google Scholar] [CrossRef]
- Bettahalli, N.M.S.; Steg, H.; Wessling, M.; Stamatialis, D. Development of poly (l-lactic acid) hollow fiber membranes for artificial vasculature in tissue engineering scaffolds. J. Membr. Sci. 2011, 371, 117–126. [Google Scholar] [CrossRef]
- Diban, N.; Haimi, S.; Bolhuis-Versteeg, L.; Teixeira, S.; Miettinen, S.; Poot, A.; Grijpma, D.; Stamatialis, D. Development and characterization of poly(ε-caprolactone) hollow fiber membranes for vascular tissue engineering. J. Membr. Sci. 2013, 438, 29–37. [Google Scholar] [CrossRef]
- Lei, B.; Shin, K.H.; Noh, D.Y.; Jo, I.H.; Koh, Y.H.; Choi, W.Y.; Kim, H.E. Nanofibrous gelatin/silica hybrid scaffolds mimicking native extracellular matrix (ECM) using thermally induced phase separation. J. Mater. Chem. 2012, 22, 14133–14140. [Google Scholar] [CrossRef]
- Gloria, A.; De Santis, R.; Ambrosio, L. Polymer-based composite scaffolds for tissue engineering. J. Appl. Biomater. Biomech. 2010, 8, 57–67. [Google Scholar] [PubMed]
- Johnson, A.J.W.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Kaplan, D.L.; Zreiqat, H. Scaffold-based regeneration of skeletal tissues to meet clinical challenges. J. Mater. Chem. B 2014, 2, 7272–7306. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014, 12, 428–440. [Google Scholar] [CrossRef] [PubMed]

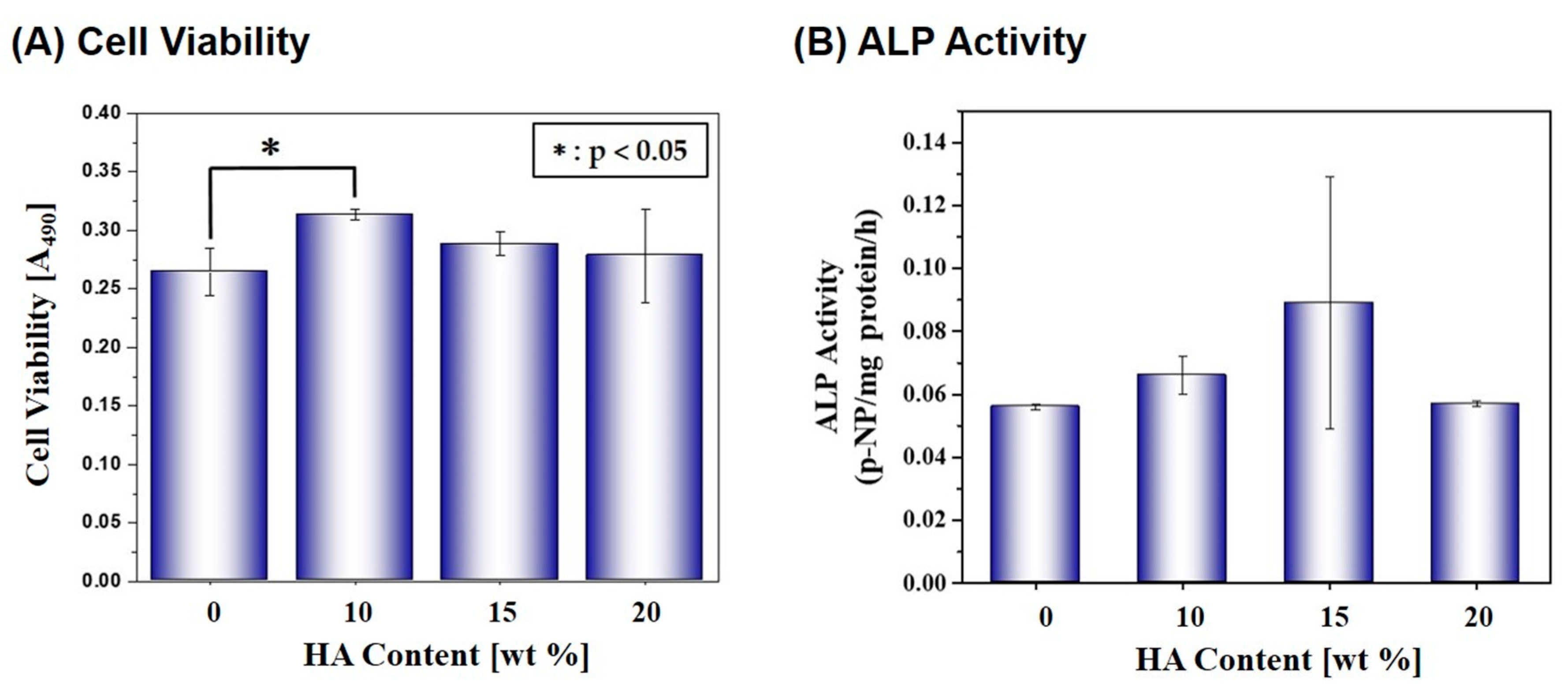
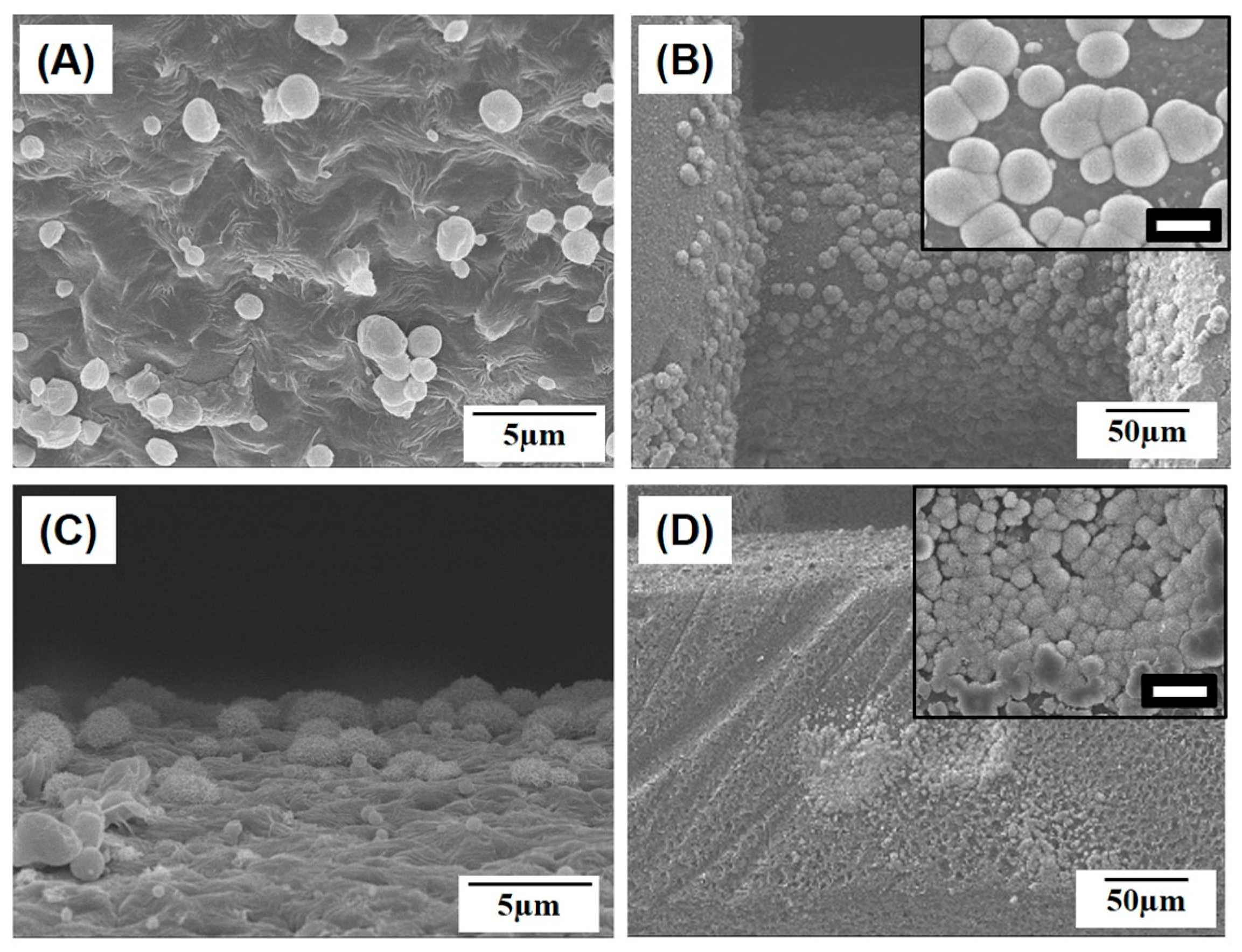
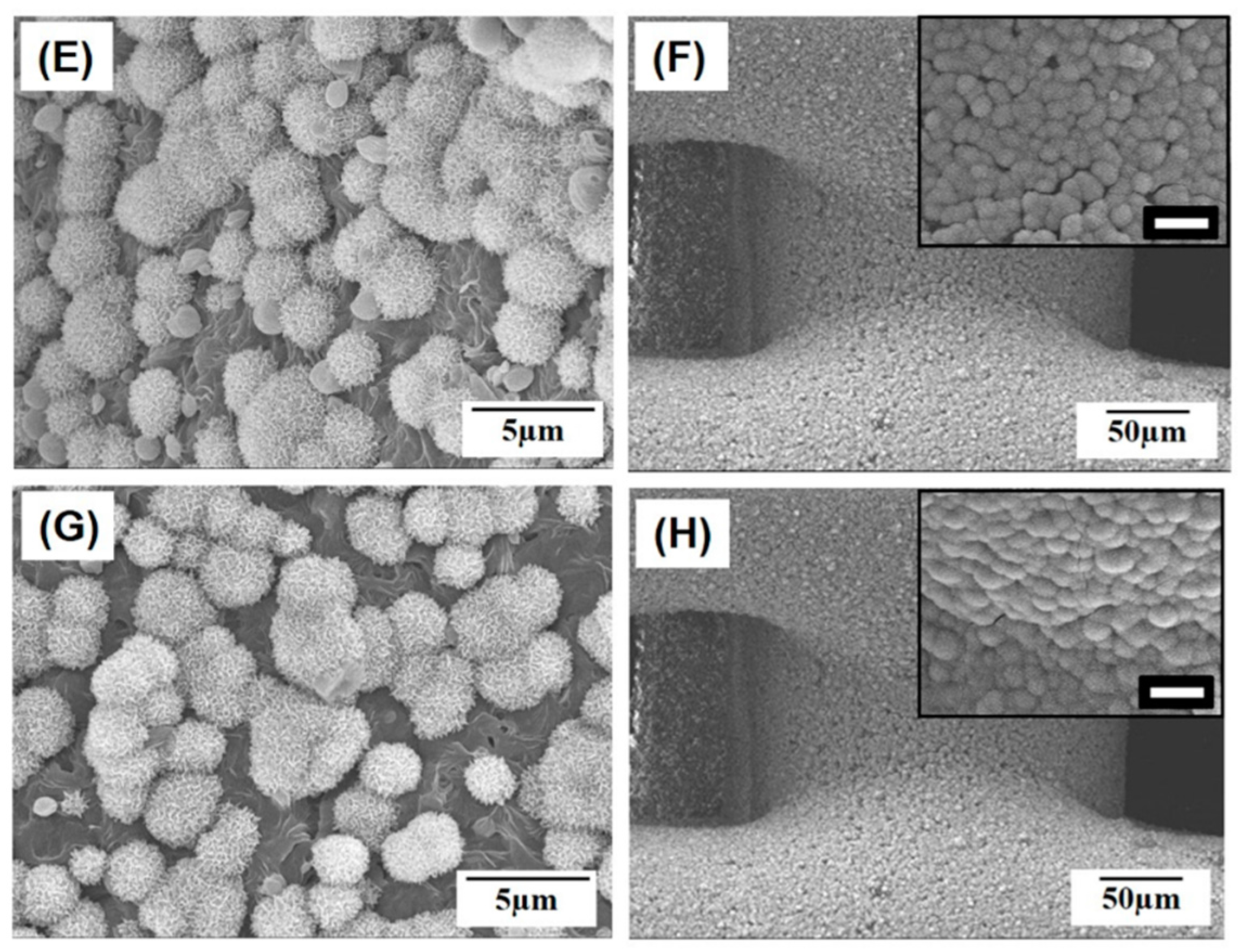
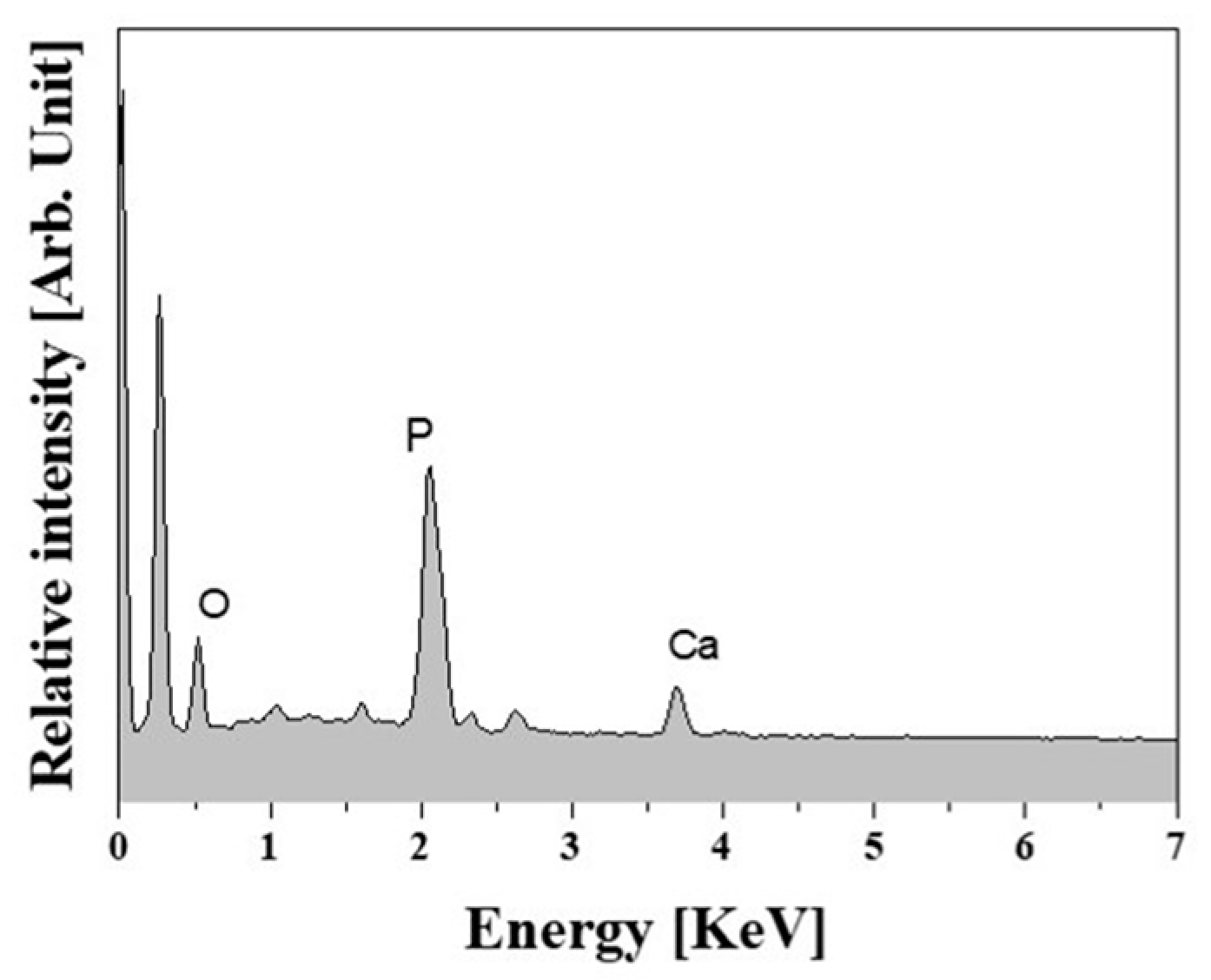
| HA Content [wt %] | 0 | 10 | 15 | 20 |
|---|---|---|---|---|
| Overall Porosity [vol %] | 78.4 ± 1.2 | 77.0 ± 3.5 | 77.9 ± 1.0 | 78.3 ± 1.6 |
| Diameter of Strut [µm] | 219 ± 16 | 270 ± 3 | 271 ± 21 | 273 ± 17 |
| Widths of Macropore [µm] | 248 ± 16 × 83 ± 18 | 184 ± 5 × 75 ± 12 | 183 ± 7 × 67 ± 9 | 184 ± 4 × 174 ± 6 |
| Size of Micropore [µm] | 2.8 ± 1.2 | 2.9 ± 1.1 | 2.3 ± 1.0 | 2.1 ± 0.8 |
| HA Content [wt %] | 0 | 10 | 15 | 20 |
|---|---|---|---|---|
| ALP Acivity [p-NP/mg protein/h] | 0.0245 ± 0.0025 | 0.0288 ± 0.002 | 0.0342 ± 0.003 | 0.0284 ± 0.005 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Shin, K.-H.; Koh, Y.-H.; Hah, M.J.; Moon, J.; Kim, H.-E. Production of Poly(ε-Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity. Materials 2017, 10, 1123. https://doi.org/10.3390/ma10101123
Kim J-W, Shin K-H, Koh Y-H, Hah MJ, Moon J, Kim H-E. Production of Poly(ε-Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity. Materials. 2017; 10(10):1123. https://doi.org/10.3390/ma10101123
Chicago/Turabian StyleKim, Jong-Woo, Kwan-Ha Shin, Young-Hag Koh, Min Jin Hah, Jiyoung Moon, and Hyoun-Ee Kim. 2017. "Production of Poly(ε-Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity" Materials 10, no. 10: 1123. https://doi.org/10.3390/ma10101123