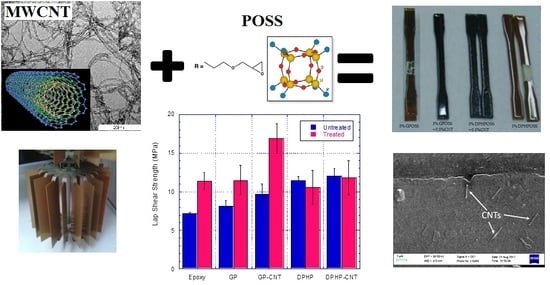
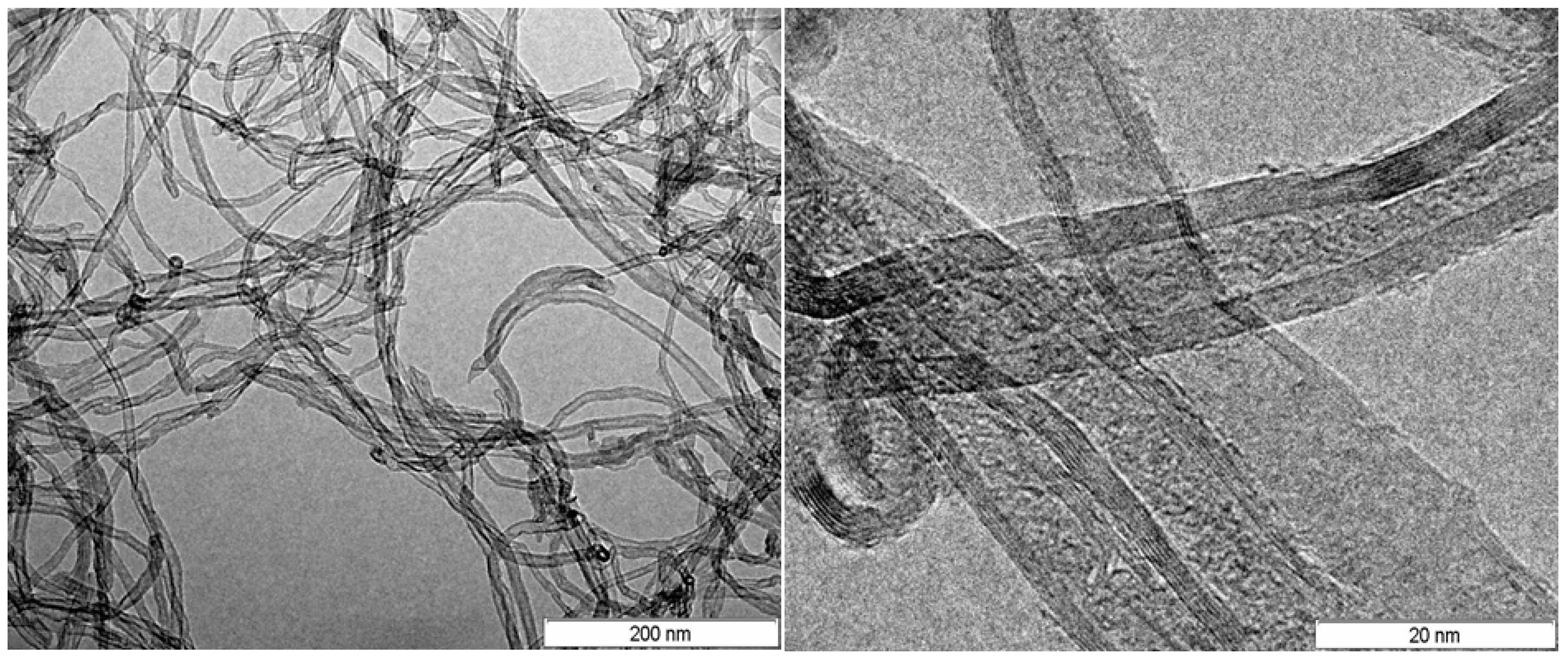
Toughening of Epoxy Adhesives by Combined Interaction of Carbon Nanotubes and Silsesquioxanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Adhesive Formulations
2.2. Adhesive Formulation Preparation
2.3. Adherends
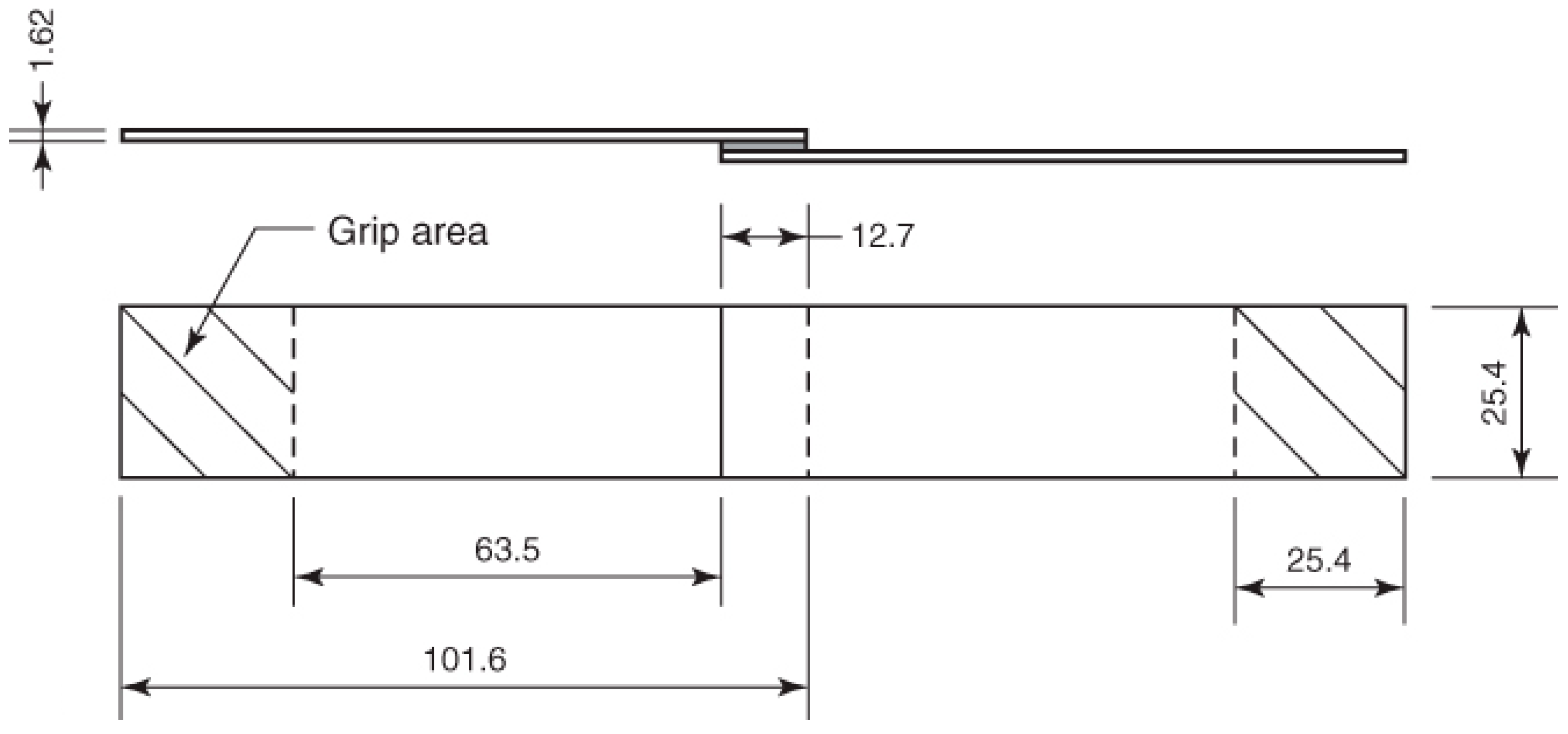
2.4. Preparation of the Adhesively Bonded Single Lap Joints
2.5. Experimental Studies
2.5.1. Characterization of the Uncured Adhesives
2.5.2. Characterization of Cured Adhesives
Tensile Test of Cured Adhesives
Dynamic Mechanical Tests
2.5.3. Characterization of the Joints
Single Lap Joint Shear Strength Tests
3. Results and Discussion
3.1. Viscosity Measurements
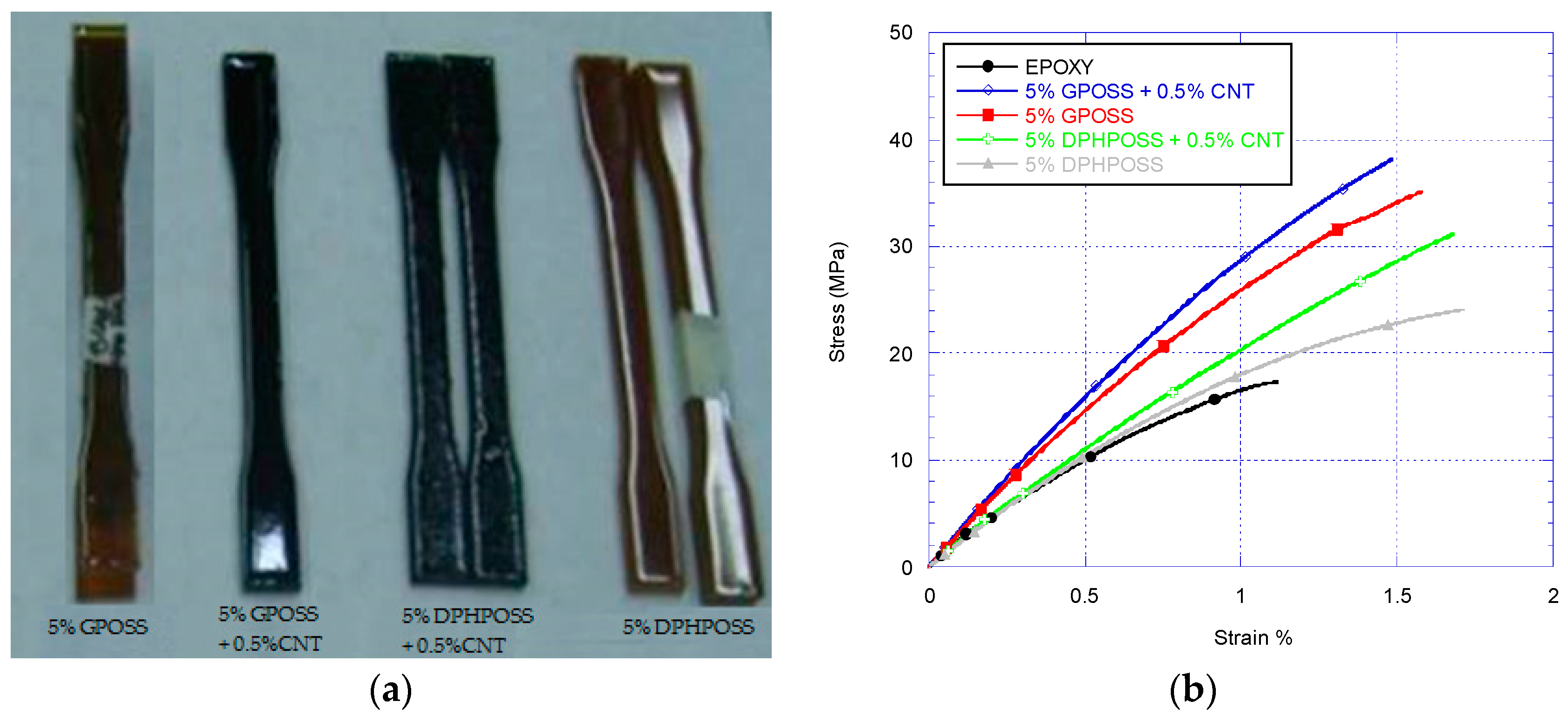
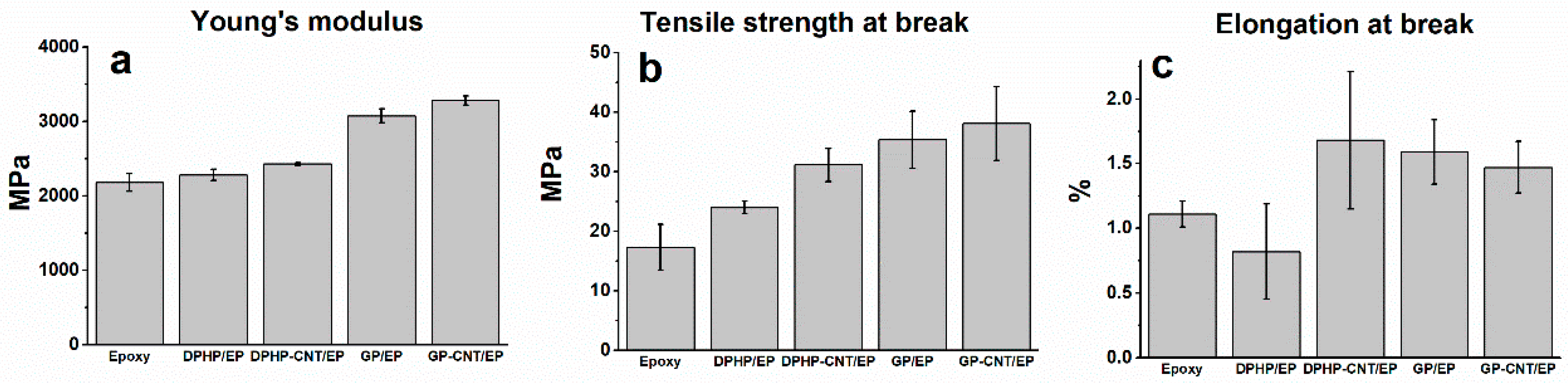
3.2. Tensile Tests
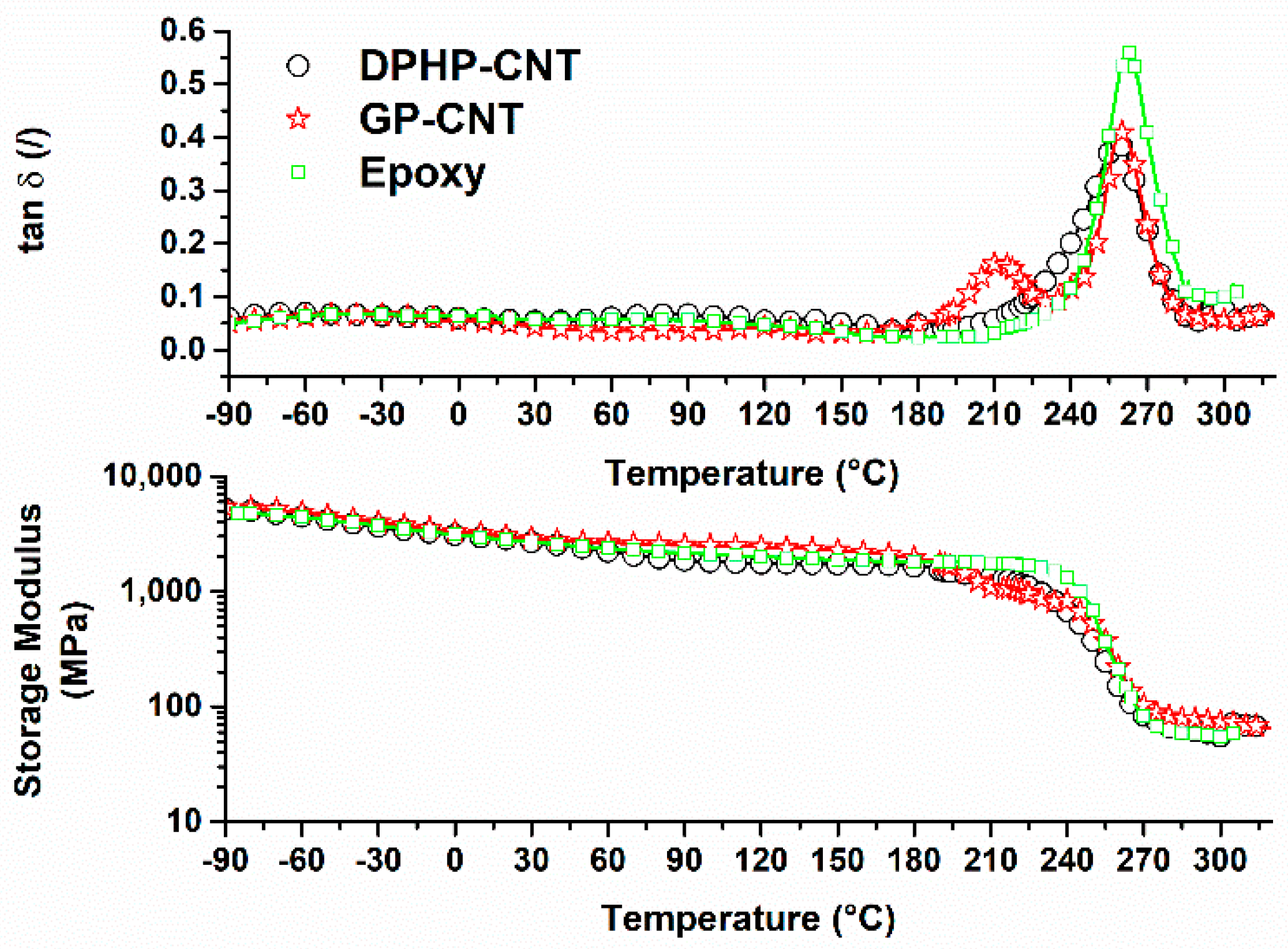
3.3. Dynamic Mechanical Tests
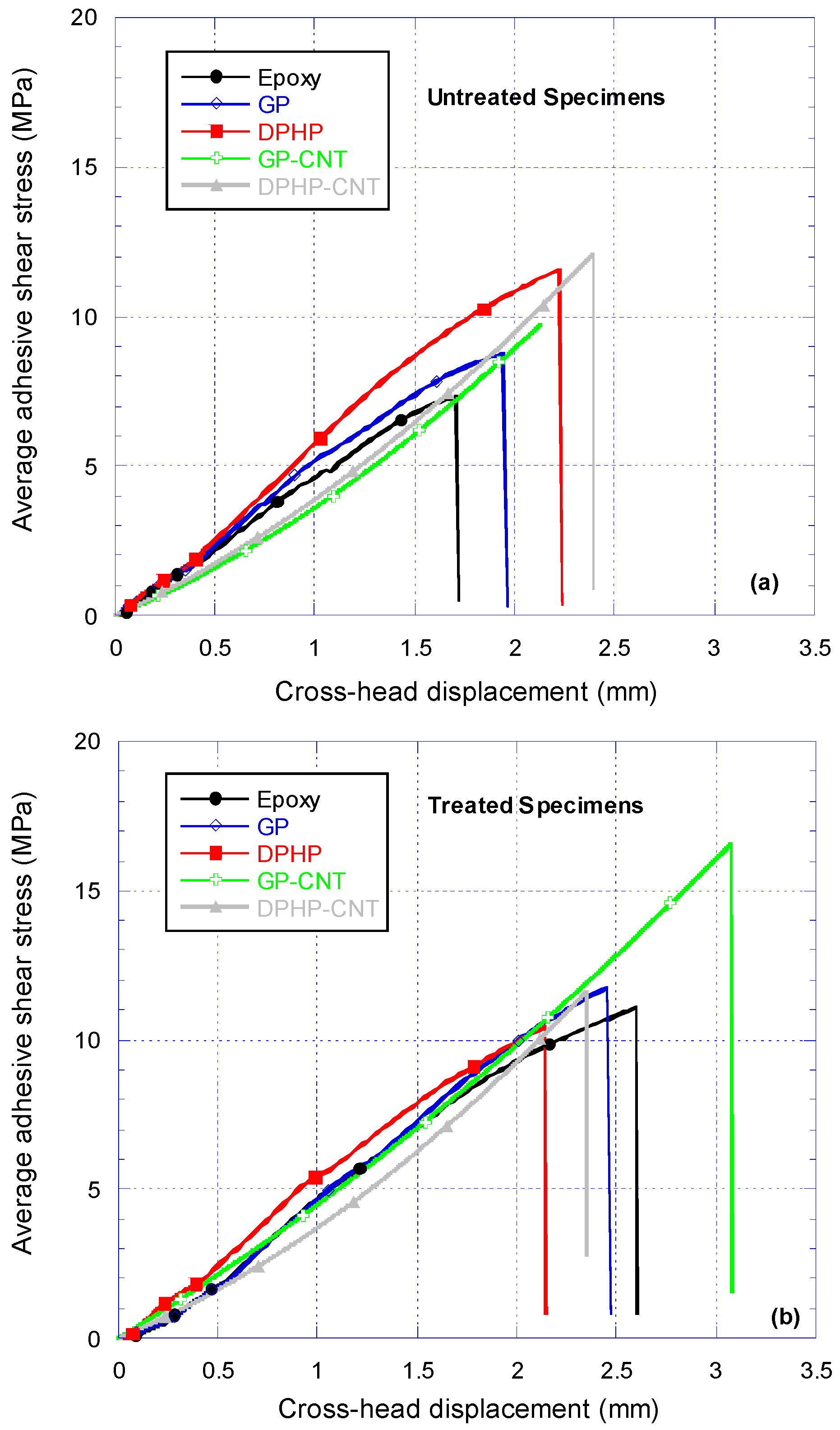
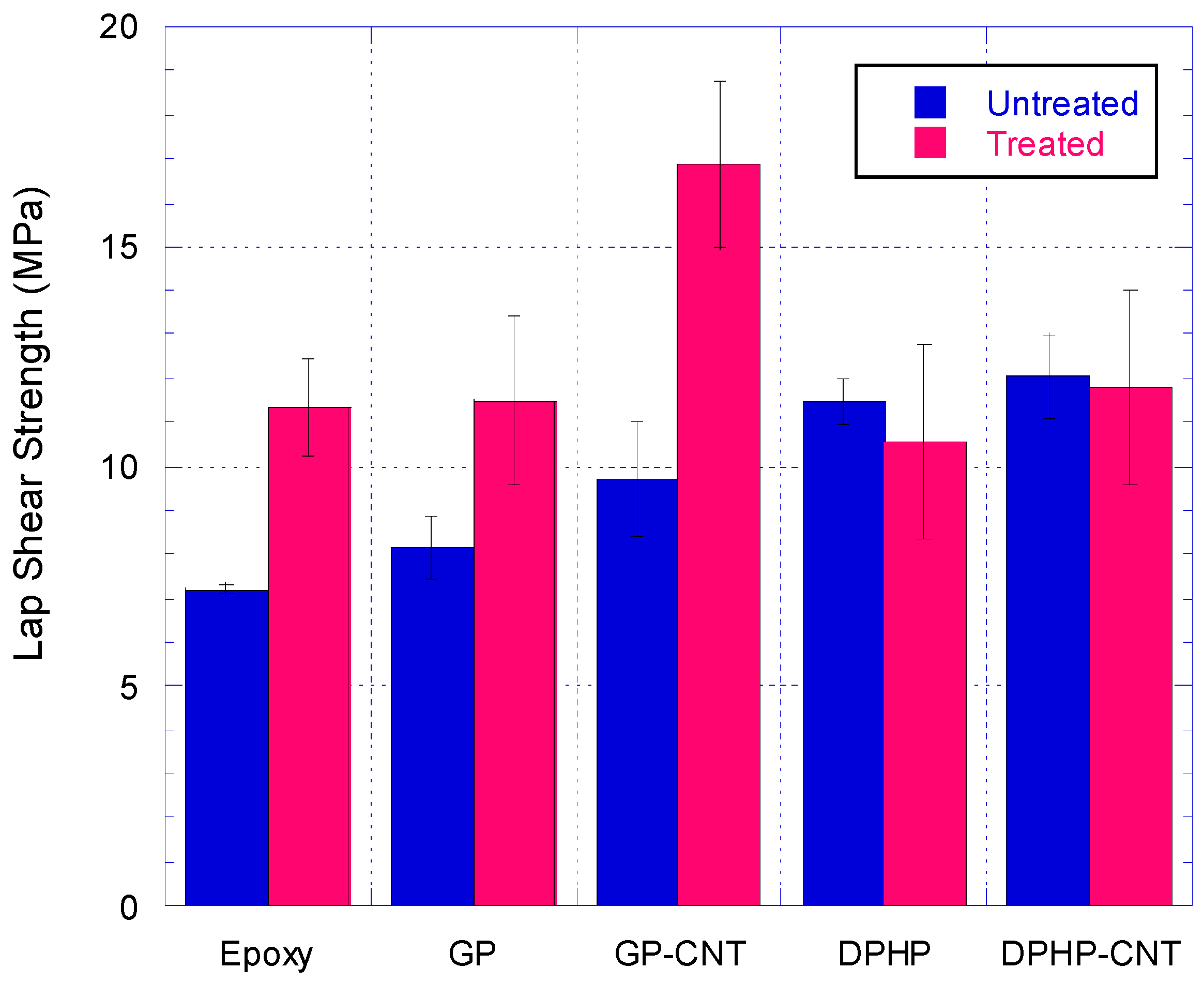
3.4. Single Lap Joint Shear Strength Tests
3.4.1. Discussion on Untreated Adherends SLJ Test Results
3.4.2. Discussion on Treated Adherends SLJ Test Results
3.4.3. Fractographic Analysis of Various Single Lap Joints
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brockmann, W.; Hennemann, O.-D.; Kollek, H.; Matz, C. Adhesion in bonded aluminium joints for aircraft construction. Int. J. Adhes. Adhes. 1986, 6, 115–143. [Google Scholar] [CrossRef]
- Bolger, J.C. Structural adhesives; today’s state of the art. In Adhesive Manufacturing; Schneberger, G.L., Ed.; Marcel Dekker: New York, NY, USA, 1983; p. 161. [Google Scholar]
- Pacchione, M.; Telgkamp, J. Challenges of the Metallic Fuselage. In Proceedings of the 25th Congress of the International Council of the Aeronautical Sciences (ICAS-Secretariat), Hamburg, Germany, 3–8 September 2006; pp. 2110–2121. [Google Scholar]
- Banea, M.D.; Da Silva, L.F.M. Adhesively bonded joints in composite materials: An overview. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2009, 223, 1–18. [Google Scholar] [CrossRef]
- Hsiao, K.T.; Alms, J.; Advani, S.G. Use of epoxy/multiwalled carbon nanotubes as adhesives to join graphite fibre reinforced polymer composites. Nanotechnology 2003, 14, 791–793. [Google Scholar] [CrossRef]
- Aldaş, K.; Sen, F. Stress analysis of hybrid joints of metal and composite plates via 3d-fem. Indian J. Eng. Mater. Sci. 2013, 20, 92–100. [Google Scholar]
- Qi, J.; Gao, L.; Liu, Y.; Liu, B.; Hashimoto, T.; Wang, Z.; Thompson, G.E. Chromate formed in a trivalent chromium conversion coating on aluminum. J. Electrochem. Soc. 2017, 164, C442–C449. [Google Scholar] [CrossRef]
- Anthony, J.I.A. New surface treatment for aluminum. J. Iron Age 1946, 158, 64–67. [Google Scholar]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Russo, S.; Iannuzzo, G. Effect of carbon nanotube and functionalized liquid rubber on mechanical and electrical properties of epoxy adhesives for aircraft structures. Compos. Part B Eng. 2017, 129, 1–10. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Naddeo, C.; Longo, P. Application of self-healing materials in aerospace engineering. In Self-Healing Polymers: From Principles to Applications; Binder, W.H., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 401–412. [Google Scholar]
- Michael, G.; Billias, M.E.B. Electrically Conductive Structural Adhesive; Luna Innovations: Roanoke, VA, USA, 1984. [Google Scholar]
- Gkikas, G.; Sioulas, D.; Lekatou, A.; Barkoula, N.M.; Paipetis, A.S. Enhanced bonded aircraft repair using nano-modified adhesives. Mater. Des. 2012, 41, 394–402. [Google Scholar] [CrossRef]
- Guadagno, L.; Sarno, M.; Vietri, U.; Raimondo, M.; Cirillo, C.; Ciambelli, P. Graphene-based structural adhesive to enhance adhesion performance. RSC Adv. 2015, 5, 27874–27886. [Google Scholar] [CrossRef]
- Vietri, U.; Guadagno, L.; Raimondo, M.; Vertuccio, L.; Lafdi, K. Nanofilled epoxy adhesive for structural aeronautic materials. Compos. Part B Eng. 2014, 61, 73–83. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Gude, M.R.; Ureña, A. Nanoreinforced adhesives. In Nanoreinforced Adhesives, Nanofibers; Kumar, A., Ed.; In-Tech: Rijeka, Croatia, 2010. [Google Scholar]
- Meguid, S.A.; Sun, Y. On the tensile and shear strength of nano-reinforced composite interfaces. Mater. Des. 2004, 25, 289–296. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Ashrafi, B.; Zhang, Y.; Martinez-Rubi, Y.; Kingston, C.T.; Johnston, A.; Simard, B. Single-walled carbon nanotube-epoxy composites for structural and conductive aerospace adhesives. Compos. Part B Eng. 2015, 69, 87–93. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Russo, S.; Lafdi, K.; Tucci, V.; Spinelli, G.; Lamberti, P. Influence of carbon nanoparticles/epoxy matrix interaction on mechanical, electrical and transport properties of structural advanced materials. Nanotechnology 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Faiella, G.; Piscitelli, F.; Lavorgna, M.; Antonucci, V.; Giordano, M. Tuning the insulator to conductor transition in a multiwalled carbon nanotubes/epoxy composite at substatistical percolation threshold. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Martone, A.; Formicola, C.; Piscitelli, F.; Lavorgna, M.; Zarrelli, M.; Antonucci, V.; Giordano, M. Thermo-mechanical characterization of epoxy nanocomposites with different carbon nanotube distributions obtained by solvent aided and direct mixing. Express Polym. Lett. 2012, 6, 520–531. [Google Scholar] [CrossRef]
- De Vivo, B.; Lamberti, P.; Tucci, V.; Guadagno, L.; Vertuccio, L.; Vittoria, V.; Sorrentino, A. Comparison of the physical properties of epoxy-based composites filled with different types of carbon nanotubes for aeronautic applications. Adv. Polym. Technol. 2012, 31, 205–218. [Google Scholar] [CrossRef]
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 2011, 49, 1919–1930. [Google Scholar] [CrossRef]
- Lee, A.; Lichtenhan, J.D. Viscoelastic responses of polyhedral oligosilsesquioxane reinforced epoxy systems. Macromolecules 1998, 31, 4970–4974. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mather, P.T. Poss polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Vittoria, V. Structural and morphological changes during uv irradiation of the trans-planar form of syndiotactic polypropylene. Polym. Degrad. Stab. 2008, 93, 176–187. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Naddeo, C.; Di Bartolomeo, A.; Lafdi, K. Influence of multiwall carbon nanotubes on morphological and structural changes during uv irradiation of syndiotactic polypropylene films. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 963–975. [Google Scholar] [CrossRef]
- Piscitelli, F.; Scamardella, A.M.; Romeo, V.; Lavorgna, M.; Barra, G.; Amendola, E. Epoxy composites based on amino-silylated mmt: The role of interfaces and clay morphology. J. Appl. Polym. Sci. 2012, 124, 616–628. [Google Scholar] [CrossRef]
- Szymańska, J.; Bakar, M.; Kostrzewa, M.; Lavorgna, M. Preparation and characterization of reactive liquid rubbers toughened epoxy-clay hybrid nanocomposites. J. Polym. Eng. 2016, 36, 43–52. [Google Scholar] [CrossRef]
- Kopesky, E.T.; Haddad, T.S.; Cohen, R.E.; McKinley, G.H. Thermomechanical properties of poly(methyl methacrylate)s containing tethered and untethered polyhedral oligomeric silsesquioxanes. Macromolecules 2004, 37, 8992–9004. [Google Scholar] [CrossRef]
- Chian, W.M.C.; Winter, R.M. Nano-Reinforced of Epoxy Adhesives with Poss; Nano Science and Technology Institute: Mohali, India, 2005; Vollume 2. [Google Scholar]
- Jones, I.K.; Zhou, Y.X.; Jeelani, S.; Mabry, J.M. Effect of polyhedral-oligomeric-sil-sesquioxanes on thermal and mechanical behavior of sc-15 epoxy. Express Polym. Lett. 2008, 2, 494–501. [Google Scholar] [CrossRef]
- Dodiuk, H.; Kenig, S.; Blinsky, I.; Dotan, A.; Buchman, A. Nanotailoring of epoxy adhesives by polyhedral-oligomeric-sil-sesquioxanes (poss). Int. J. Adhes. Adhes. 2005, 25, 211–218. [Google Scholar] [CrossRef]
- Khoramishad, H.; Khakzad, M. Toughening epoxy adhesives with multi-walled carbon nanotubes. J. Adhes. 2016, 1–15. [Google Scholar] [CrossRef]
- Barra, G.; De Nicola, F.; De Vivo, B.; Egiziano, L.; Guadagno, L.; Lamberti, P.; Raimondo, M.; Spinelli, G.; Tucci, V.; Vertuccio, L.; et al. Enhanced electrical properties of carbon fiber reinforced composites obtained by an effective infusion process. In Proceedings of the IEEE 9th Nanotechnology Materials and Devices Conference (NMDC), Sicily, Italy, 12–15 October 2014; pp. 88–91. [Google Scholar]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Naddeo, C.; Vittoria, V.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Electrical and dynamic mechanical properties of mwcnts/epoxy composite for high performance aerospace applications. In Proceedings of the 15th European Conference on Composite Materials (ECCM), Venice, Italy, 24–28 June 2012. [Google Scholar]
- Guadagno, L.; Vietri, U.; Raimondo, M.; Vertuccio, L.; Barra, G.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V.; De Nicola, F.; et al. Correlation between electrical conductivity and manufacturing processes of nanofilled carbon fiber reinforced composites. Compos. Part B Eng. 2015, 80, 7–14. [Google Scholar] [CrossRef]
- Raimondo, M.; Russo, S.; Guadagno, L.; Longo, P.; Chirico, S.; Mariconda, A.; Bonnaud, L.; Murariu, O.; Dubois, P. Effect of incorporation of poss compounds and phosphorous hardeners on thermal and fire resistance of nanofilled aeronautic resins. RSC Adv. 2015, 5, 10974–10986. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. Part B Eng. 2017, 128, 30–38. [Google Scholar] [CrossRef]
- Kashiwagi, T. Progress in flammability studies of nanocomposites with new types of nanoparticles. In Flame Retardant Polymer Nanocomposites; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 285–324. [Google Scholar]
- Guadagno, L.; Raimondo, M.; Vietri, U.; Vertuccio, L.; Barra, G.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V.; Volponi, R.; et al. Effective formulation and processing of nanofilled carbon fiber reinforced composites. RSC Adv. 2015, 5, 6033–6042. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Lafdi, K.; Fierro, A.; Rosolia, S.; Nobile, M.R. Influence of nanofiller morphology on the viscoelastic properties of cnf/epoxy resins. AIP Conf. Proc. 2014, 386, 386–389. [Google Scholar]
- Barra, G.; Guadagno, L.; Simonet, B.; Santos, B. The influence of different dispersion methods on the size of the aggregate of cnts in epoxy resin for the manufacturing of carbon fiber reinforced composites. AIP Conf. Proc. 2016. [Google Scholar] [CrossRef]
- ASTM D1002–10. Standard test method for apparent shear strength of single-lap-joint adhesively bonded metal specimens by tension loading (metal-to-metal). In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM D638–14. Standard test method for tensile properties of plastics, astm international. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Kim, K.S.; Yoo, J.S.; Yi, Y.M.; Kim, C.G. Failure mode and strength of uni-directional composite single lap bonded joints with different bonding methods. Compos. Struct. 2006, 72, 477–485. [Google Scholar] [CrossRef]
- Buchman, A.; Dodiuk-Kenig, H.; Dotan, A.; Tenne, R.; Kenig, S. Toughening of epoxy adhesives by nanoparticles. J. Adhes. Sci. Technol. 2009, 23, 753–768. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, L.; Toghiani, H.; Daulton, T.L.; Koyama, K.; Pittman, C.U., Jr. Viscoelastic and mechanical properties of epoxy/multifunctional polyhedral oligomeric silsesquioxane nanocomposites and epoxy/ladderlike polyphenylsilsesquioxane blends. Macromolecules 2001, 34, 8686–8693. [Google Scholar] [CrossRef]
- Weickmann, H.; Delto, R.; Thomann, R.; Brenn, R.; Döll, W.; Mülhaupt, R. Pmma nanocomposites and gradient materials prepared by means of polysilsesquioxane (poss) self-assembly. J. Mater. Sci. 2007, 42, 87–92. [Google Scholar] [CrossRef]
- Critchlow, G. Surface treatments for moisture resistance. In Design of Adhesive Joints under Humid Conditions; Lucas, F.M., da Silva, C.S., Eds.; Springer: Heidelberg, Germany, 2013; p. 62. [Google Scholar]
- Critchlow, G.W.; Yendall, K.A.; Bahrani, D.; Quinn, A.; Andrews, F. Strategies for the replacement of chromic acid anodising for the structural bonding of aluminium alloys. Int. J. Adhes. Adhes. 2006, 26, 419–453. [Google Scholar] [CrossRef]
- Abrahami, S.T.; de Kok, J.M.; Gudla, V.C.; Ambat, R.; Terryn, H.; Mol, J.M. Interface strength and degradation of adhesively bonded porous aluminum oxides. NPJ Mater. Degrad. 2017, 1. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Little, M.S.G.; Watts, J.F. Role of the interphase in the environmental failure of adhesive joints. Acta Mater. 2000, 48, 4543–4553. [Google Scholar] [CrossRef]


| Acronym | Configuration | Characteristics |
|---|---|---|
| Glycidyl Oligomeric Silsesquioxanes (GPOSS) | 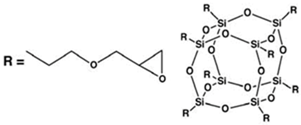 | (1) Appearance: viscous liquid |
| (2) Color: colorless to slightly yellow | ||
| (3) Molecular/chemical formula: (C6H11O2)n(SiO1.5)n n = 8, 10, 12 | ||
| (4) Molecular weight: 1337.88 FW | ||
| DodecaPhenyl Oligomeric Silsesquioxanes (DPHPOSS) |  | (1) Appearance: powder |
| (2) Color: white | ||
| (3) Molecular/chemical formula: C72H60O18Si12 | ||
| (4) Molecular weight: 1550.26 FW |
| Adhesive Composition | Epoxy Blend wt % | MWCNT wt % | GPOSS wt % | DPHPOSS wt % |
|---|---|---|---|---|
| Epoxy | 100 | - | - | - |
| CNT/EP | 99.5 | 0.5 | - | - |
| GP/EP | 95 | - | 5 | - |
| GP-CNT/EP | 94.5 | 0.5 | 5 | - |
| DPHP/EP | 95 | - | - | 5 |
| DPHP-CNT/EP | 94.5 | 0.5 | - | 5 |
| Sample Label | Adherend | Adhesive Composition | Adhesive Thickness [mm] |
|---|---|---|---|
| Al-Epoxy | Aluminium | Epoxy | 0.16 ± 0.02 |
| Tr-Al-Epoxy | Treated aluminium | Epoxy | 0.18 ± 0.03 |
| Al-GP | Aluminium | GP/EP | 0.20 ± 0.01 |
| Tr-Al-GP | Treated aluminium | GP/EP | 0.21 ± 0.01 |
| Al-DPHP | Aluminium | DPHP/EP | 0.19 ± 0.01 |
| Tr-Al-DPHP | Treated aluminium | DPHP/EP | 0.20 ± 0.01 |
| Al-GP-CNT | Aluminium | GP-CNT/EP | 0.23 ± 0.05 |
| Tr-Al-GP-CNT | Treated aluminium | GP-CNT/EP | 0.22 ± 0.02 |
| Al-DPHP-CNT | Aluminium | DPHP-CNT/EP | 0.25 ± 0.05 |
| Tr-Al-DPHP-CNT | Treated aluminium | DPHP-CNT/EP | 0.25 ± 0.03 |
| Temperature (°C) | Epoxy η (Pa·s) | CNT/EP η (Pa·s) | GP-CNT/EP η (Pa·s) | DPH-CNT/EP η (Pa·s) |
|---|---|---|---|---|
| 80 | 0.40 | 7.00 | 3.11 | 7.5 |
| 90 | 0.11 | 5.00 | 1.34 | 5.6 |
| 100 | 0.10 | 3.50 | 0.65 | 4.9 |
| 110 | 0.07 | 2.10 | 0.35 | 5.0 * |
| 120 | 0.05 | 3.05 * | 0.22 | 6.9 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra, G.; Vertuccio, L.; Vietri, U.; Naddeo, C.; Hadavinia, H.; Guadagno, L. Toughening of Epoxy Adhesives by Combined Interaction of Carbon Nanotubes and Silsesquioxanes. Materials 2017, 10, 1131. https://doi.org/10.3390/ma10101131
Barra G, Vertuccio L, Vietri U, Naddeo C, Hadavinia H, Guadagno L. Toughening of Epoxy Adhesives by Combined Interaction of Carbon Nanotubes and Silsesquioxanes. Materials. 2017; 10(10):1131. https://doi.org/10.3390/ma10101131
Chicago/Turabian StyleBarra, Giuseppina, Luigi Vertuccio, Umberto Vietri, Carlo Naddeo, Homayoun Hadavinia, and Liberata Guadagno. 2017. "Toughening of Epoxy Adhesives by Combined Interaction of Carbon Nanotubes and Silsesquioxanes" Materials 10, no. 10: 1131. https://doi.org/10.3390/ma10101131