Biocompatibility and Biocorrosion of Hydroxyapatite-Coated Magnesium Plate: Animal Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
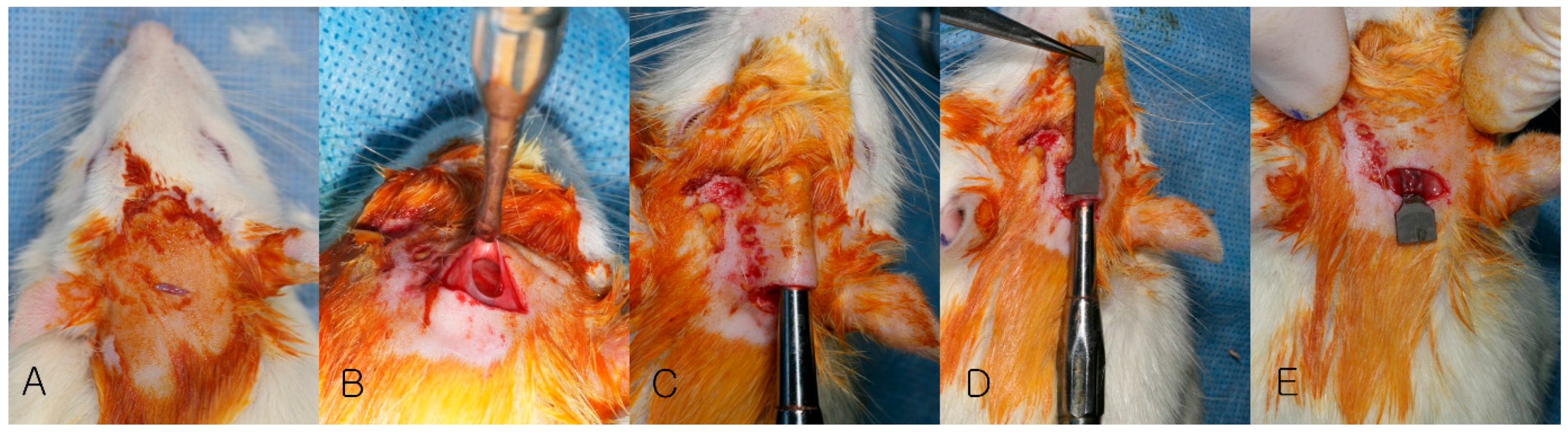
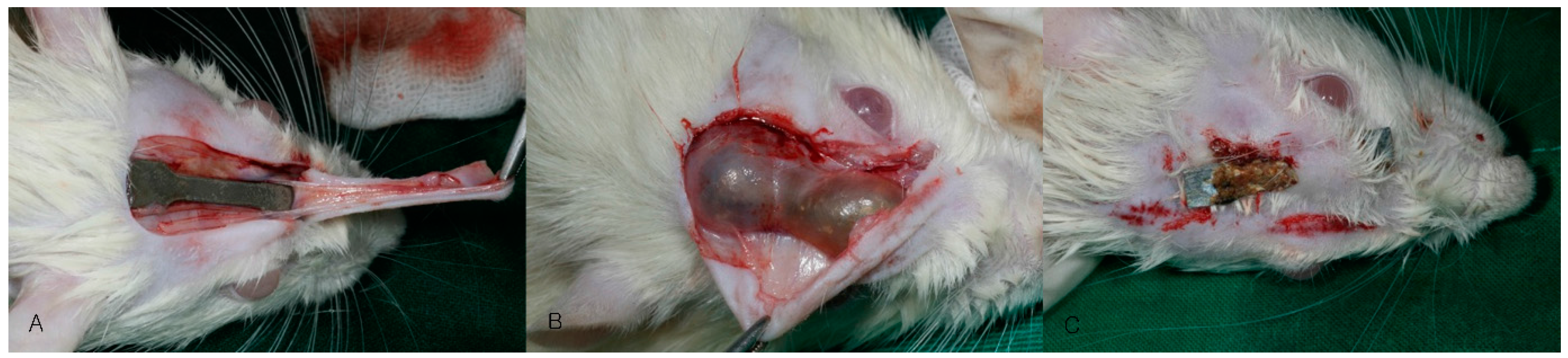
2.2. Animal Surgery
2.3. Evaluation
2.3.1. Clinical Evaluation
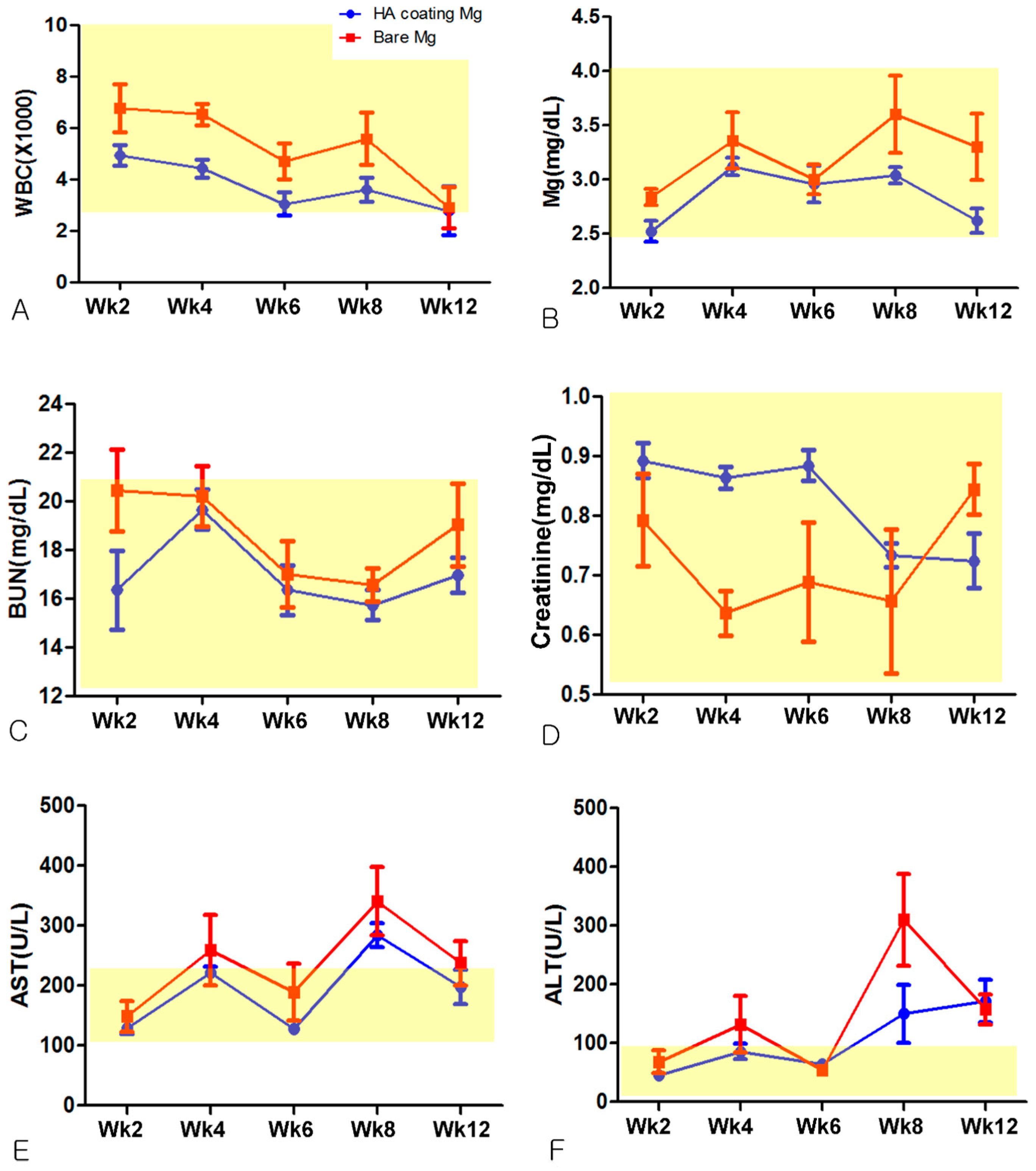
2.3.2. Hematological Evaluation
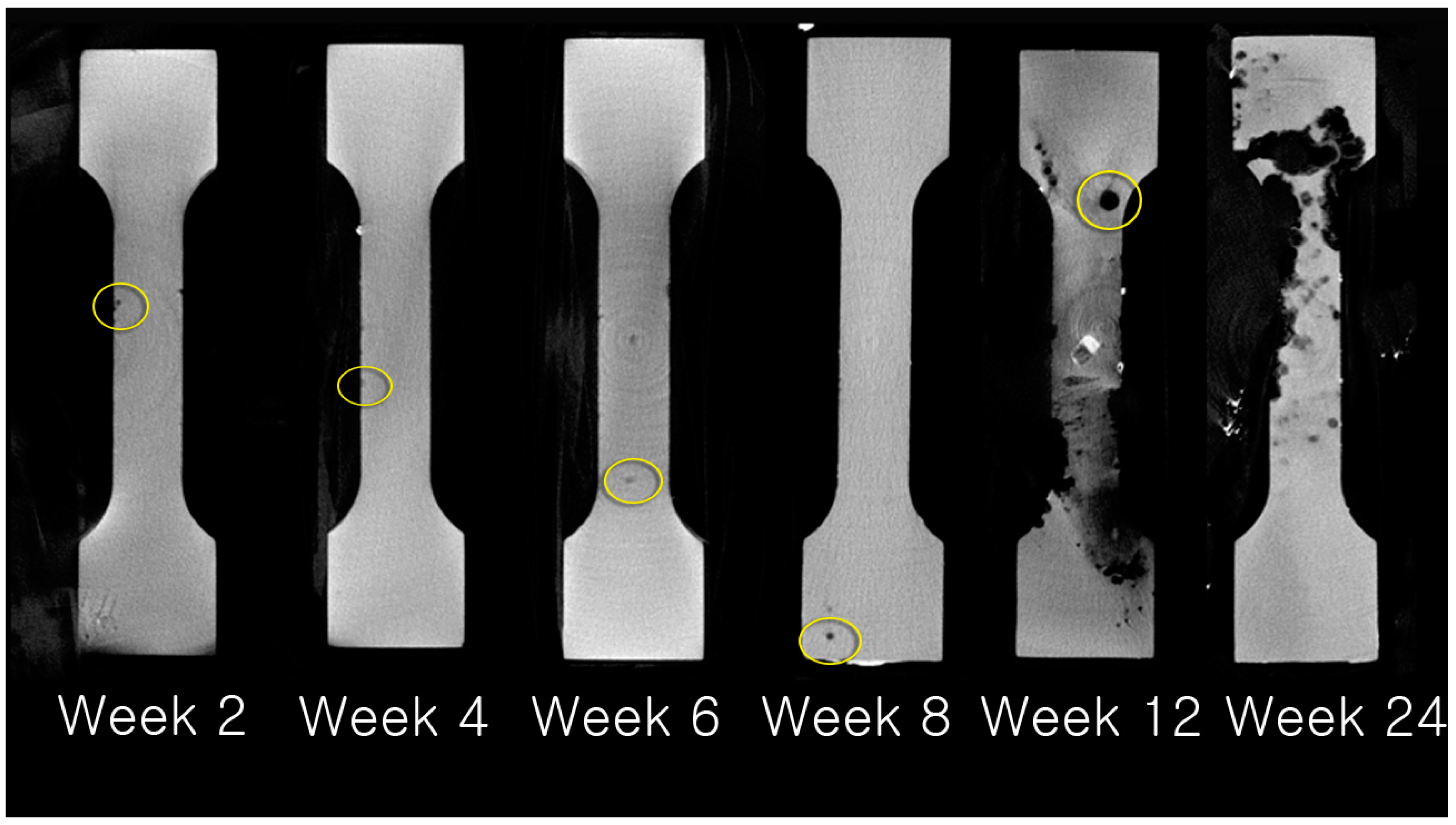
2.3.3. Evaluation of Absorption Rate using µCT
2.3.4. Change of Mechanical Strength
2.3.5. Statistical Analysis
3. Results
3.1. Clinical Evaluation
3.2. Hematological Evaluation
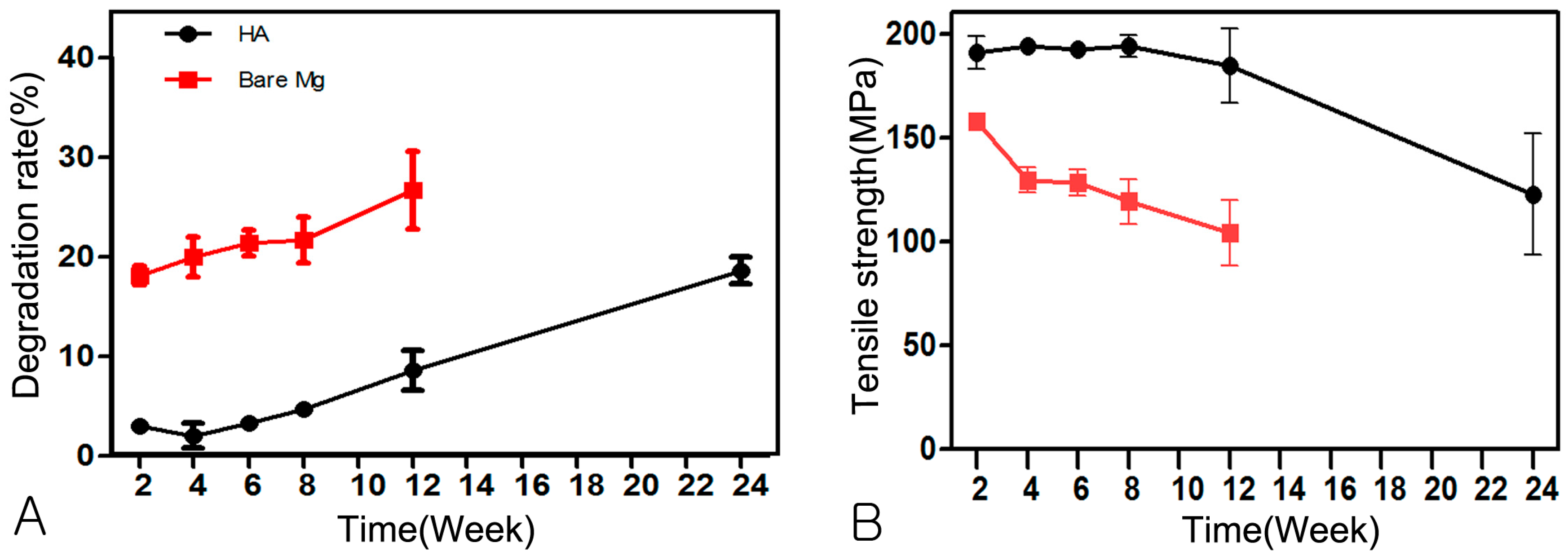
3.3. Evaluation of Absorption Rate Using µCT
3.4. Change of Mechanical Strength
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilhan, H.; Bural, C.; Geckili, O. Titanium hypersensitivity. A hidden threat for dental implant patients? N. Y. State Dent. J. 2013, 79, 38–43. [Google Scholar] [PubMed]
- Dolanmaz, D.; Uckan, S.; Isik, K.; Saglam, H. Comparison of stability of absorbable and titanium plate and screw fixation for sagittal split ramus osteotomy. Br. J. Oral Maxillofac. Surg. 2004, 42, 127–132. [Google Scholar] [CrossRef]
- Edwards, R.C.; Kiely, K.D.; Eppley, B.L. The fate of resorbable poly-l-lactic/polyglycolic acid (LactoSorb) bone fixation devices in orthognathic surgery. J. Oral Maxillofac. Surg. 2001, 59, 19–25. [Google Scholar] [CrossRef] [PubMed]
- McBane, J.E.; Battiston, K.G.; Wadhwani, A.; Sharifpoor, S.; Labow, R.S.; Santerre, J.P. The effect of degradable polymer surfaces on co-cultures of monocytes and smooth muscle cells. Biomaterials 2011, 32, 3584–3595. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jo, J.H.; Lee, S.M.; Kang, M.H.; Kim, H.E.; Estrin, Y.; Lee, J.H.; Lee, J.W.; Koh, Y.H. Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. A 2014, 102, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, S.; Shishido, T.; Yamamoto, A.; Maruyama, N.; Somekawa, H.; Mukai, T. Precipitation control of calcium phosphate on pure magnesium by anodization. Corros. Sci. 2008, 50, 2906–2913. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Salih, V.; Knowles, J.C. Sol-gel-modified titanium with hydroxyapatite thin films and effect on osteoblast-like cell responses. J. Biomed. Mater. Res. A 2005, 74, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Kim, I.S.; Kim, H.; Chou, A.H.K.; Hahn, B.D.; Li, L.H.; Hwang, S.J. Improved biocompatibility of hydroxyapatite thin film prepared by aerosol deposition. J. Biomed. Mater. Res. B 2010, 94, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.W.; Kwon, T.Y.; Yang, Y.Z.; Ong, J.L.; Kim, K.H. Effects of applied voltages on hydroxyapatite coating of titanium by electrophoretic deposition. J. Biomed. Mater. Res. B 2006, 78, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, S.H.; Kim, H.W.; Kong, Y.M.; Kim, H.E. Fluoridated apatite coatings on titanium obtained by electron-beam deposition. Biomaterials 2005, 26, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Muraleedharan, C.V.; Komath, M.; Varma, H. Pulsed laser deposition of hydroxyapatite on titanium substrate with titania interlayer. J. Mater. Sci. Mater. Med. 2011, 22, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Byun, S.H.; Lee, J.Y.; Lee, J.W.; Kim, S.M.; Lee, S.M.; Kim, H.E.; Lee, J.H. Radiological, histological, and hematological evaluation of hydroxyapatite-coated resorbable magnesium alloy screws placed in rabbit tibia. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 105, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.L.; Chen, H.Y.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Fischerauer, S.F.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hanzi, A.C.; Loffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo degradation behaviour and biocompatibility of the magnesium alloy zek100 for use as a biodegradable bone implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Youn, S.; Kim, J.T. Early complete bone union after condylar fracture in a child. J. Craniofac Surg. 2011, 22, 1516–1517. [Google Scholar] [CrossRef] [PubMed]

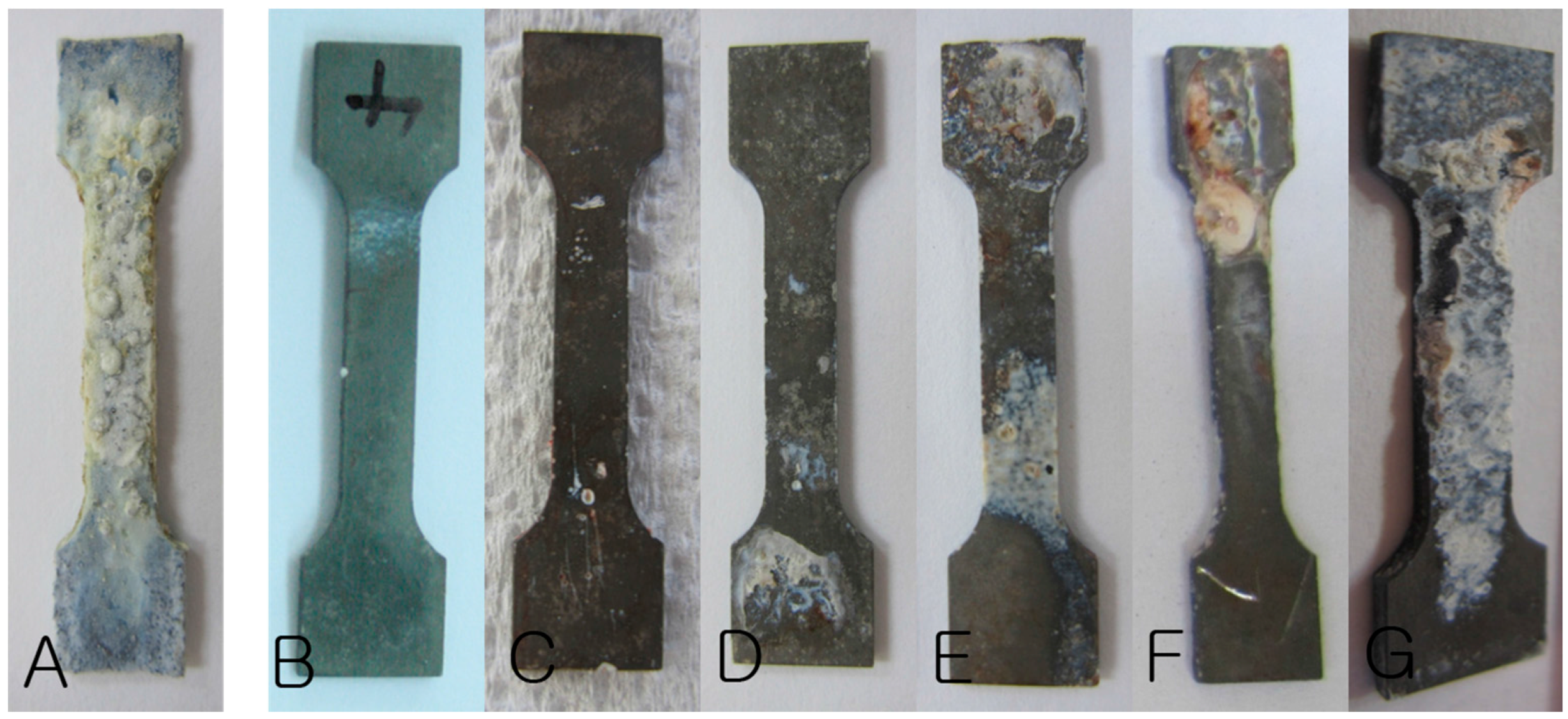
| Time | Hydrogen Gas Formation | Plate Exposure | ||
|---|---|---|---|---|
| HA-Mg | Bare-Mg | HA-Mg | Bare-Mg | |
| Week 2 | 0 | 4 | 0 | 4 |
| Week 4 | 0 | 3 | 0 | 2 |
| Week 6 | 0 | 2 | 0 | 3 |
| Week 8 | 0 | 4 | 0 | 1 |
| Week 12 | 0 | 4 | 0 | 3 |
| Week 24 | 2 | - | 0 | - |
| Time | Week 2 | Week 4 | Week 6 | Week 8 | Week 12 | Week 24 |
|---|---|---|---|---|---|---|
| HA-Mg | 2.99 ± 0.59 | 2.01 ± 2.77 | 3.33 ± 1.25 | 4.71 ± 1.49 | 8.58 ± 4.57 | 18.35 ± 3.03 |
| Bare-Mg | 18.10 ± 2.12 * | 20.00 ± 4.47 * | 21.40 ± 2.96 * | 21.71 ± 5.20 * | 26.70 ± 8.72 * | - |
| Time | Week 2 | Week 4 | Week 6 | Week 8 | Week 12 | Week 24 |
|---|---|---|---|---|---|---|
| HA-Mg | 190.10 ± 17.46 | 193.89 ± 5.19 | 192.16 ± 7.82 | 193.77 ± 11.77 | 184.58 ± 35.69 | 122.78 ± 65.07 |
| Bare-Mg | 157.94 ± 6.18 * | 129.65 ± 13.70 * | 128.40 ± 14.58 * | 119.39 ± 24.15 * | 104.22 ± 31.17 * | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-K.; Byun, S.-H.; Woo, J.-M.; Kim, S.-M.; Lee, S.-M.; Kim, B.-J.; Kim, H.-E.; Lee, J.-W.; Kim, S.-M.; Lee, J.-H. Biocompatibility and Biocorrosion of Hydroxyapatite-Coated Magnesium Plate: Animal Experiment. Materials 2017, 10, 1149. https://doi.org/10.3390/ma10101149
Lim H-K, Byun S-H, Woo J-M, Kim S-M, Lee S-M, Kim B-J, Kim H-E, Lee J-W, Kim S-M, Lee J-H. Biocompatibility and Biocorrosion of Hydroxyapatite-Coated Magnesium Plate: Animal Experiment. Materials. 2017; 10(10):1149. https://doi.org/10.3390/ma10101149
Chicago/Turabian StyleLim, Ho-Kyung, Soo-Hwan Byun, Jae-Man Woo, Sae-Mi Kim, Sung-Mi Lee, Bong-Ju Kim, Hyoun-Ee Kim, Jung-Woo Lee, Soung-Min Kim, and Jong-Ho Lee. 2017. "Biocompatibility and Biocorrosion of Hydroxyapatite-Coated Magnesium Plate: Animal Experiment" Materials 10, no. 10: 1149. https://doi.org/10.3390/ma10101149





