Hydrogen Separation by Natural Zeolite Composite Membranes: Single and Multicomponent Gas Transport
Abstract
:1. Introduction
2. Results and Discussion
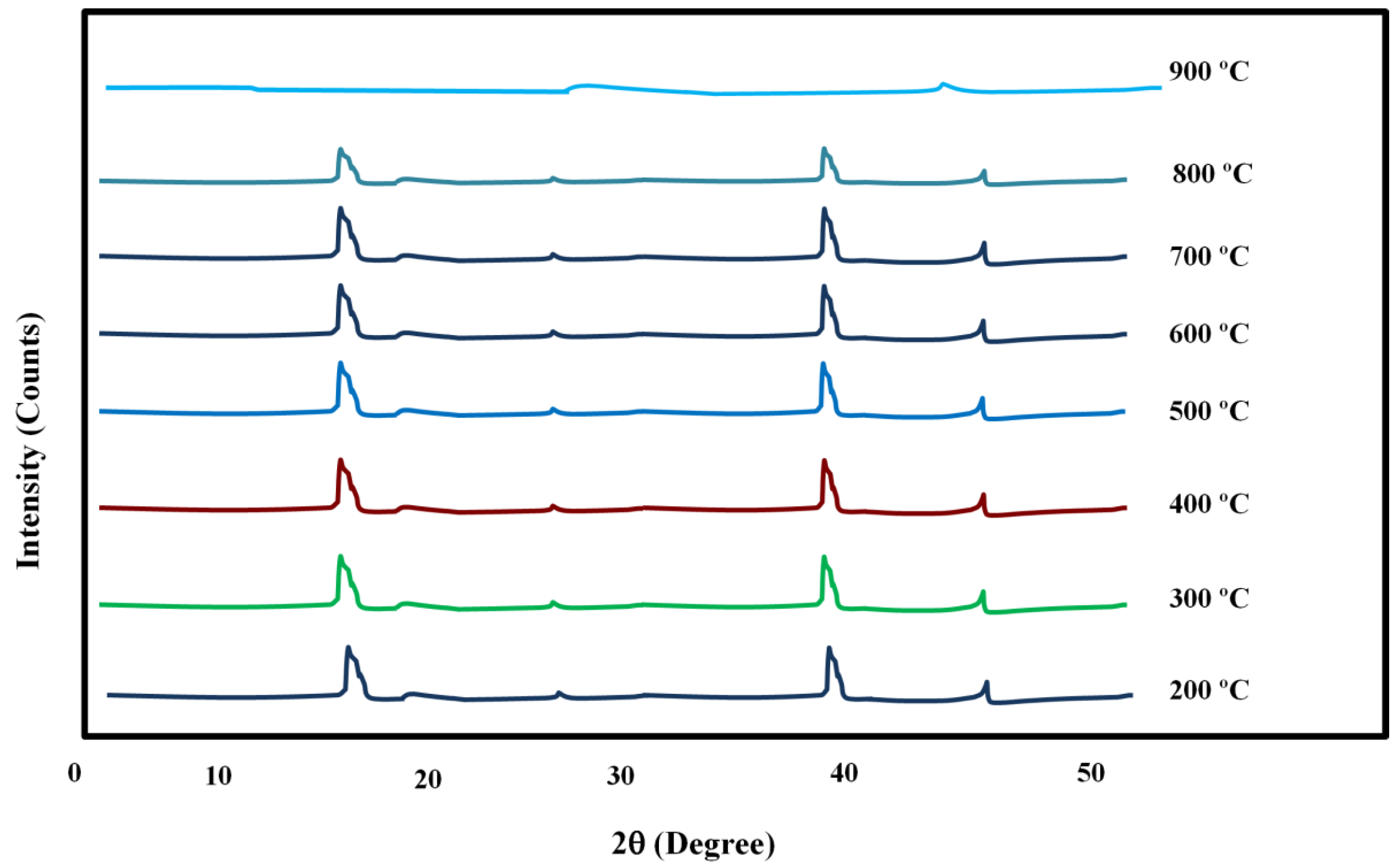
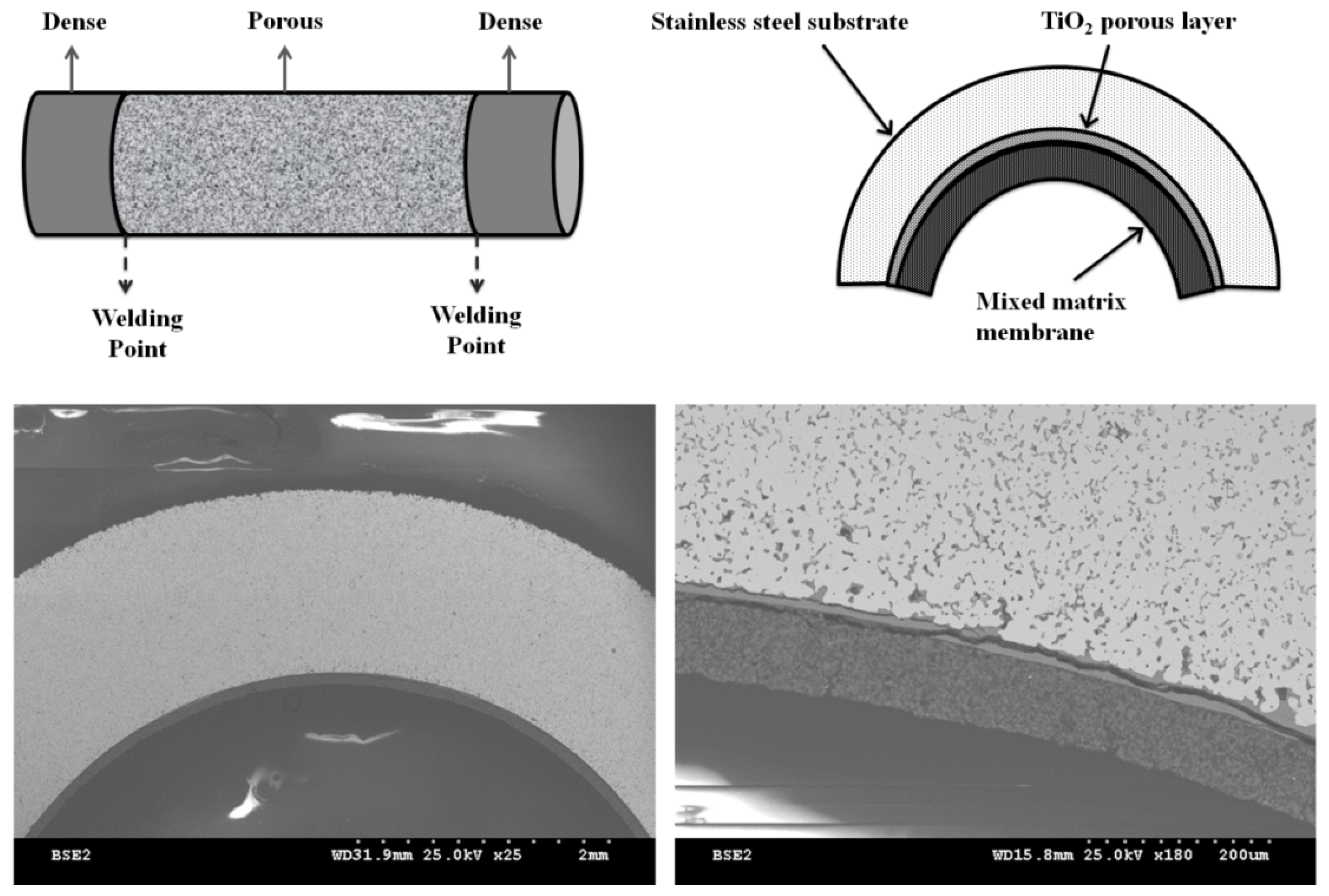
2.1. X-ray Diffraction (XRD) Results
2.2. Adsorption Parameters
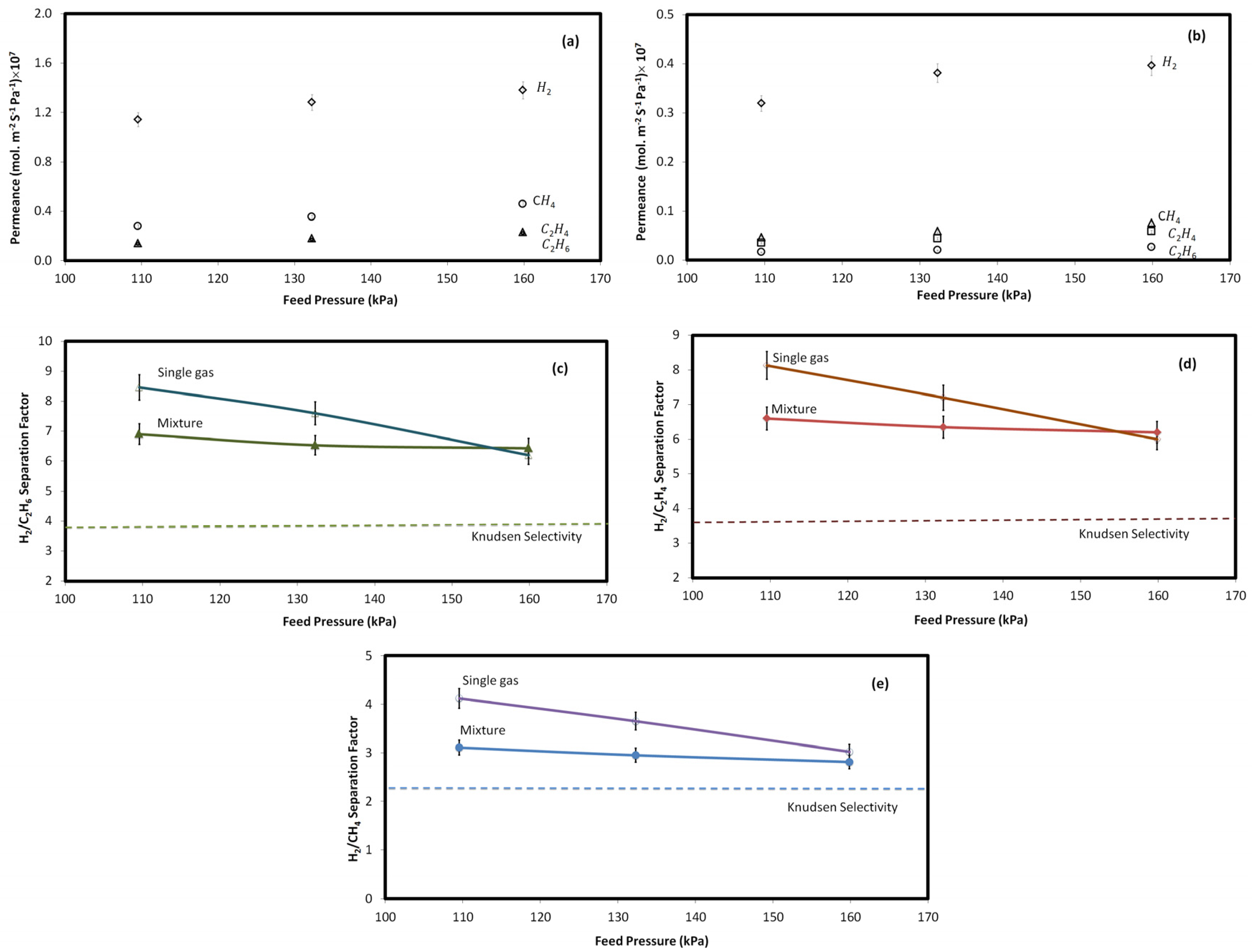
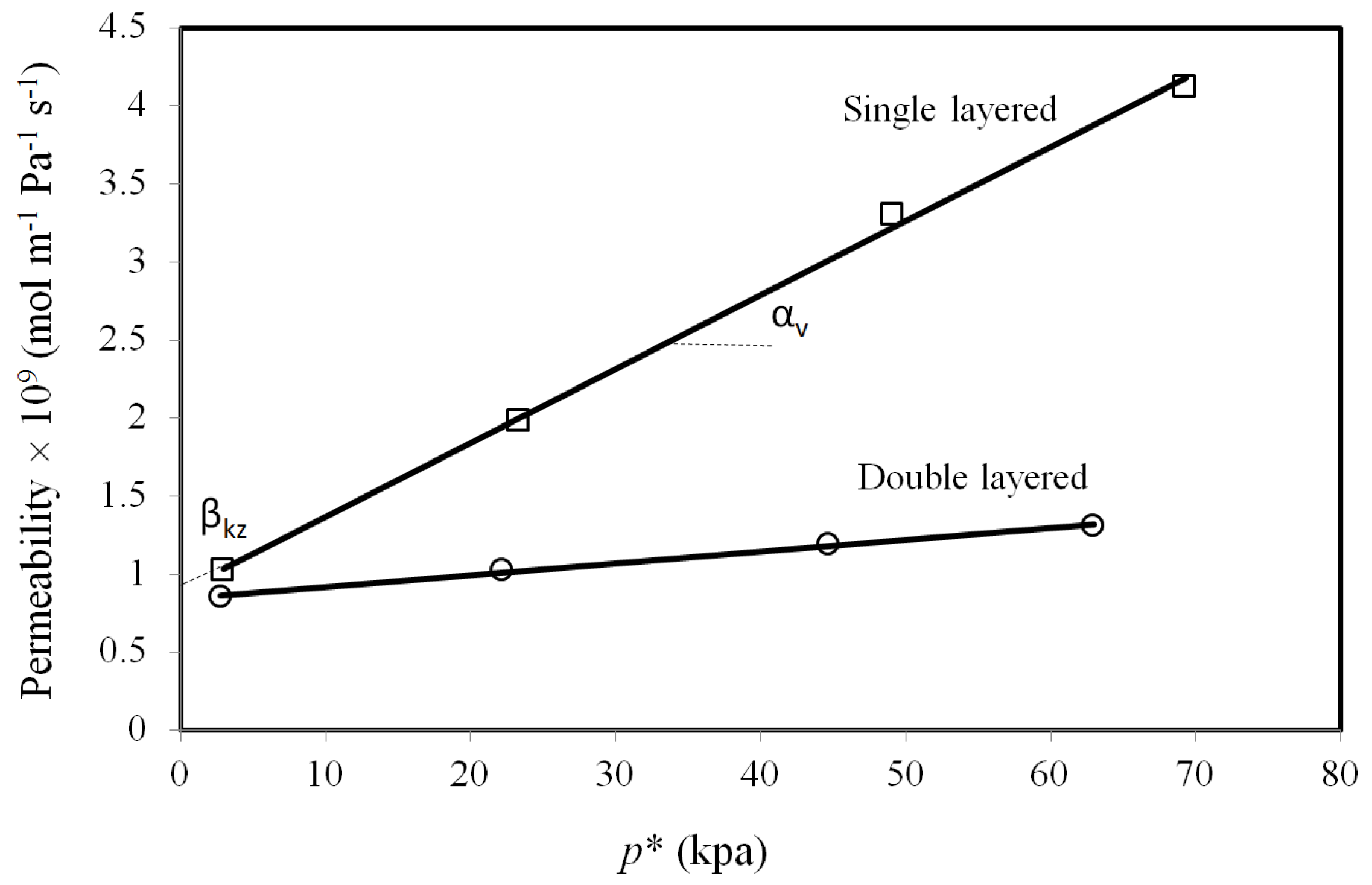
2.3. Effect of Pressure
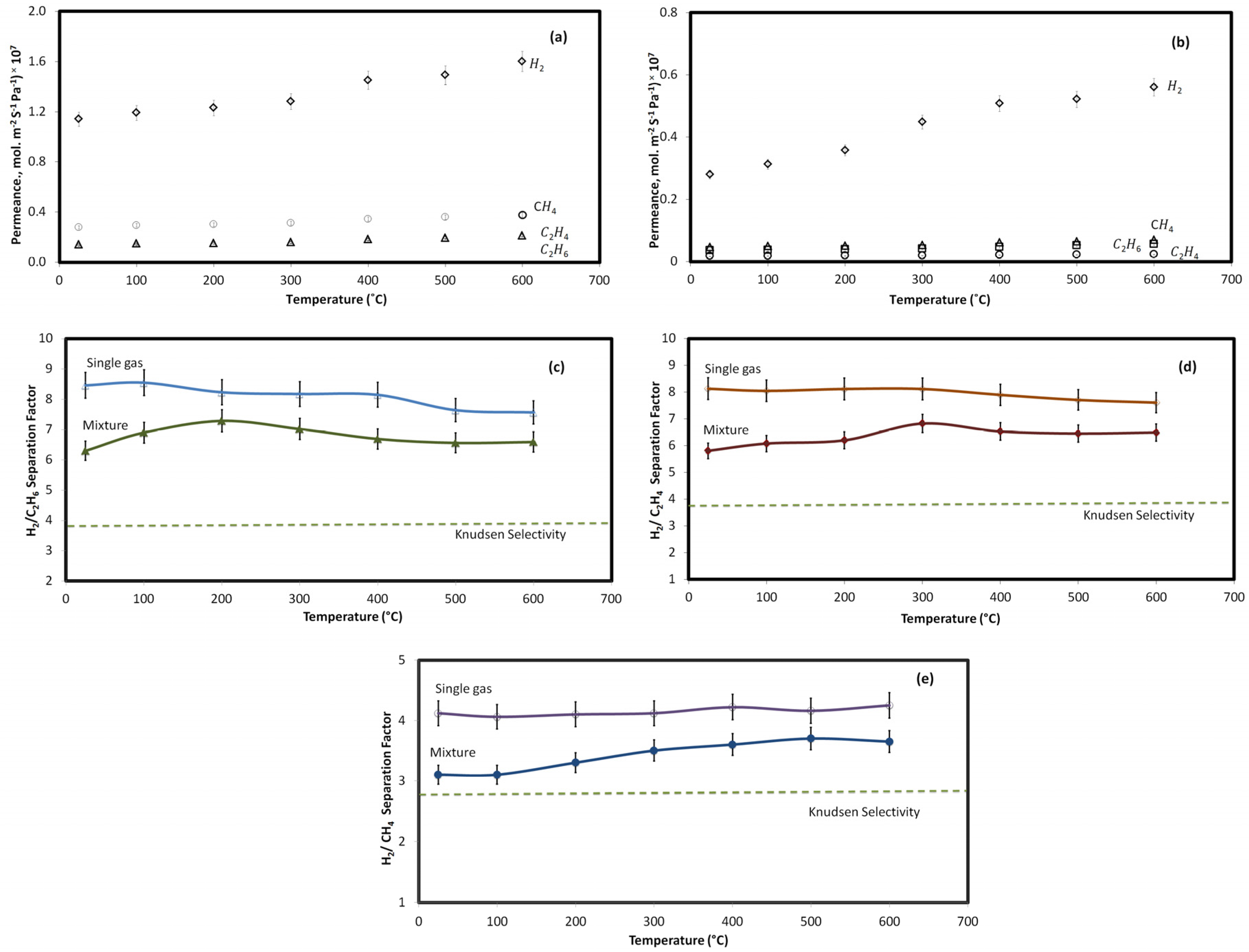
2.4. Effect of Temperature
2.5. Average Defect Size
3. Materials and Methods
3.1. Materials
3.2. Membrane Preparation Method
3.3. Characterization Methods
3.3.1. X-Ray Diffraction (XRD)
3.3.2. Adsorption Isotherms
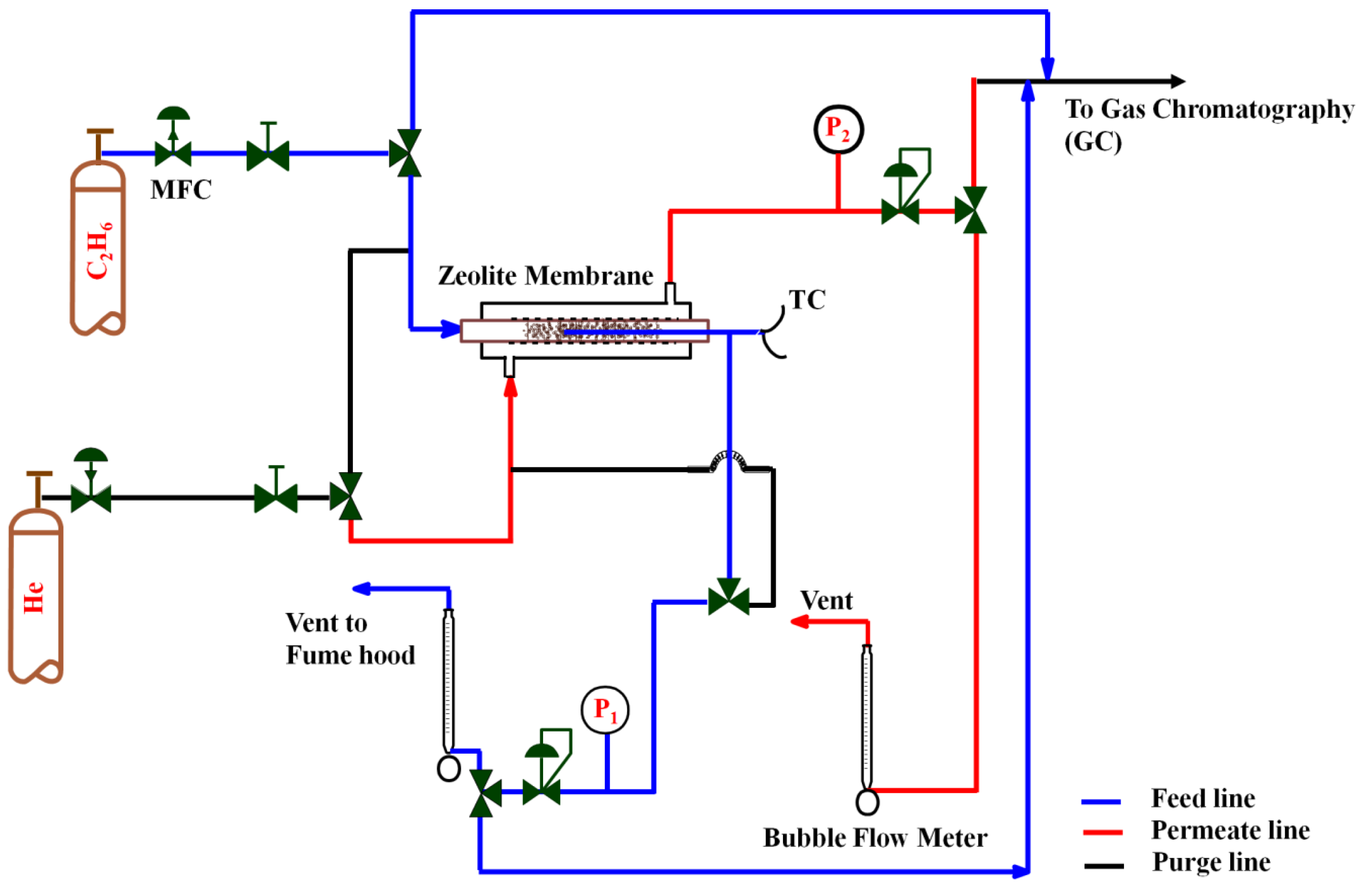
3.3.3. Gas Permeation Tests
3.3.4. Relative Average Defect Size
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1974. [Google Scholar]
- Tsitsishvili, G.V.; Skhirtladze, N.S.; Andronikashvili, T.G.; Tsitsishvili, V.G.; Dolidze, A.V. Natural zeolites of Georgia: Occurrences, properties, and application. In Porous Materials in Environmentally Friendly Processes; Elsevier: Amsterdam, Netherlands, 1999; Volume 125, pp. 715–722. [Google Scholar]
- Ackley, M.W.; Giese, R.F.; Yang, R.T. Clinoptilolite: Untapped potential for kinetic gas separations. Zeolites 1992, 12, 780–788. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite Membranes—Status and Prospective. In Advances in Nanoporous Materials; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 1–96. [Google Scholar]
- Bish, D.L.; Carey, J.W. Thermal Behavior of Natural Zeolites. Rev. Mineral. Geochem. 2001, 45, 403–452. [Google Scholar] [CrossRef]
- Lin, Y.S.; Kumakiri, I.; Nair, B.N.; Alsyouri, H. Microporous Inorganic Membranes. Sep. Purif. Methods 2002, 31, 229–379. [Google Scholar] [CrossRef]
- Bakker, W.J.W.; Kapteijn, F.; Poppe, J.; Moulijn, J.A. Permeation characteristics of a metal-supported silicalite-1 zeolite membrane. J. Membr. Sci. 1996, 117, 57–78. [Google Scholar] [CrossRef]
- Van de Graaf, J.M.; Kapteijn, F.; Moulijn, J.A. Modeling Permeation of Binary Mixtures Through Zeolite Membranes. AIChE J. 1999, 45, 497–511. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, B.; Chen, R.; Zhang, Q.; Huang, W.; Gu, C.R.; Chen, C.L. A computer simulation study on the diffusion and permeation of dimethylformamide/water mixtures through poly(vinyl alcohol)/poly(acrylic acid) blend membranes. Chem. Eng. Res. Des. 2015, 94, 681–690. [Google Scholar] [CrossRef]
- Jia, W.; Murad, S. Separation of gas mixtures using a range of zeolite membranes: A molecular-dynamics study. J. Chem. Phys. 2005, 122, 234708. [Google Scholar] [CrossRef] [PubMed]
- Funke, H.H.; Kovalchick, M.G.; Falconer, J.L.; Noble, R.D. Separation of Hydrocarbon Isomer Vapors with Silicalite Zeolite Membranes. Ind. Eng. Chem. Res. 1996, 35, 1575–1582. [Google Scholar] [CrossRef]
- Vroon, Z.A.E.P.; Keizer, K.; Gilde, M.J.; Verweij, H.; Burggraaf, A.J. Transport properties of alkanes through ceramic thin zeolite MFI membranes. J. Membr. Sci. 1996, 113, 293–300. [Google Scholar] [CrossRef]
- Geus, E.R.; Den Exter, M.J.; van Bekkum, H. Synthesis and Characterization of Zeolite (MFI) Membranes on Porous Ceramic Supports. J. Chem. Soc. Faraday Trans. 1992, 88, 3101–3109. [Google Scholar] [CrossRef]
- Geusa, E.R.; van Bekkum, H.; Bakkerb, W.J.W.; Moulijnb, J.A. High-temperature stainless steel supported zeolite (MFI) membranes: Preparation, module construction, and permeation experiments. Microporous Mater. 1993, 1, 131–147. [Google Scholar] [CrossRef]
- Bai, C.; Jia, M.D.; Falconer, J.L.; Noble, R.D. Preparation and separation properties of silicalite composite membranes. J. Membr. Sci. 1995, 105, 79–87. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.S.; Kanezashi, M.; Tang, Z. Microporous inorganic membranes for high temperature hydrogen purification. J. Appl. Phys. 2008, 104, 121301. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Lombardo, E.A.; Avila, A.M. Xylene permeation transport through composite Ba-ZSM-5/SS tubular membranes: Modeling the steady-state permeation. Ind. Eng. Chem. Res. 2008, 47, 2377–2385. [Google Scholar] [CrossRef]
- Krishna, R.; Baur, R. Modelling issues in zeolite based separation processes. Sep. Purif. Technol. 2003, 33, 213–254. [Google Scholar] [CrossRef]
- Farjoo, A.; Kuznicki, S.M. H2 separation using tubular stainless steel supported natural clinoptilolite membranes. Can. J. Chem. Eng. 2016, 94, 2219–2224. [Google Scholar] [CrossRef]
- Triebe, R.W.; Tezel, F.H.; Khulbe, K.C. Adsorption of methane, ethane and ethylene on molecular sieve zeolites. Gas Sep. Purif. 1996, 10, 81–84. [Google Scholar] [CrossRef]
- Farjoo, A.; Sawada, J.A.; Kuznicki, S.M. Manipulation of the pore size of clinoptilolite for separation of ethane from ethylene. Chem. Eng. Sci. 2015, 138, 685–688. [Google Scholar] [CrossRef]
- Mofarahi, M.; Salehi, S.M. Pure and binary adsorption isotherms of ethylene and ethane on zeolite 5A. Adsorption 2012, 19, 101–110. [Google Scholar] [CrossRef]
- Kroon, M.C.; Vega, L.F. Selective paraffin removal from ethane/ethylene mixtures by adsorption into aluminum methylphosphonate-alpha: A molecular simulation study. Langmuir 2009, 25, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Barrer, R.M. Porous Crystal Membranes. J. Chem. Soc. 1990, 86, 1123–1130. [Google Scholar] [CrossRef]
- Lin, C.C. Advances in Molecular Sieves and Their Applications in Adsorptive Gas Separation Processes. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2009. [Google Scholar]
- Ruthven, D.M. Principle of Adsorption and Adsorption Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984. [Google Scholar]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hejazi, S.A.H.; Avila, A.M.; Kuznicki, T.M.; Weizhu, A.; Kuznicki, S.M. Characterization of natural zeolite membranes for H2/CO2 separations by single gas permeation. Ind. Eng. Chem. Res. 2011, 50, 12717–12726. [Google Scholar] [CrossRef]
- Farjoo, A.; Avila, A.M.; Kuznicki, S.M. H2 separation using pressed clinoptilolite and mixed copper-clinoptilolite disc membranes. Can. J. Chem. Eng. 2016, 95, 500–507. [Google Scholar] [CrossRef]
- Cot, L.; Durand, J.; Guizard, C.; Hovnanian, N.; Julbe, A. Inorganic membranes and solid state sciences. Solid State Sci. 2000, 2, 313–334. [Google Scholar] [CrossRef]
- Abedini, R.; Nezhadmoghadam, A. Application of membranes in gas separation processes: Its suitability and mechanism. Pet. Coal 2010, 52, 69–80. [Google Scholar]
- Bowen, T.C.; Li, S.; Noble, R.D.; Falconer, J.L. Driving force for pervaporation through zeolite membranes. J. Membr. Sci. 2003, 225, 165–176. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, J. Diffusion mechanism of hydrocarbons in zeolites—I. Theory. Chem. Eng. Sci. 1992, 47, 1123–1141. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Webb, S.W. Gas Phase Diffusion in Porous Media: Comparison of Models; Sandia National Labs: Albuquerque, NM, USA, 1998. [Google Scholar]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Berlin, Germany, 2015. [Google Scholar]
- Choudary, N.V.; Kumar, P.; Bhat, T.S.G.; Cho, S.H.; Han, S.S.; Kim, J.N. Adsorption of Light Hydrocarbon Gases on Alkene-Selective Adsorbent. Ind. Eng. Chem. Res. 2002, 41, 2728–2734. [Google Scholar] [CrossRef]
- Yama, S.T.O.; Yamada, M.; Sugawara, T.; Takagaki, A.; Kikuchi, R. Review on Mechanisms of Gas Permeation through Inorganic Membranes. J. Jpn. Pet. Inst. 2011, 54, 298–309. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, J. Diffusion mechanism of hydrocarbons in zeolites—II. Analysis of experimental observations. Chem. Eng. Sci. 1992, 47, 1143–1159. [Google Scholar] [CrossRef]
- Mason, E.A.; Malinauskas, A.P. Gas Transport in Porous Media: The Dusty-Gas Model; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Zornoza, B.; Casado, C.; Navajas, A. Advances in Hydrogen Separation and Purification with Membrane Technology. In Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 245–268. [Google Scholar]
- Lin, Y.S.; Burggraaf, A.J. Experimental studies on pore size change of porous ceramic membranes after modification. J. Membr. Sci. 1993, 79, 65–82. [Google Scholar] [CrossRef]
- Tsederberg, N.V.; Cess, R.D. Thermal Conductivity of Gases and Liquids; The MIT Press: Cambridge, MA, USA, 1965. [Google Scholar]
- Caro, J.; Noack, M.; Kolsch, P.; Schafer, R. Zeolite membranes—State of their development and perspective. Micorporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Thomas, S.; Schäfer, R.; Caro, J.; Seidel-Morgenstern, A. Investigation of mass transfer through inorganic membranes with several layers. Catal. Today 2001, 67, 205–216. [Google Scholar] [CrossRef]
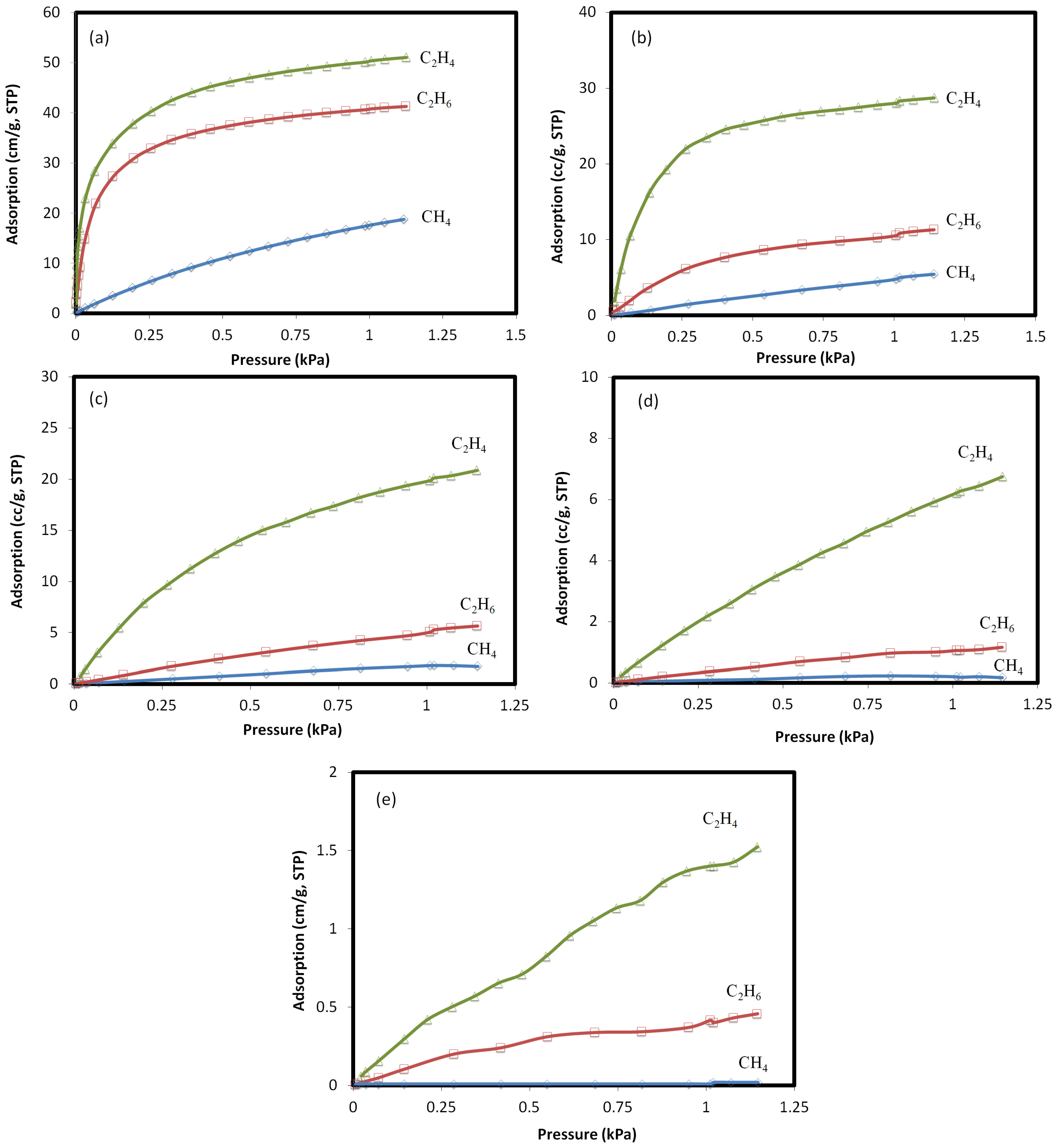
| Component | K cc/(g bar) | cc/g | K |
|---|---|---|---|
| CH4 | 5.22 | 15.2 | 0.041 |
| C2H6 | 21.5 | 6.13 | 3.54 |
| C2H4 | 243.9 | 24.4 | 16.0 |
| Membrane | λ × 102 (kPa−1) |
|---|---|
| Single-layered membrane | 5.2 |
| Double-layered membranes | 0.9 |
| Gas | Thermal Conductivity (mW/m·K) |
|---|---|
| N2 | 26.0 |
| Ar | 17.9 |
| H2 | 186.9 |
| He | 156.7 |
| C2H4 | 20.5 |
| CH4 | 34.1 |
| C2H6 | 21.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farjoo, A.; Kuznicki, S.M.; Sadrzadeh, M. Hydrogen Separation by Natural Zeolite Composite Membranes: Single and Multicomponent Gas Transport. Materials 2017, 10, 1159. https://doi.org/10.3390/ma10101159
Farjoo A, Kuznicki SM, Sadrzadeh M. Hydrogen Separation by Natural Zeolite Composite Membranes: Single and Multicomponent Gas Transport. Materials. 2017; 10(10):1159. https://doi.org/10.3390/ma10101159
Chicago/Turabian StyleFarjoo, Afrooz, Steve M. Kuznicki, and Mohtada Sadrzadeh. 2017. "Hydrogen Separation by Natural Zeolite Composite Membranes: Single and Multicomponent Gas Transport" Materials 10, no. 10: 1159. https://doi.org/10.3390/ma10101159






