Photocatalytic Performance of a Novel MOF/BiFeO3 Composite
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of MOF
2.2. Preparation of MOF/BiFeO3 Composite
2.3. Characterization of Phase and Microstructure
2.4. Measurements of Photocatalytic Performance
3. Results and Discussion
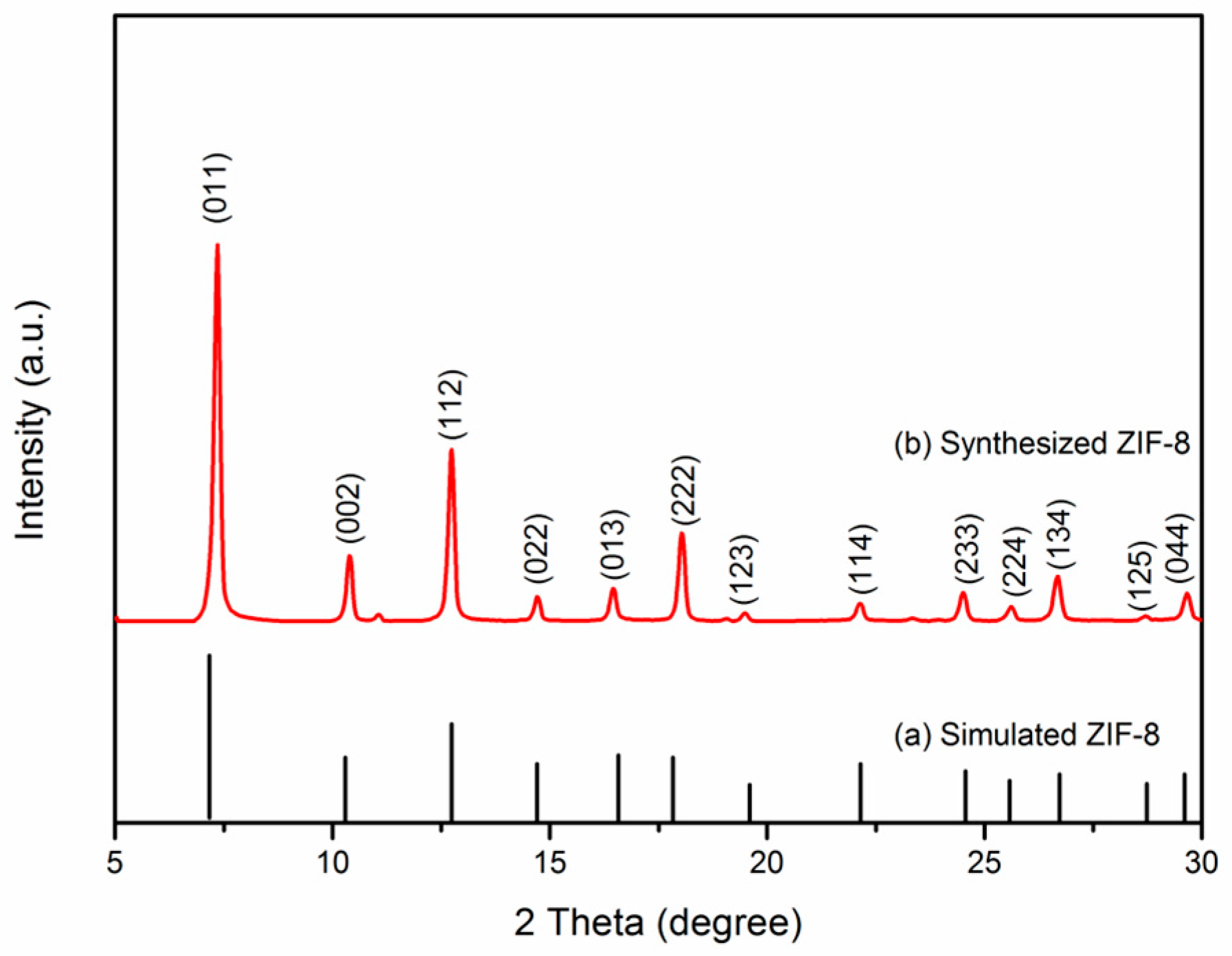
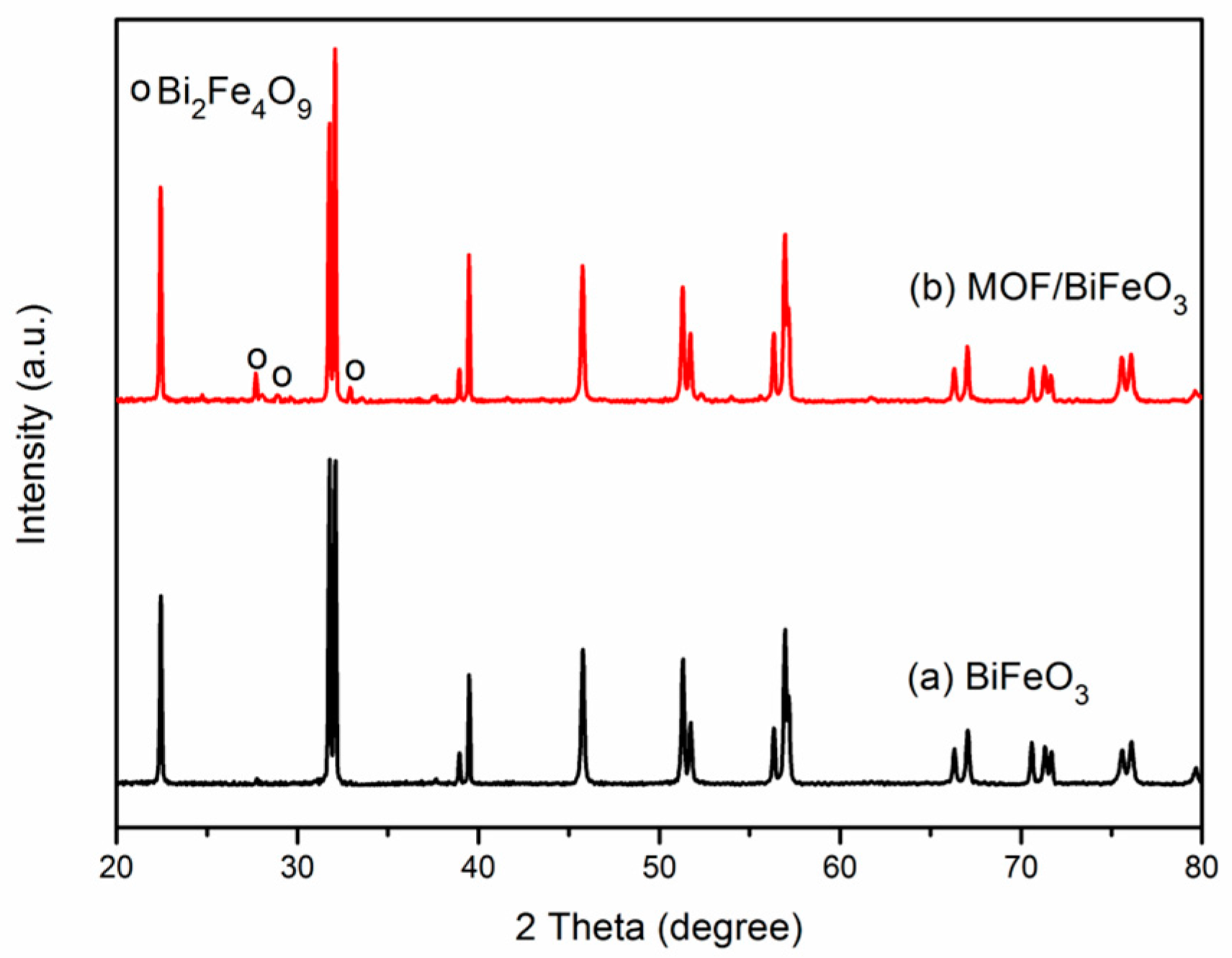
3.1. X-ray Diffraction Analysis
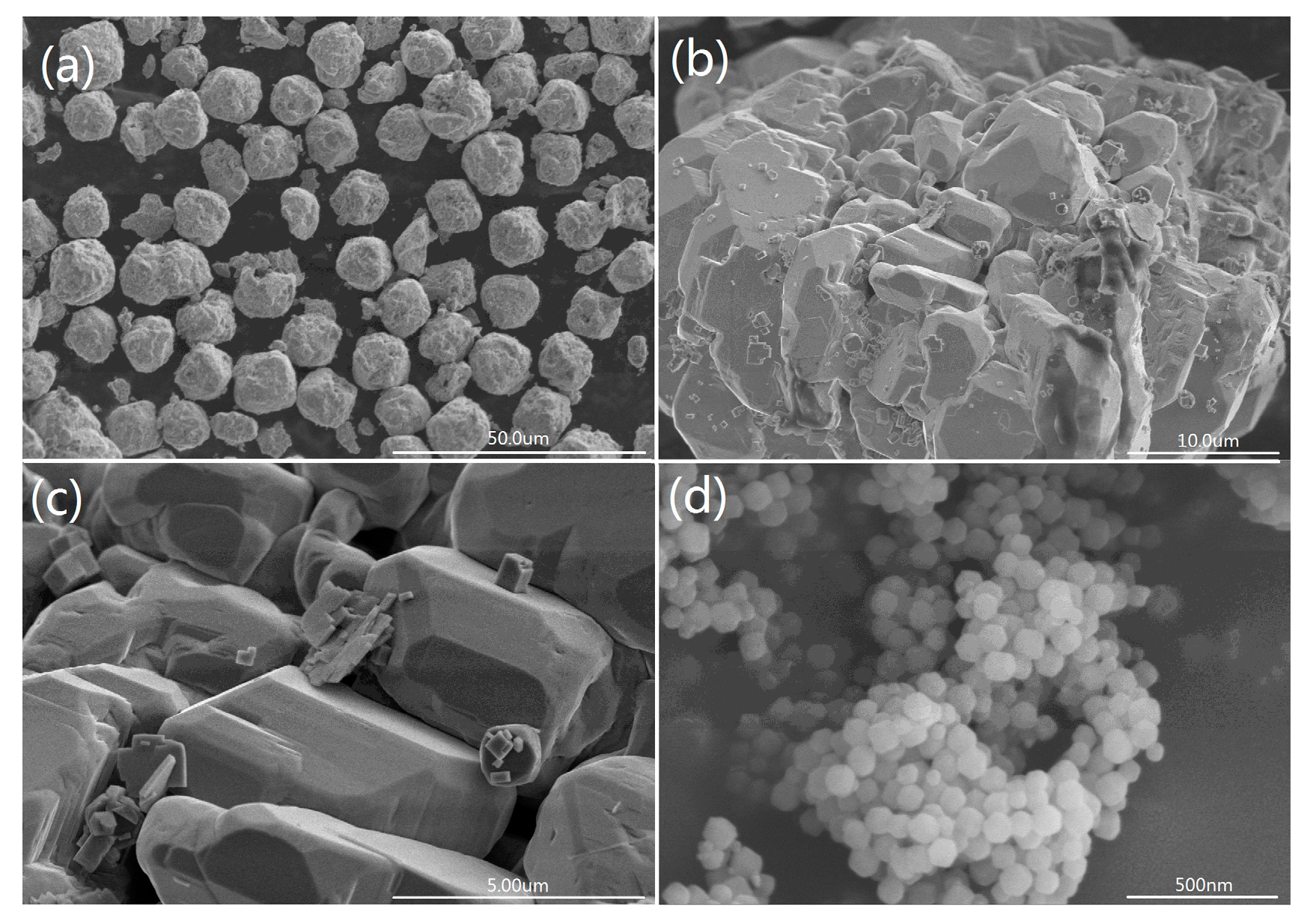
3.2. Morphology and Microstructure Analysis
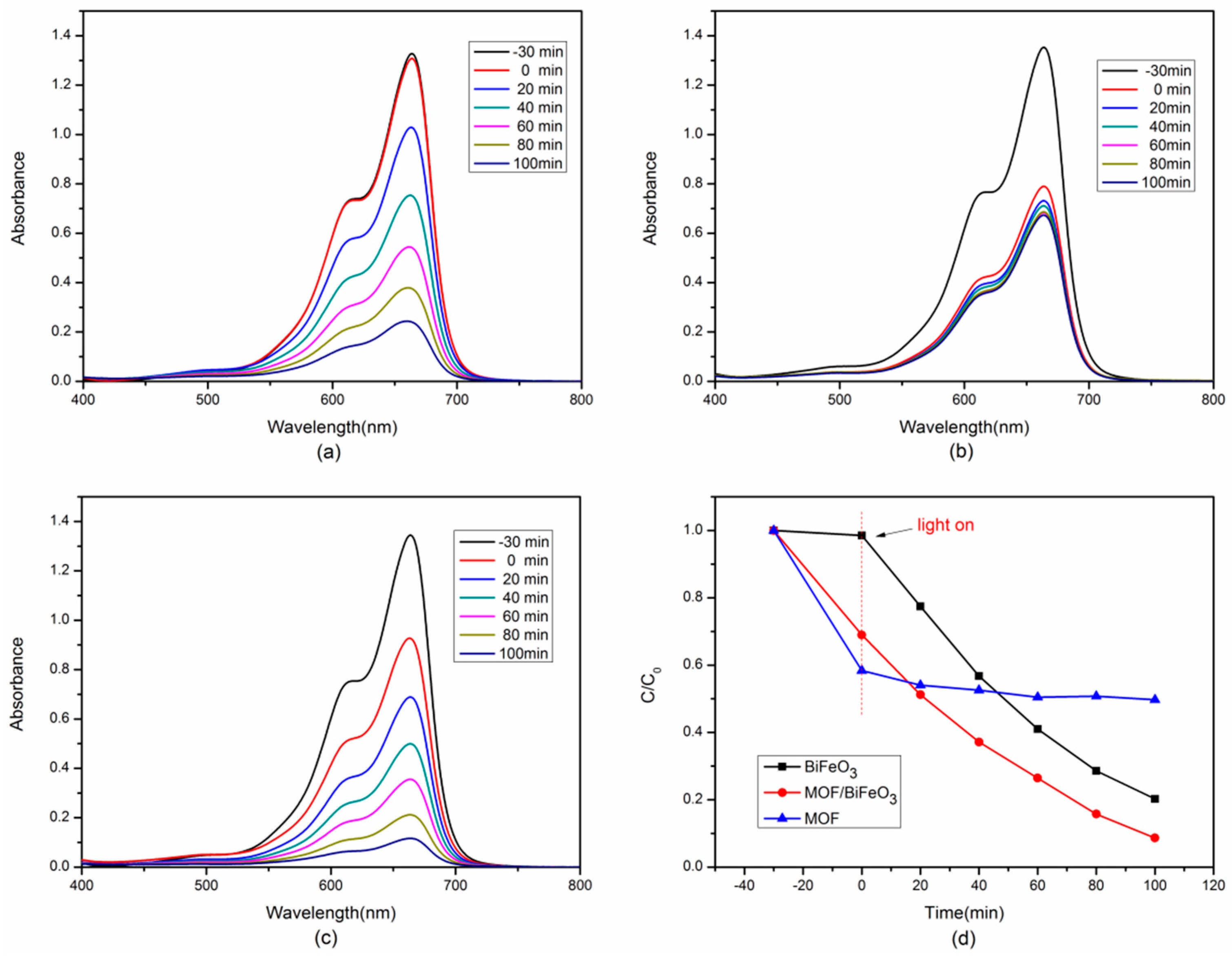
3.3. Enhanced Photocatalytic Performance
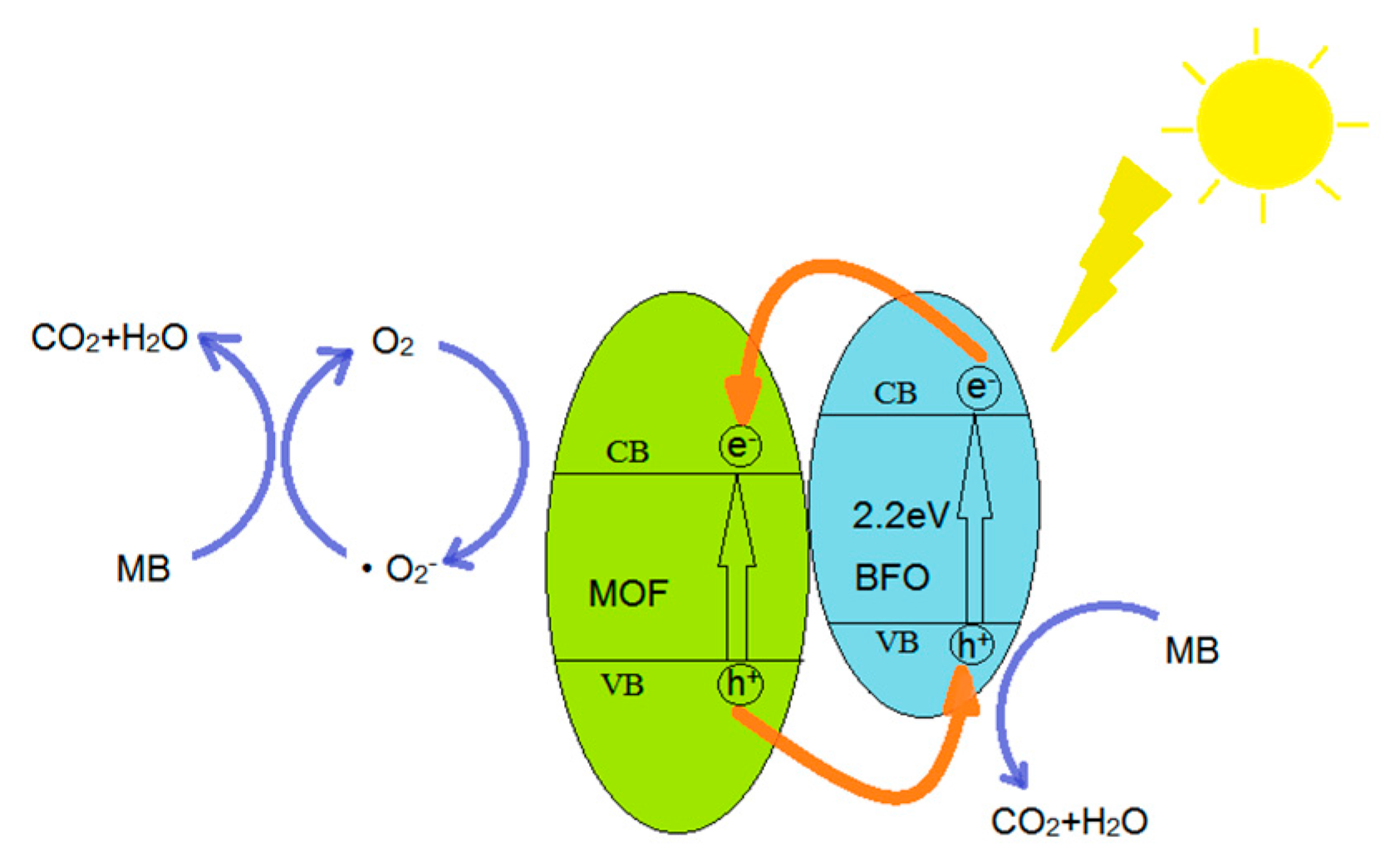
4. Photocatalytic Reaction Mechanism of MB over MOF/BiFeO3 Composite
- Absorption of efficient photons by MOF/BiFeO3 composite photocatalyst
- Holes (h+) in valence band combine with absorbed H2O molecules which produces hydroxyl radical (·OH)
- Oxygen ionosorption
- Neutralization of by protons
- Transient hydrogen peroxide formation and dismutation of oxygen
- Decomposition of hydrogen peroxide
- Hydroxyl radical is further generated
- Oxidation of the MB molecules via successive attacks by ·OH and direct oxidation by reaction with holes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abe, R. Recent progress visible light irradiation on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Xu, J.H.; Wang, W.Z.; Sun, S.M.; Wang, L. Enhancing visible-light-induced photocatalytic activity by coupling with wide-band-gap semiconductor: A case study on Bi2WO6/TiO2. Appl. Catal. B Environ. 2012, 111, 126–132. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on nonmetal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140, 559–587. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Li, J.M. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Xue, Z.H.; Wang, T.; Chen, B.D.; Malkoske, T.; Yu, S.L.; Tang, Y.L. Degradation of tetracycline with BiFeO3 prepared by a simple hydrothermal method. Materials 2015, 8, 6360–6378. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.Q.; Fang, L.; Dong, W.; Zheng, F.G.; Shen, M.G. Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Wang, X.; Xiao, X.; Wu, Z.W.; Zheng, J.; Ren, F.; Jiang, C.Z. Preparation of M@BiFeO3 nanocomposites (M = Ag, Au) bowl arrays with enhanced visible light photocatalytic activity. J. Am. Ceram. Soc. 2015, 98, 2255–2263. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.M.; Gong, L.X.; Zhou, Z.F. Structural, magnetic and photocatalytic properties of Sr2+-doped BiFeO3 nanoparticles based on an ultrasonic irradiation assisted self-combustion method. Ceram. Int. 2012, 38, 6643–6649. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, M.; Chhoker, S.; Katyal, S.C.; Singh, H.; Jewariya, M.; Yadav, K.L. Multiferroic, magnetoelectric and optical properties of Mn doped BiFeO3 nanoparticles. Solid State Commun. 2012, 152, 525–529. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Y.; Tang, H. Influence of adsorption on the photodegradation of various dyes using surface bond-conjugated TiO2 /SiO2 photocatalyst. Appl. Catal. B Environ. 2001, 35, 95–105. [Google Scholar]
- Oshikiri, M.; Boero, M.; Matsushita, A.; Ye, J. Water adsorption onto Y and V sites at the surface of the YVO4 photocatalyst and related electronic properties. J. Chem. Phys. 2009, 131, 034701. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, W.; Lu, Z.; Huo, P.; Yao, G.X.; Yan, Y.S. Surface molecular imprinting modified TiO2 photocatalyst for adsorption and photocatalytic degradation of salicylic acid. Fresen. Environ. Bull. 2014, 23, 1626–1634. [Google Scholar]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Duren, T.; Sarkisov, L.; Yaghi, O.M.; Snurr, R.Q. Design of new materials for methane storage. Langmuir 2004, 20, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Kuesgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal organic frameworks by water adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Wang, Y.G.; Xu, G.; Ren, Z.H.; Wei, X.; Weng, W.J.; Du, P.Y.; Shen, G.; Han, G.R. Mineralizer-assisted hydrothermal synthesis and characterization of BiFeO3 nanoparticles. J. Am. Ceram. Soc. 2007, 90, 2615–2617. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.H.; Zhang, B.P.; Nan, C.W.; Wang, Y. Photocatalytic and magnetic behaviors observed in nanostructured BiFeO3 particles. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Li, Y.; Zou, J.; Xiong, X.; Zeng, X.; Zhou, J. Photocatalytic Performance of a Novel MOF/BiFeO3 Composite. Materials 2017, 10, 1161. https://doi.org/10.3390/ma10101161
Si Y, Li Y, Zou J, Xiong X, Zeng X, Zhou J. Photocatalytic Performance of a Novel MOF/BiFeO3 Composite. Materials. 2017; 10(10):1161. https://doi.org/10.3390/ma10101161
Chicago/Turabian StyleSi, Yunhui, Yayun Li, Jizhao Zou, Xinbo Xiong, Xierong Zeng, and Ji Zhou. 2017. "Photocatalytic Performance of a Novel MOF/BiFeO3 Composite" Materials 10, no. 10: 1161. https://doi.org/10.3390/ma10101161



