Advanced Materials in Polymer Electrolyte Fuel Cells
1. Introduction
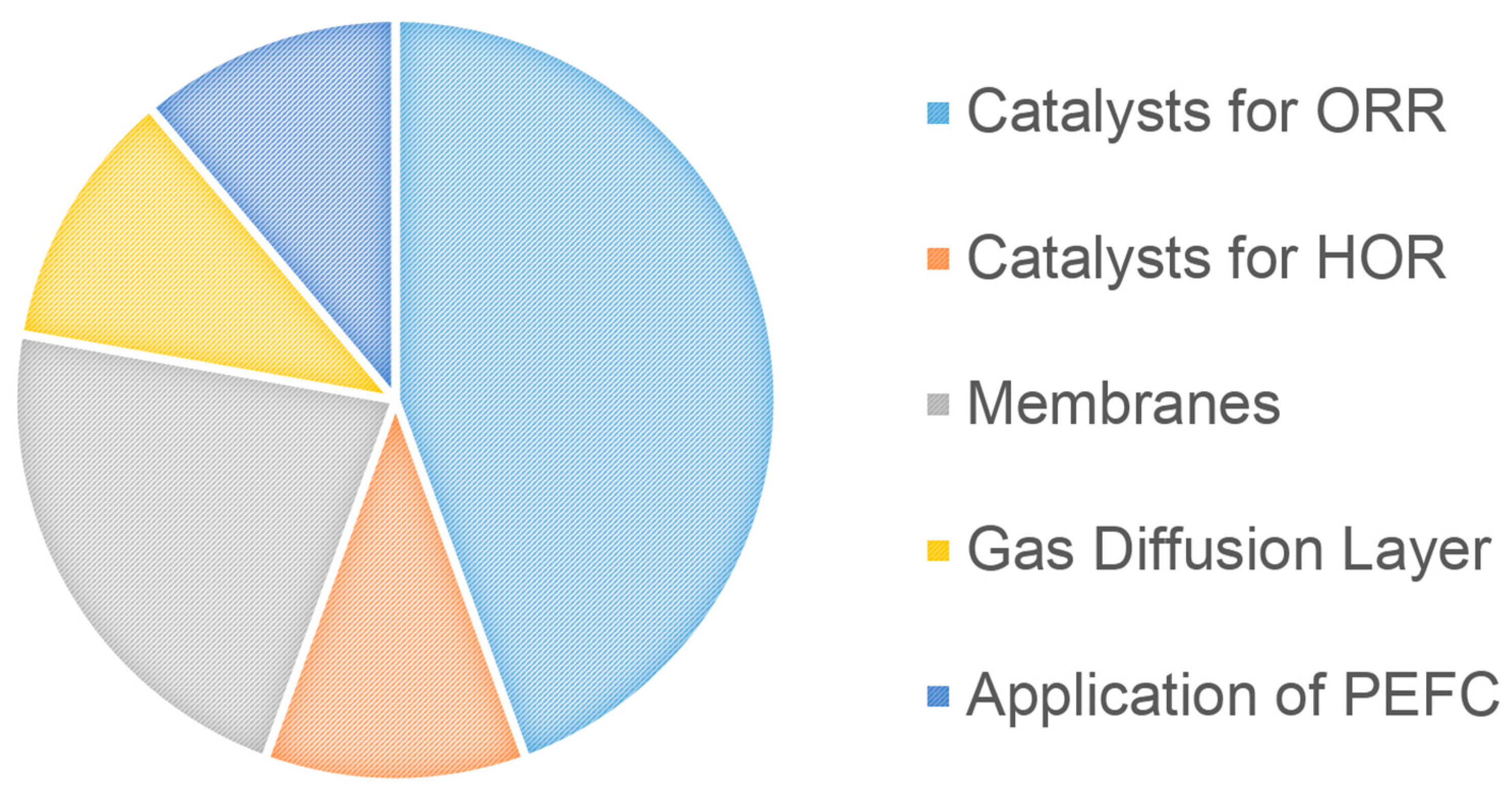
2. This Special Issue
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Dyer, C.K. Fuel cells for portable applications. J. Power Sources 2002, 106, 31–34. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Antonucci, V. Direct Methanol Fuel Cells; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs directly fed with ethanol: A current status comparative review. J. Appl. Electrochem. 2013, 43, 119–136. [Google Scholar] [CrossRef]
- Sgroi, M.F.; Zedde, F.; Barbera, O.; Stassi, A.; Sebastián, D.; Lufrano, F.; Baglio, V.; Aricò, A.S.; Bonde, J.L.; Schuster, M. Cost analysis of direct methanol fuel cell stacks for mass production. Energies 2016, 9, 1008. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Staiti, P.; Stassi, A.; Aricò, A.S.; Antonucci, V. Investigation of sulfonated polysulfone membranes as electrolyte in a passive-mode direct methanol fuel cell mini-stack. J. Power Sources 2010, 195, 7727–7733. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of direct methanol fuel cell membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Sebastián, D.; Baglio, V.; Aricò, A.S.; Serov, A.; Atanassov, P. Performance analysis of a non-platinum group metal catalyst based on iron-aminoantipyrine for direct methanol fuel cells. Appl. Catal. B Environ. 2016, 182, 297–305. [Google Scholar] [CrossRef]
- Yu, Z.; Piao, J.; Liang, Z. Synthesis of 2D Nitrogen-Doped Mesoporous Carbon Catalyst for Oxygen Reduction Reaction. Materials 2017, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, T.; Madkikar, P.; Wang, X.; Gasteiger, H.; Piana, M. Probing Transition-Metal Silicides as PGM-Free Catalysts for Hydrogen Oxidation and Evolution in Acidic Medium. Materials 2017, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Zignani, S.; Baglio, V.; Sebastián, D.; Saccà, A.; Gatto, I.; Aricò, A. Towards Highly Performing and Stable PtNi Catalysts in Polymer Electrolyte Fuel Cells for Automotive Application. Materials 2017, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Alegre, C.; Sebastián, D.; Gálvez, M.; Baquedano, E.; Moliner, R.; Aricò, A.; Baglio, V.; Lázaro, M. N-Doped Carbon Xerogels as Pt Support for the Electro-Reduction of Oxygen. Materials 2017, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Stassi, A.; Gatto, I.; Baglio, V.; Passalacqua, E.; Aricò, A.S.S. Investigation of Pd-based electrocatalysts for oxygen reduction in PEMFCs operating under automotive conditions. J. Power Sources 2013, 222, 390–399. [Google Scholar] [CrossRef]
- Rivera Gavidia, L.; Sebastián, D.; Pastor, E.; Aricò, A.; Baglio, V. Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes. Materials 2017, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Cárdenas, A.L.; Koper, G.J. Transport in Proton Exchange Membranes for Fuel Cell Applications—A Systematic Non-Equilibrium Approach. Materials 2017, 10, 576. [Google Scholar] [CrossRef]
- Jayakumar, A.; Singamneni, S.; Ramos, M.; Al-Jumaily, A.; Pethaiah, S. Manufacturing the Gas Diffusion Layer for PEM Fuel Cell Using a Novel 3D Printing Technique and Critical Assessment of the Challenges Encountered. Materials 2017, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, H.; Ogawa, R.; Shibuya, S.; Ueda, M. Novel PEFC Application for Deuterium Isotope Separation. Materials 2017, 10, 303. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastián, D.; Baglio, V. Advanced Materials in Polymer Electrolyte Fuel Cells. Materials 2017, 10, 1163. https://doi.org/10.3390/ma10101163
Sebastián D, Baglio V. Advanced Materials in Polymer Electrolyte Fuel Cells. Materials. 2017; 10(10):1163. https://doi.org/10.3390/ma10101163
Chicago/Turabian StyleSebastián, David, and Vincenzo Baglio. 2017. "Advanced Materials in Polymer Electrolyte Fuel Cells" Materials 10, no. 10: 1163. https://doi.org/10.3390/ma10101163





