Carbon-Based Oxamate Cobalt(III) Complexes as Bioenzyme Mimics for Contaminant Elimination in High Backgrounds of Complicated Constituents
Abstract
:1. Introduction
2. Results and Discussion
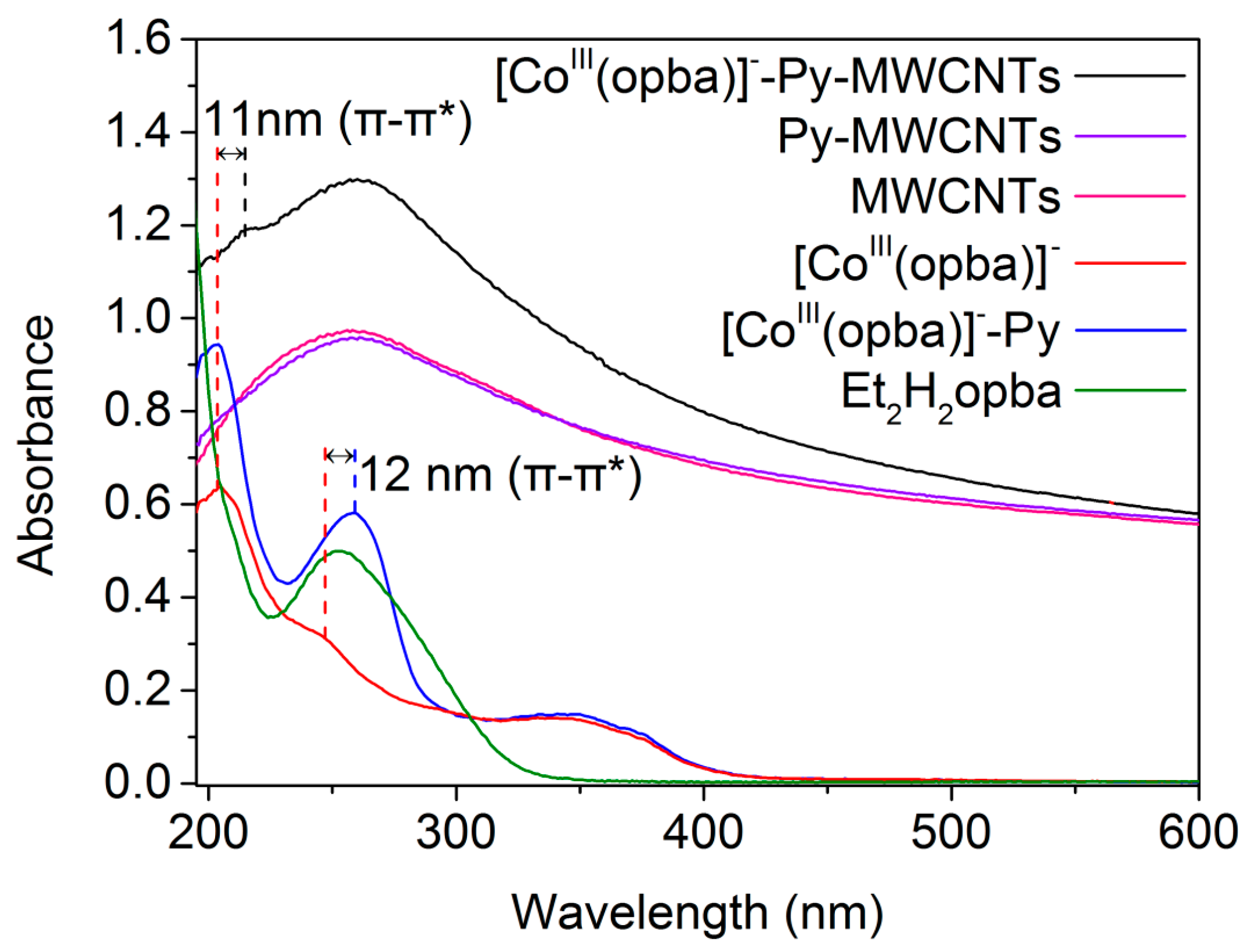
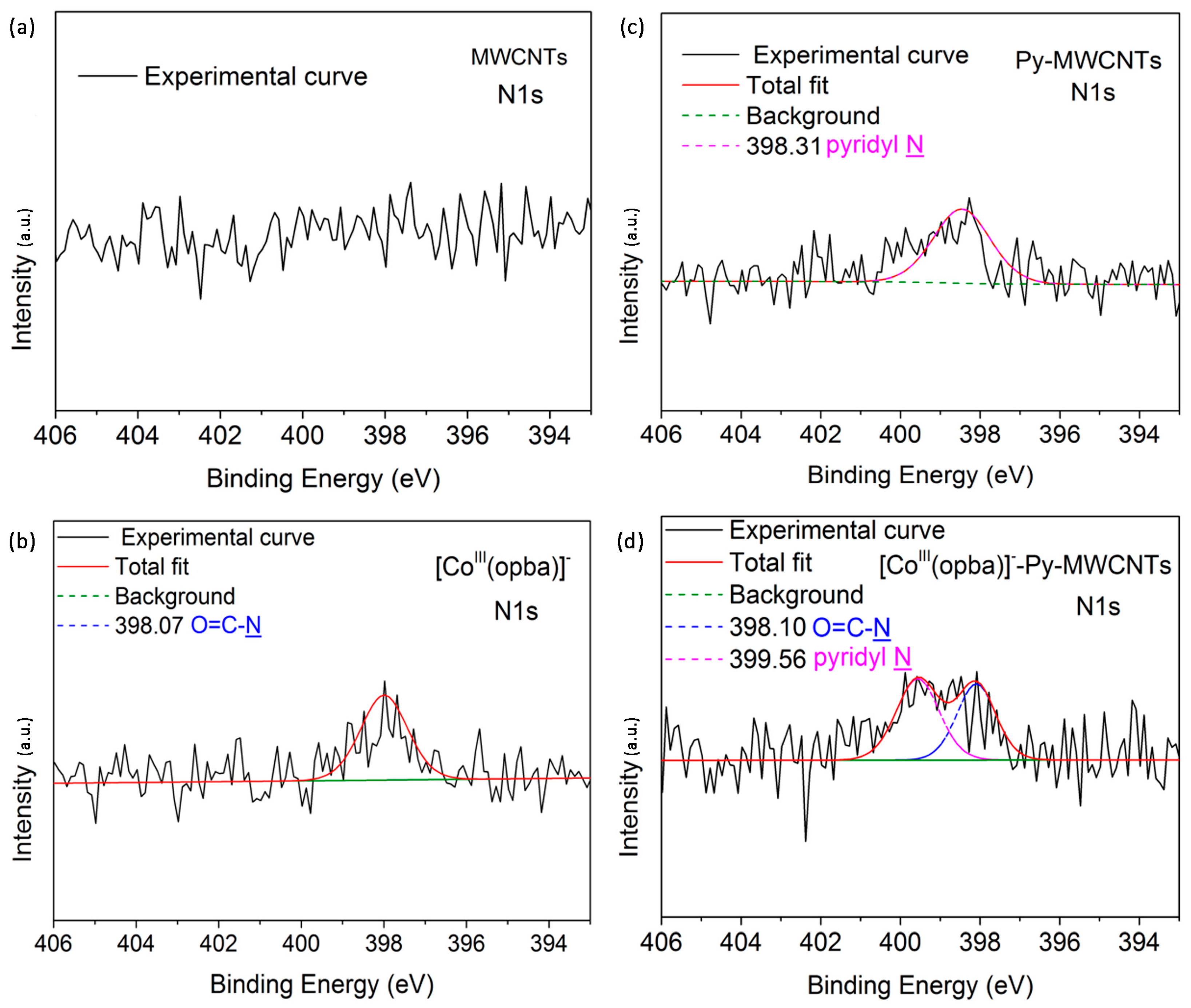
2.1. Characterization
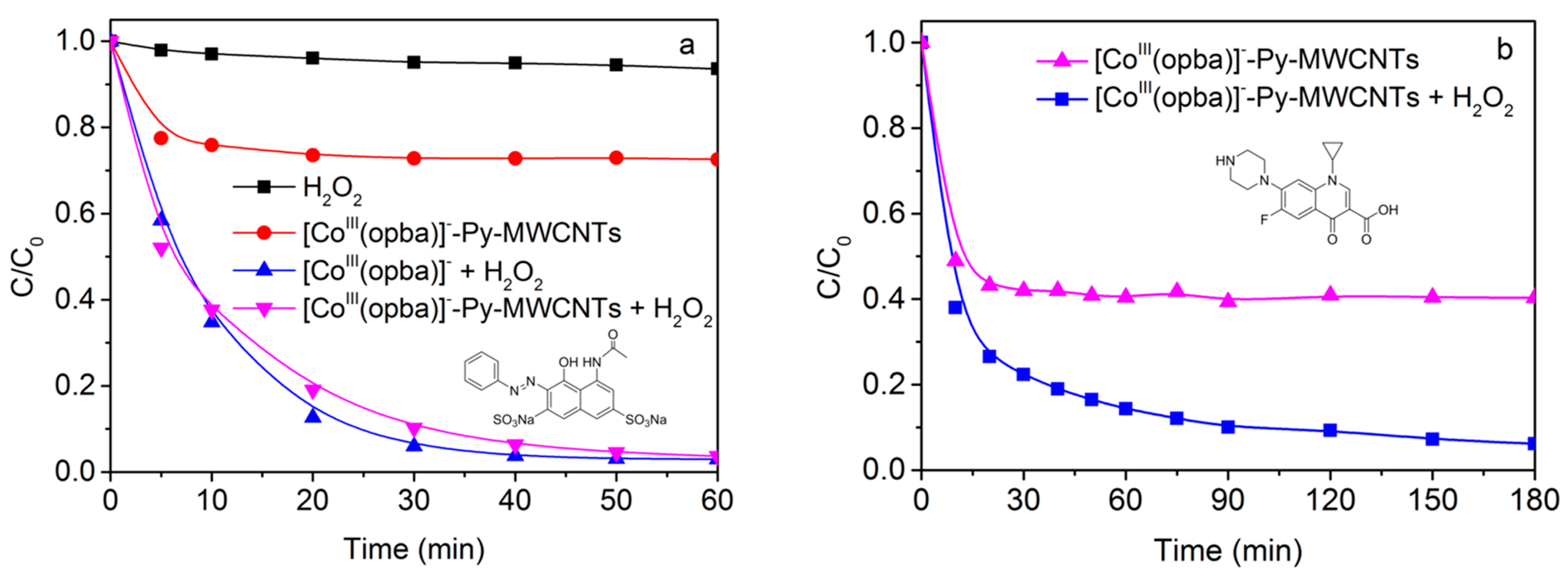
2.2. Catalytic Performance
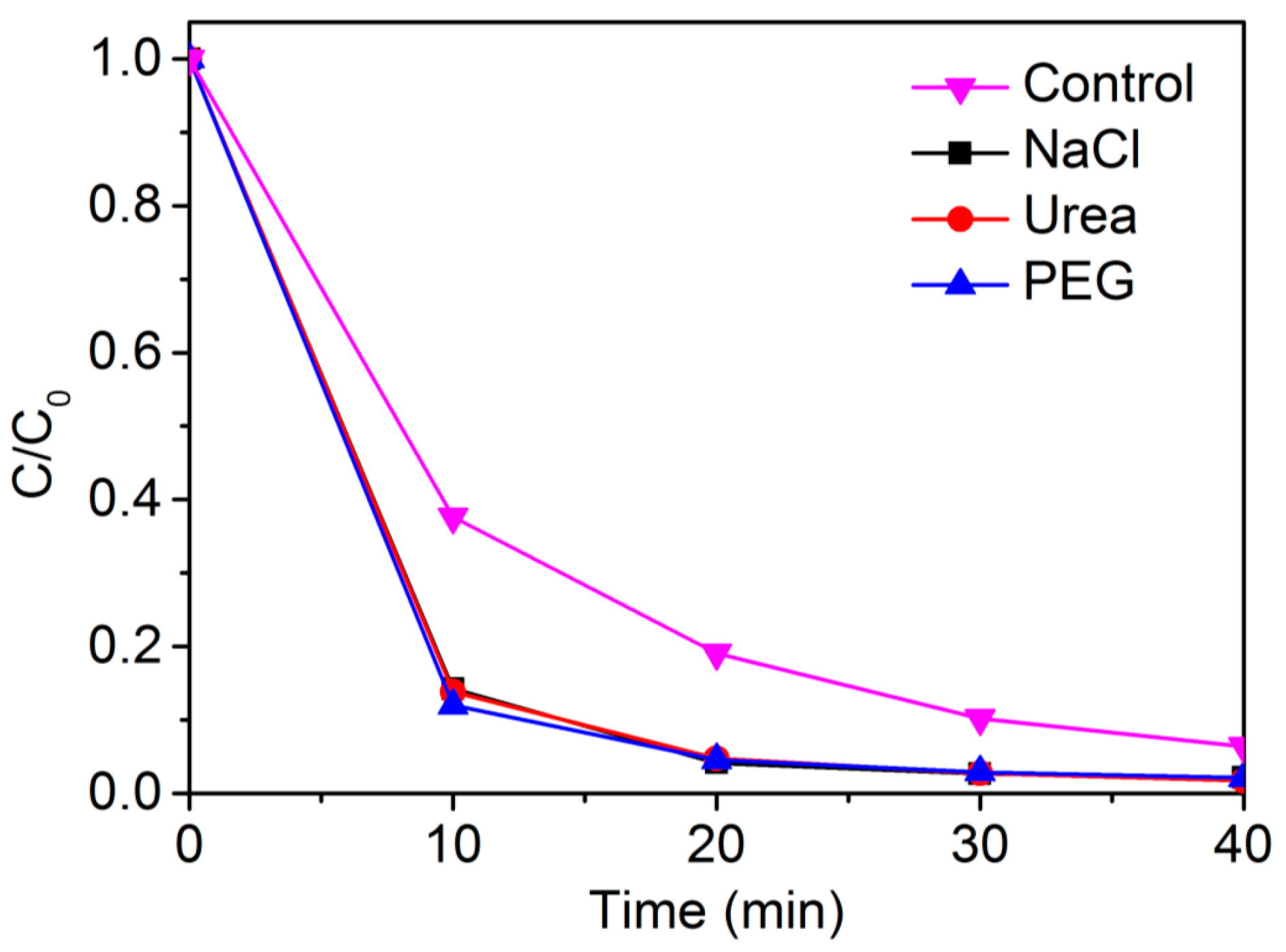
2.3. Effect of High Backgrounds of Complex Constituents
2.4. Cyclic Catalytic Oxidation
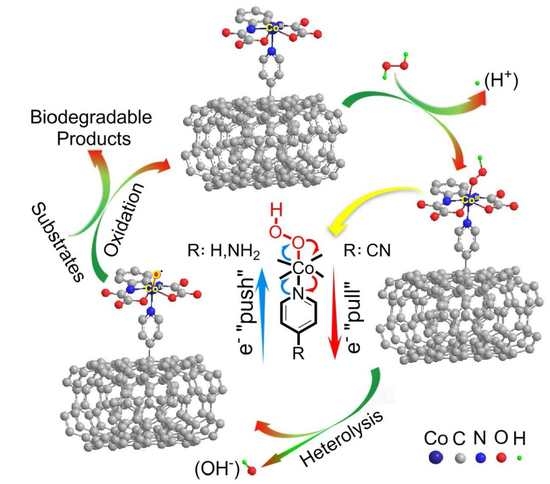
2.5. Mechanism and Pathway
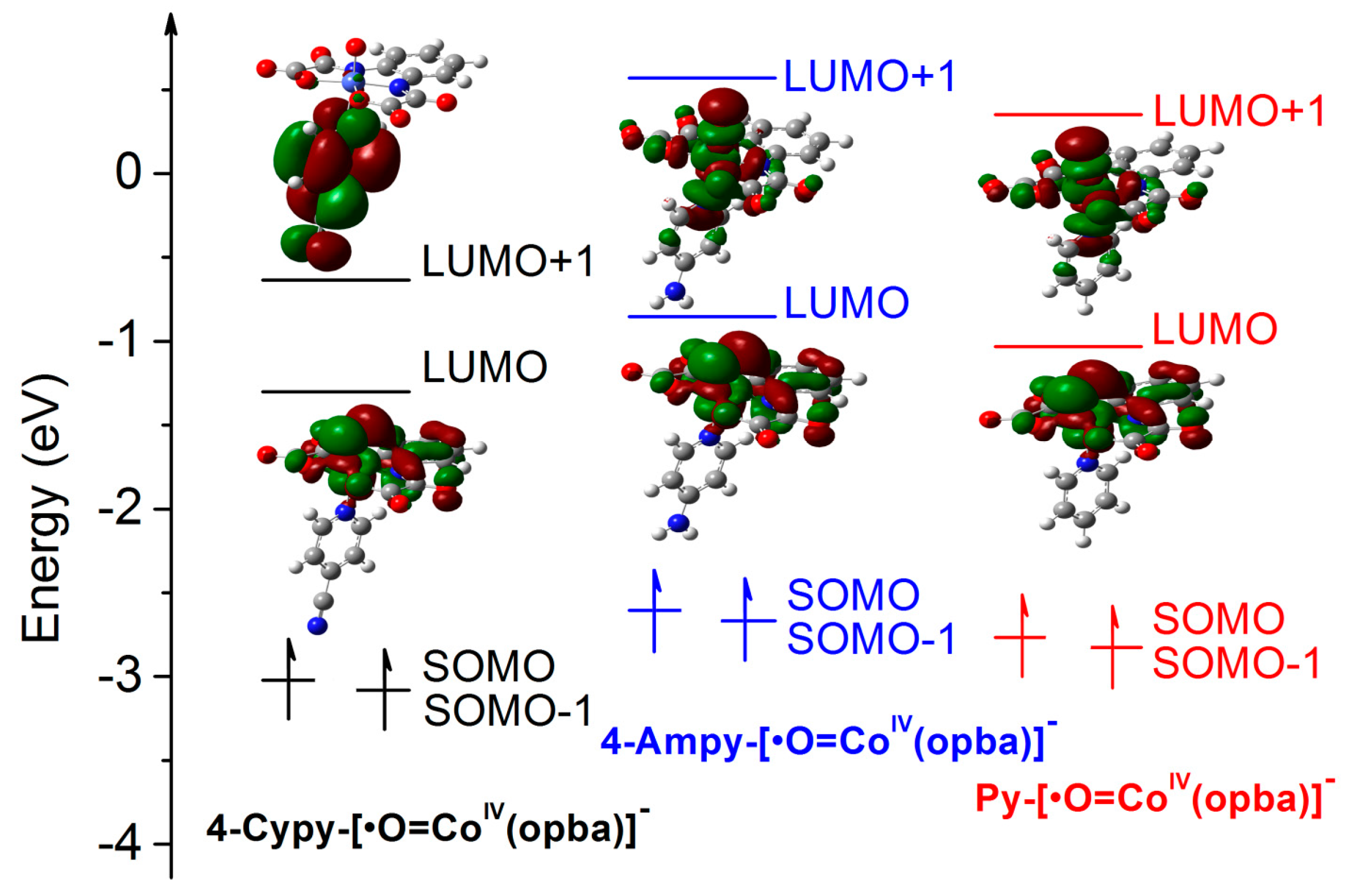
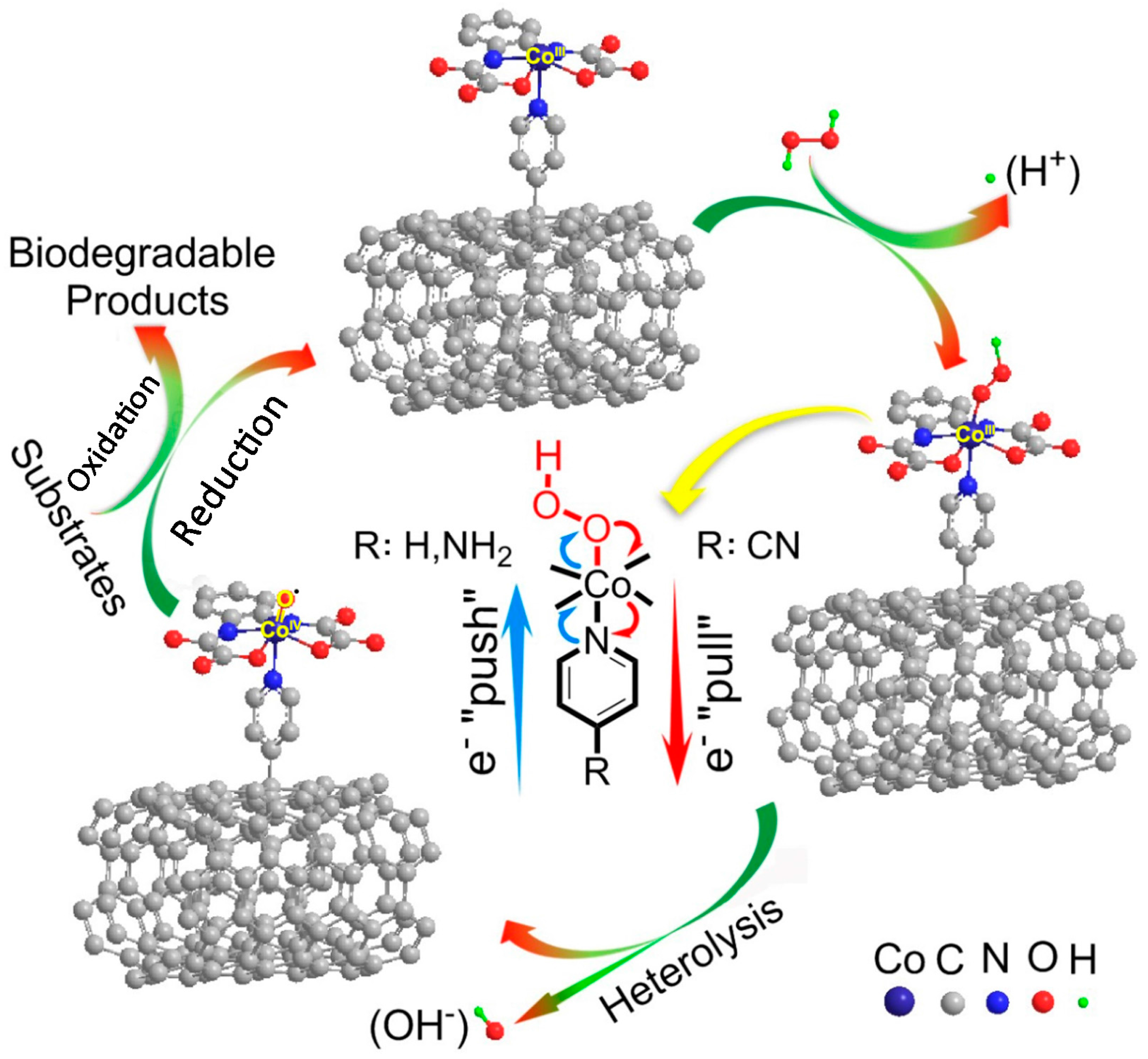
2.5.1. Mechanism
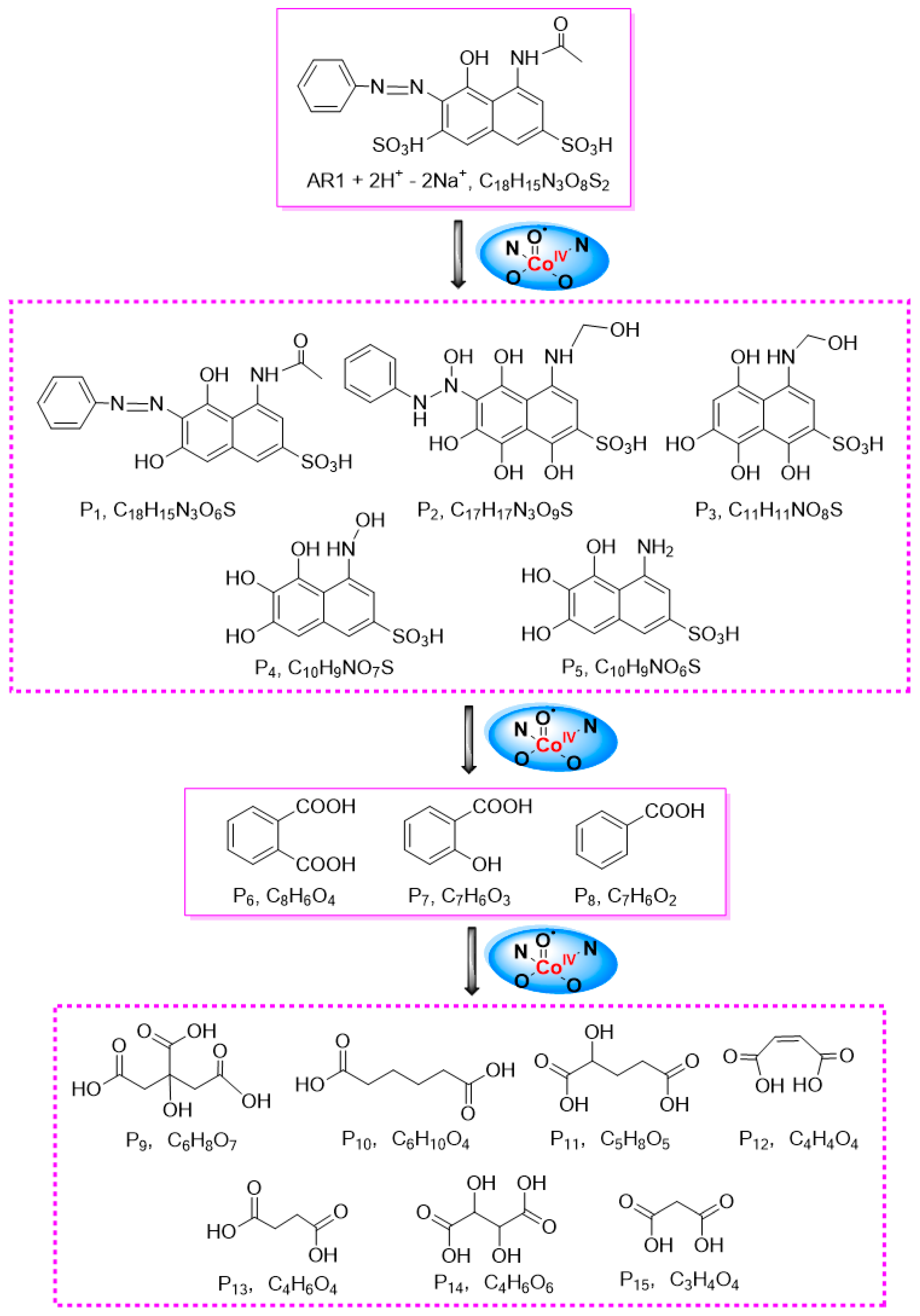
2.5.2. Detoxification Pathway
3. Materials and Methods
3.1. Synthesis of Catalyst
3.2. Catalytic Experiments and Analysis
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Homaeigohar, S.; Zillohu, A.; Abdelaziz, R.; Hedayati, M.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Thao, L.; Dang, T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Photocatalytic Degradation of Organic Dye under UV-A Irradiation Using TiO2-Vetiver Multifunctional Nano Particles. Materials 2017, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, A.; Persson, K. Bioinspired Materials for Water Purification. Materials 2016, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.C.; We, N.Y.; Chou, L.S. Bioaccumulation of persistent organic pollutants in stranded cetaceans from Taiwan coastal waters. J. Hazard. Mater. 2014, 277, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Solla, S.R.; Gilroy, E.A.; Klinck, J.S.; King, L.E.; McInnis, R.; Struger, J.; Backus, S.M.; Gillis, P.L. Bioaccumulation of pharmaceuticals and personal care products in the unionid mussel Lasmigona costata in a river receiving wastewater effluent. Chemosphere 2016, 146, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.A.; Elimelech, M. Water and sanitation in developing countries: Including health in the equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, J.; Liu, W.; Wen, Y.; Rashid, S. Selective conversion of organic pollutant p-chlorophenol to formic acid using zeolite Fenton catalyst. Chemosphere 2016, 161, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Sligar, S.G. Chemistry. Glimpsing the critical intermediate in cytochrome P450 oxidations. Science 2010, 330, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.T. Enzymatic C-H bond activation: Using push to get pull. Nat. Chem. 2014, 6, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Hlavica, P. Evaluation of structural features in fungal cytochromes P450 predicted to rule catalytic diversification. Biochim. Biophys. Acta 2013, 1834, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.N.; Yanamala, N.V.K.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic Investigations of Horseradish Peroxidase-Catalyzed Degradation of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef] [PubMed]
- Aldag, C.; Gromov, I.A.; Garcia-Rubio, I.; von Koenig, K.; Schlichting, I.; Jaun, B.; Hilvert, D. Probing the role of the proximal heme ligand in cytochrome P450cam by recombinant incorporation of selenocysteine. Proc. Natl. Acad. Sci. USA 2009, 106, 5481–5486. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeung, N.; Sieracki, N.; Marshall, N.M. Design of functional metalloproteins. Nature 2009, 460, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, A.; Serdyuk, O.V.; Tsogoeva, S.B. Non-heme iron catalysts for epoxidation and aziridination reactions of challenging terminal alkenes: Towards sustainability. Green Chem. 2015, 17, 2042–2058. [Google Scholar] [CrossRef]
- Berggren, G.; Adamska, A.; Lambertz, C.; Simmons, T.R.; Esselborn, J.; Atta, M.; Gambarelli, S.; Mouesca, J.M.; Reijerse, E.; Lubitz, W.; et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 2013, 499, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Datta, A.; Nguyen, K.T.; Zhao, Y. VOPO4·2H2O encapsulated in graphene oxide as a heterogeneous catalyst for selective hydroxylation of benzene to phenol. Green Chem. 2016, 18, 397–401. [Google Scholar] [CrossRef]
- Brown-Marshall, C.D.; Diebold, A.R.; Solomon, E.I. Reaction coordinate of isopenicillin N synthase: Oxidase versus oxygenase activity. Biochemistry 2010, 49, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Lu, W.Y.; Yao, Y.Y.; Xu, M.H. Highly efficient decomposition of organic dyes by aqueous-fiber phase transfer and in situ catalytic oxidation, using fiber-supported cobalt phthalocyanine. Environ. Sci. Technol. 2007, 41, 6240–6245. [Google Scholar] [CrossRef] [PubMed]
- Lendzion-Bieluń, Z.; Bettahar, M.M.; Monteverdi, S.; Moszyński, D.; Narkiewicz, U. Effect of Cobalt on the Activity of CuO/CeO2 Catalyst for the Selective Oxidation of CO. Catal. Lett. 2010, 134, 196–203. [Google Scholar] [CrossRef]
- Taguchi, T.; Stone, K.L.; Gupta, R.; Kaiser-Lassalle, B.; Yano, J.; Hendrich, M.P.; Borovik, A.S. Preparation and Properties of an MnIV-Hydroxide Complex: Proton and Electron Transfer at a Mononuclear Manganese Site and its Relationship to the Oxygen Evolving Complex within Photosystem II. Chem. Sci. 2014, 5, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Simões, M.M.; Santos, I.C.; Rebelo, S.L.; Paz, F.A.A.; Neves, M.G.P.; Cavaleiro, J.A. Oxidation of organosulfur compounds using an iron(III) porphyrin complex: An environmentally safe and efficient approach. Appl. Catal. B Environ. 2014, 160–161, 80–88. [Google Scholar] [CrossRef]
- Sannino, F.; Spaccini, R.; Savy, D.; Piccolo, A. Remediation of highly contaminated soils from an industrial site by employing a combined treatment with exogeneous humic substances and oxidative biomimetic catalysis. J. Hazard. Mater. 2013, 261, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Serrano, M.; García-Argüelles, S.; Maia Da Costa, M.; Ferrer, M.; Gutiérrez, M. Ice as a Green-Structure-Directing Agent in the Synthesis of Macroporous MWCNTs and Chondroitin Sulphate Composites. Materials 2017, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hilarius, K.; Liebscher, M.; Lellinger, D.; Alig, I.; Pötschke, P. Effect of Graphite Nanoplate Morphology on the Dispersion and Physical Properties of Polycarbonate Based Composites. Materials 2017, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bao, H.; Yao, Y.; Lu, W.; Chen, W. Novel green activation processes and mechanism of peroxymonosulfate based on supported cobalt phthalocyanine catalyst. Appl. Catal. B Environ. 2014, 154–155, 36–43. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Performance Enhancement and Mechanistic Studies of Room-Temperature Sodium–Sulfur Batteries with a Carbon-Coated Functional Nafion Separator and a Na2S/Activated Carbon Nanofiber Cathode. Chem. Mater. 2016, 28, 896–905. [Google Scholar] [CrossRef]
- Schaetz, A.; Zeltner, M.; Stark, W.J. Carbon Modifications and Surfaces for Catalytic Organic Transformations. ACS Catal. 2012, 2, 1267–1284. [Google Scholar] [CrossRef]
- Lendzion-Bielun, Z.; Narkiewicz, U.; Arabczyk, W. Cobalt-based Catalysts for Ammonia Decomposition. Materials 2013, 6, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, W.; Pei, K.; Yao, Y.; Chen, W. Formation of high-valent cobalt-oxo phthalocyanine species in a cellulose matrix for eliminating organic pollutants. Appl. Catal. B Environ. 2015, 163, 105–112. [Google Scholar] [CrossRef]
- Tuci, G.; Zafferoni, C.; Rossin, A.; Milella, A.; Luconi, L.; Innocenti, M.; Truong Phuoc, L.; Duong-Viet, C.; Pham-Huu, C.; Giambastiani, G. Chemically Functionalized Carbon Nanotubes with Pyridine Groups as Easily Tunable N-Decorated Nanomaterials for the Oxygen Reduction Reaction in Alkaline Medium. Chem. Mater. 2014, 26, 3460–3470. [Google Scholar] [CrossRef]
- Bahr, J.L.; Yang, J.P.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.; Fernandez, I.; Pedro, J.R.; Ottenwaelder, X.; Ruiz, R.; Journaux, Y. Aerobic epoxidation of olefins catalysed by square-planar cobalt(III) complexes of bis-N,N′-disubstituted oxamides and related ligands. Tetrahedron Lett. 1997, 38, 2377–2380. [Google Scholar] [CrossRef]
- Usachov, D.; Fedorov, A.; Vilkov, O.; Senkovskiy, B.; Adamchuk, V.K.; Yashina, L.V.; Volykhov, A.A.; Farjam, M.; Verbitskiy, N.I.; Gruneis, A.; et al. The chemistry of imperfections in N-graphene. Nano Lett. 2014, 14, 4982–4988. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Huang, G.; Wang, X.; Weng, J. Solvothermal syntheses of hollow carbon microspheres modified with –NH2 and –OH groups in one-step process. Carbon 2010, 48, 3145–3156. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Kiwi, J.; Lopez, A.; Nadtochenko, V. Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environ. Sci. Technol. 2000, 34, 2162–2168. [Google Scholar] [CrossRef]
- Hamlin, J.; Phillips, D.; Whiting, A. UV/Visible spectroscopic studies of the effects of common salt and urea upon reactive dye solutions. Dyes Pigments 1999, 41, 137–142. [Google Scholar] [CrossRef]
- Sun, S.P.; Li, C.J.; Sun, J.H.; Shi, S.H.; Fan, M.H.; Zhou, Q. Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: Effect of system parameters and kinetic study. J. Hazard. Mater. 2009, 161, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Olkowska, E.; Polkowska, Z.; Namiesnik, J. Analytics of surfactants in the environment: Problems and challenges. Chem. Rev. 2011, 111, 5667–5700. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Teel, A.L.; Watts, R.J. Identification of the reactive oxygen species responsible for carbon tetrachloride degradation in modified Fenton’s systems. Environ. Sci. Technol. 2004, 8, 5465–5469. [Google Scholar] [CrossRef]
- Wang, L.; Cao, M.; Ai, Z.; Zhang, L. Dramatically enhanced aerobic atrazine degradation with Fe@Fe2O3 core-shell nanowires by tetrapolyphosphate. Environ. Sci. Technol. 2014, 48, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Nam, W.; Han, H.J.; Oh, S.-Y.; Lee, Y.J.; Choi, M.-H.; Han, S.-Y.; Kim, C.; Woo, S.K.; Shin, W. New insights into the mechanisms of O–O bond cleavage of hydrogen peroxide and tert-alkyl hydroperoxides by iron (III) porphyrin complexes. J. Am. Chem. Soc. 2000, 122, 8677–8684. [Google Scholar] [CrossRef]
- Kusamoto, T.; Kume, S.; Nishihara, H. Realization of SOMO–HOMO Level Conversion for a TEMPO-Dithiolate Ligand by Coordination to Platinum (II). J. Am. Chem. Soc. 2008, 130, 13844–13845. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.A.; Mashiko, T.; Bentley, S.P.; Kastner, M.E.; Scheidt, W.R.; Spartalian, K.; Lang, G. The missing heme spin state and a model for cytochrome c’. The mixed S = 3/2, 5/2 intermediate spin ferric porphyrin: Perchlorato (meso-tetraphenylporphinato) iron (III). J. Am. Chem. Soc. 1979, 101, 2948–2958. [Google Scholar] [CrossRef]
- Zhong, R.-L.; Xu, H.-L.; Muhammad, S.; Zhang, J.; Su, Z.-M. The stability and nonlinear optical properties: Encapsulation of an excess electron compound LiCN…Li within boron nitride nanotubes. J. Mater. Chem. 2012, 22, 2196–2202. [Google Scholar] [CrossRef]
- Hong, S.; Lee, Y.-M.; Cho, K.-B.; Seo, M.S.; Song, D.; Yoon, J.; Garcia-Serres, R.; Clémancey, M.; Ogura, T.; Shin, W.; et al. Conversion of high-spin iron(III)–alkylperoxo to iron(IV)–oxo species via O–O bond homolysis in nonheme iron models. Chem. Sci. 2014, 5, 156–162. [Google Scholar] [CrossRef]
- Fernández, I.; Pedro, J.R.; Roselló, A.L.; Ruiz, R.; Castro, I.; Ottenwaelder, X.; Journaux, Y. Alcohol Oxidation by Dioxygen and Aldehydes Catalysed by Square-Planar Cobalt (III) Complexes of Disubstituted Oxamides and Related Ligands. Eur. J. Org. Chem. 2001, 2001, 1235–1247. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Louloudi, M.; Deligiannakis, Y. Complete dechlorination of pentachlorophenol by a heterogeneous SiO2–Fe–porphyrin catalyst. Appl. Catal. B Environ. 2010, 95, 297–302. [Google Scholar] [CrossRef]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Kim, M.G.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zheng, Y.; Jiang, X.; Zhang, R.; Pei, K.; Chen, W. Carbon-Based Oxamate Cobalt(III) Complexes as Bioenzyme Mimics for Contaminant Elimination in High Backgrounds of Complicated Constituents. Materials 2017, 10, 1169. https://doi.org/10.3390/ma10101169
Li N, Zheng Y, Jiang X, Zhang R, Pei K, Chen W. Carbon-Based Oxamate Cobalt(III) Complexes as Bioenzyme Mimics for Contaminant Elimination in High Backgrounds of Complicated Constituents. Materials. 2017; 10(10):1169. https://doi.org/10.3390/ma10101169
Chicago/Turabian StyleLi, Nan, Yun Zheng, Xuemei Jiang, Ran Zhang, Kemei Pei, and Wenxing Chen. 2017. "Carbon-Based Oxamate Cobalt(III) Complexes as Bioenzyme Mimics for Contaminant Elimination in High Backgrounds of Complicated Constituents" Materials 10, no. 10: 1169. https://doi.org/10.3390/ma10101169