Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ch/G Scaffolds
2.3. Preparation of Nanoparticle Solutions
2.4. Scaffold Characterization
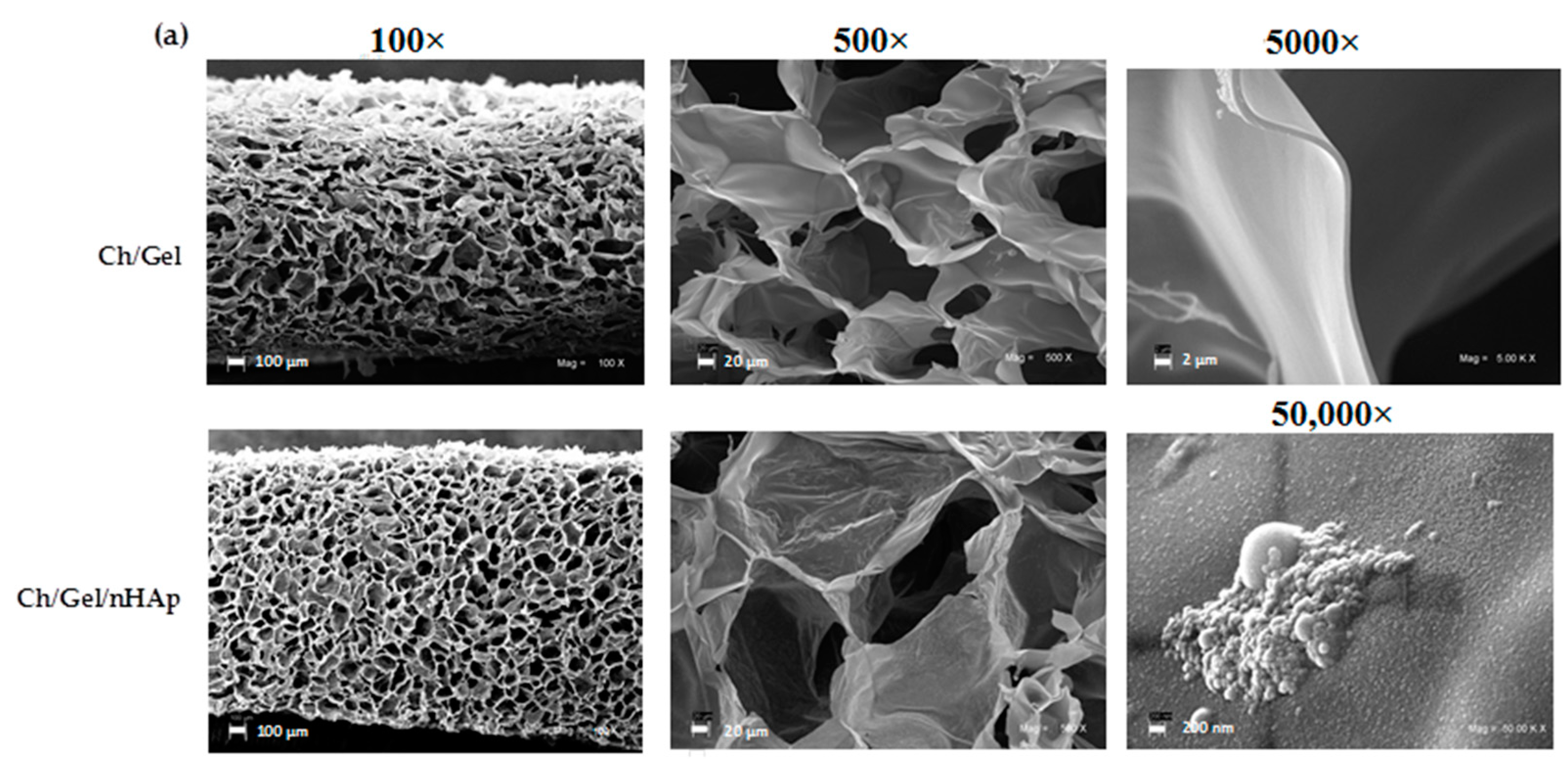
2.4.1. Microstructure, Porosity and Roughness
2.4.2. Determination of Glass Transition Temperature (Tg)
2.5. Cell Culture Studies
2.5.1. Cell Seeding and Culture on Scaffolds
2.5.2. Cell Attachment and Proliferation
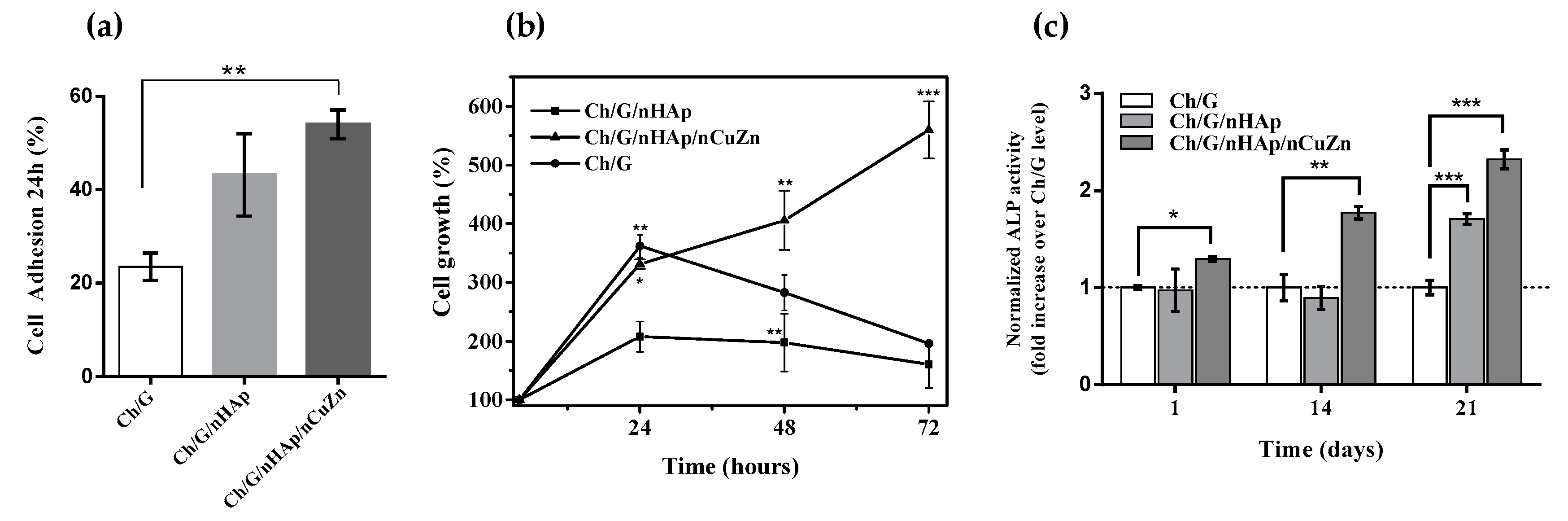
2.5.3. Alkaline Phosphatase Activity
2.6. In Vivo Biocompatibility Assay
2.6.1. Surgical Procedure
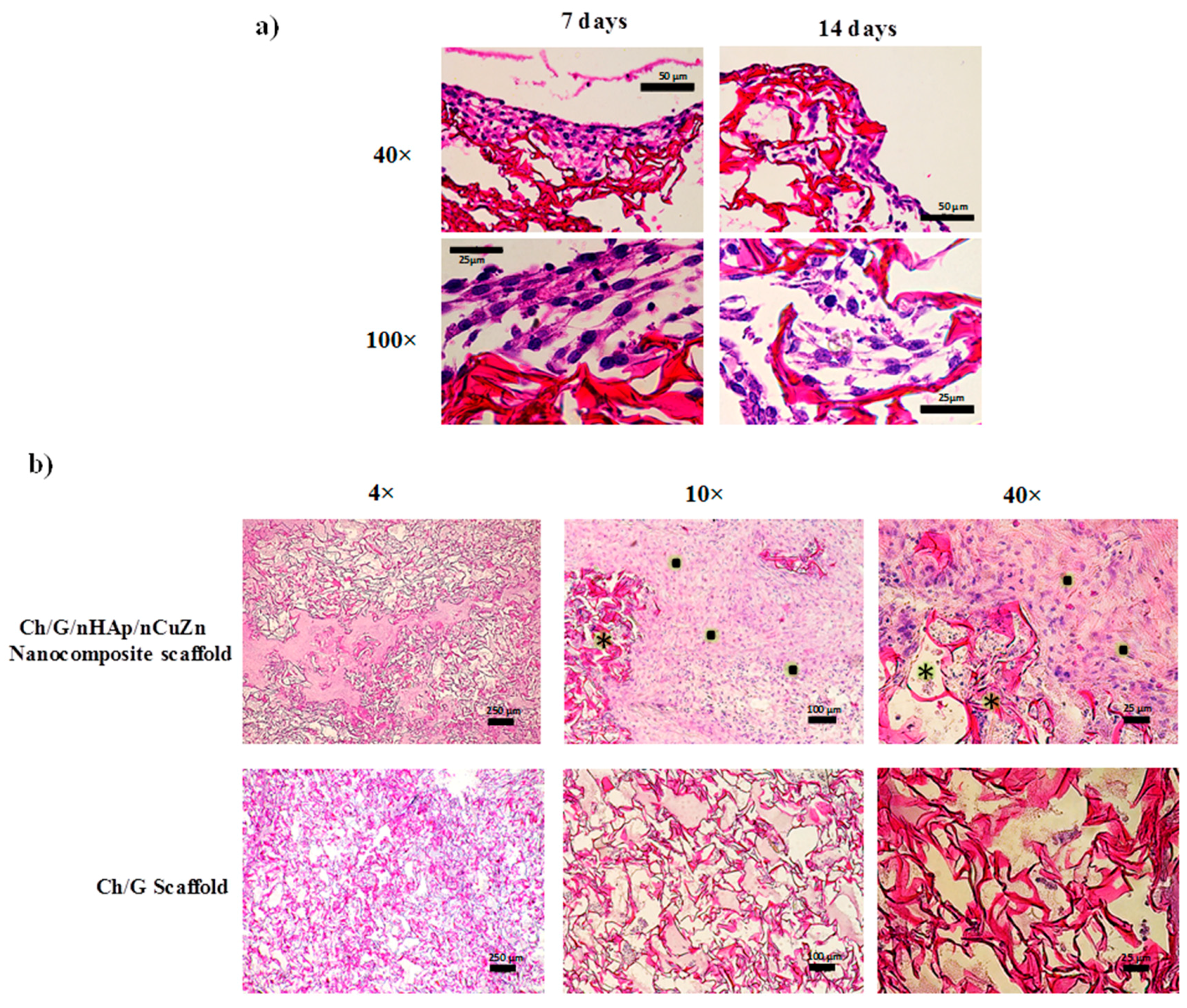
2.6.2. Histochemistry
2.7. Statistical Analysis and Experimental Design
3. Results
3.1. Ch/G Scaffold and Nanocomposite Scaffold Characterization
3.2. Influence of Nanoparticle Content and Crosslinker on Glass Transition Temperature (Tg)
3.3. Cell Attachment, Proliferation and Alkaline Phosphatase Activity
3.4. Biocompatibility Analysis of Ch/G/nHAp/nCuZn Scaffold
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Schonwalder, S.M.; Bally, F.; Heinke, L.; Azucena, C.; Bulut, O.D.; Heissler, S.; Kirschhofer, F.; Gebauer, T.P.; Neffe, A.T.; Lendlein, A.; et al. Interaction of human plasma proteins with thin gelatin-based hydrogel films: A QCM-D and ToF-SIMS study. Biomacromolecules 2014, 15, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Mohanty, C.; Harilal, A.; Maheswari, U.K.; Sahoo, S.K.; Krishnakumar, S. A novel in vitro three-dimensional retinoblastoma model for evaluating chemotherapeutic drugs. Mol. Vis. 2012, 18, 1361–1378. [Google Scholar] [PubMed]
- Zhan, J.; Lan, P. The review on electrospun gelatin fiber scaffold. J. Res. Updat. Polym. Sci. 2012, 1, 59–71. [Google Scholar]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and evaluation of gelatin-chitosan-nanobioglass 3D porous scaffold for bone tissue engineering. Int. J. Biomater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Mokhtar, M.; Hassan, M.I.; Jin, R.M.; Roozbahani, F.; Khan, T.H. Chitosan-based nanocomposite scaffolds for tissue engineering applications. Mater. Manuf. Process. 2015, 30, 273–278. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.K. Nano-hydroxyapatite composite biomaterials for bone tissue engineering—A review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Kim, K.; Patel, M.; Fisher, J.P. Nanomaterials for musculoskeletal tissue engineering. In Nanobiomaterials Handbook; Sitharaman, B., Ed.; CRC Press: Boca Raton, FL, USA, 2011; Volume 1. [Google Scholar]
- Mathur, V.; Sharma, K. Evaluation of morphological effect on thermal and mechanical performance of PS/PMMA/CdS nanocomposite systems. Adv. Nanopart. 2013, 2, 205–216. [Google Scholar] [CrossRef]
- Michel, J.; Penna, M.; Kochen, J.; Cheung, H. Recent advances in hydroxyapatite scaffolds containing mesenchymal stem cells. Stem Cells Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Sahoo, S.; Ng, K.S.; Chen, K.; Toh, S.L.; Goh, J.C. Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. J. Biomed. Mater. Res. A 2013, 101, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Elgendy, E.A.; Abo Shady, T.E. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J. Indian Soc. Periodontol. 2015, 19, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Saranya, N.; Saravanan, S.; Ramasamy, K.; Srinivasan, N.; Selvamurugan, N. Synthesis, characterization, and antimicrobial activity of nano-hydroxyapatite-zinc for bone tissue engineering applications. J. Nanosci. Nanotechnol. 2012, 12, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sahithi, K.; Swetha, M.; Prabaharan, M.; Moorthi, A.; Saranya, N.; Ramasamy, K.; Srinivasan, N.; Partridge, N.C.; Selvamurugan, N. Synthesis and characterization of nanoscale-hydroxyapatite-copper for antimicrobial activity towards bone tissue engineering applications. J. Biomed. Nanotechnol. 2010, 6, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Bizios, R. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J. Biomed. Mater. Res. 2002, 59, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Kappel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. A 2012, 100, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Cho, Y.E.; Kim, T.; Shin, H.I.; Kwun, I.S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K.; Onuma, K.; Kanzaki, N.; Sogo, Y.; Ichinose, N. Zinc-releasing calcium phosphate for stimulating bone formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper-zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan-gelatin-alginate-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2016, 64, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Genove, E.; Borros, S.; Semino, C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006, 12, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar] [PubMed]
- Jiang, X.; Yu, F.; Wang, Z.; Li, J.; Tan, H.; Ding, M.; Fu, Q. Fabrication and characterization of waterborne biodegradable polyurethanes 3-dimensional porous scaffolds for vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2010, 21, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Chesnutt, B.M.; Viano, A.M.; Yuan, Y.; Yang, Y.; Guda, T.; Appleford, M.R.; Ong, J.L.; Haggard, W.O.; Bumgardner, J.D. Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration. J. Biomed. Mater. Res. A 2009, 88, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Schwartz, Z.; Raines, A.L.; Boyan, B.D. The effect of substrate microtopography on osseointegration of titanium implants. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 343–352. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.T.; Marquez, M.; Xia, Y. Highly porous fibers by electrospinning into a cryogenic liquid. J. Am. Chem. Soc. 2006, 128, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Bag, S.; Pal, S. Development of porous ultra high molecular weight polyethylene scaffolds for the fabrication of orbital implant. J. Porous Mater. 2008, 15, 53–59. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef] [PubMed]
- Pazmino Betancourt, B.A.; Douglas, J.F.; Starr, F.W. Fragility and cooperative motion in a glass-forming polymer-nanoparticle composite. Soft Matter 2013, 9, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Ash, B.J.; Schadler, L.S.; Siegel, R.W. Glass transition behavior of alumina/polymethylmethacrylate nanocomposites. Mater. Lett. 2002, 55, 83–87. [Google Scholar] [CrossRef]
- Putz, K.W.; Palmeri, M.J.; Cohn, R.B.; Andrews, R.; Brinson, L.C. Effect of cross-link density on interphase creation in polymer nanocomposites. Macromolecules 2008, 41, 6752–6756. [Google Scholar] [CrossRef]
- Yu, J.W.; Jung, J.; Choi, Y.-M.; Choi, J.H.; Yu, J.; Lee, J.K.; You, N.-H.; Goh, M. Enhancement of the crosslink density, glass transition temperature, and strength of epoxy resin by using functionalized graphene oxide co-curing agents. Polym. Chem. 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Maitra, J.; Kumar, S. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-Y.; Noh, I.-S.; Zhang, S.-M. Silicate-doped hydroxyapatite and its promotive effect on bone mineralization. Front. Mater. Sci. 2013, 7, 40–50. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Mohamed, K.R.; Beherei, H.H.; El-Rashidy, Z.M. In vitro study of nano-hydroxyapatite/chitosan—Gelatin composites for bio-applications. J. Adv. Res. 2014, 5, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Chiang, H.; Kuo, T.F.; Lee, H.S.; Jiang, C.C.; Tuan, R.S. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: A pilot study. J. Tissue Eng. Regen. Med. 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010, 6, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dong, W.J.; He, F.M.; Wang, X.X.; Zhao, S.F.; Yang, G.L. Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, I.; Luthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.G.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Kumbicak, U.; Cavas, T.; Cinkilic, N.; Kumbicak, Z.; Vatan, O.; Yilmaz, D. Evaluation of in vitro cytotoxicity and genotoxicity of copper-zinc alloy nanoparticles in human lung epithelial cells. Food Chem. Toxicol. 2014, 73, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Diaz-Calderon, P.; Weinstein-Oppenheimer, C.R.; Sanchez, E.; Fuentes, M.A.; Brown, D.I.; Herrera, H.; Acevedo, C.A. Designing a gelatin/chitosan/hyaluronic acid biopolymer using a thermophysical approach for use in tissue engineering. Bioprocess Biosyst. Eng. 2013, 36, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Tommila, M.; Jokinen, J.; Wilson, T.; Forsback, A.P.; Saukko, P.; Penttinen, R.; Ekholm, E. Bioactive glass-derived hydroxyapatite-coating promotes granulation tissue growth in subcutaneous cellulose implants in rats. Acta Biomater. 2008, 4, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef] [PubMed]



| Factor | Glass Transition (First Scan) | |||
|---|---|---|---|---|
| Onset | Tg | Endpoint | ∆Cp | |
| A | 0.62 | 0.32 | 0.06 | 0.05 * |
| B | 0.19 | 0.12 | 0.04 * | 0.03 * |
| A × B | 0.16 | 0.06 | 0.01 * | 0.06 |
| Scaffold Designation | Composition | |||
|---|---|---|---|---|
| Chitosan (Ch) 1% | Gelatin (Gel) 1% | nHAp 0.25% | nCuZn Alloy 0.01% | |
| Final Concentrations in Blend (%) | ||||
| Ch/Gel | 0.5 | 0.25 | - | - |
| Ch/Gel/nHAp | 0.5 | 0.25 | 0.0625 | - |
| Ch/Gel/nHAp/nCuZn | 0.5 | 0.25 | 0.0625 | 0.00025 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. https://doi.org/10.3390/ma10101177
Forero JC, Roa E, Reyes JG, Acevedo C, Osses N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials. 2017; 10(10):1177. https://doi.org/10.3390/ma10101177
Chicago/Turabian StyleForero, Juan Carlos, Eduardo Roa, Juan G. Reyes, Cristian Acevedo, and Nelson Osses. 2017. "Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold" Materials 10, no. 10: 1177. https://doi.org/10.3390/ma10101177





