Effect of Dentin Wetness on the Bond Strength of Universal Adhesives
Abstract
:1. Introduction
2. Results
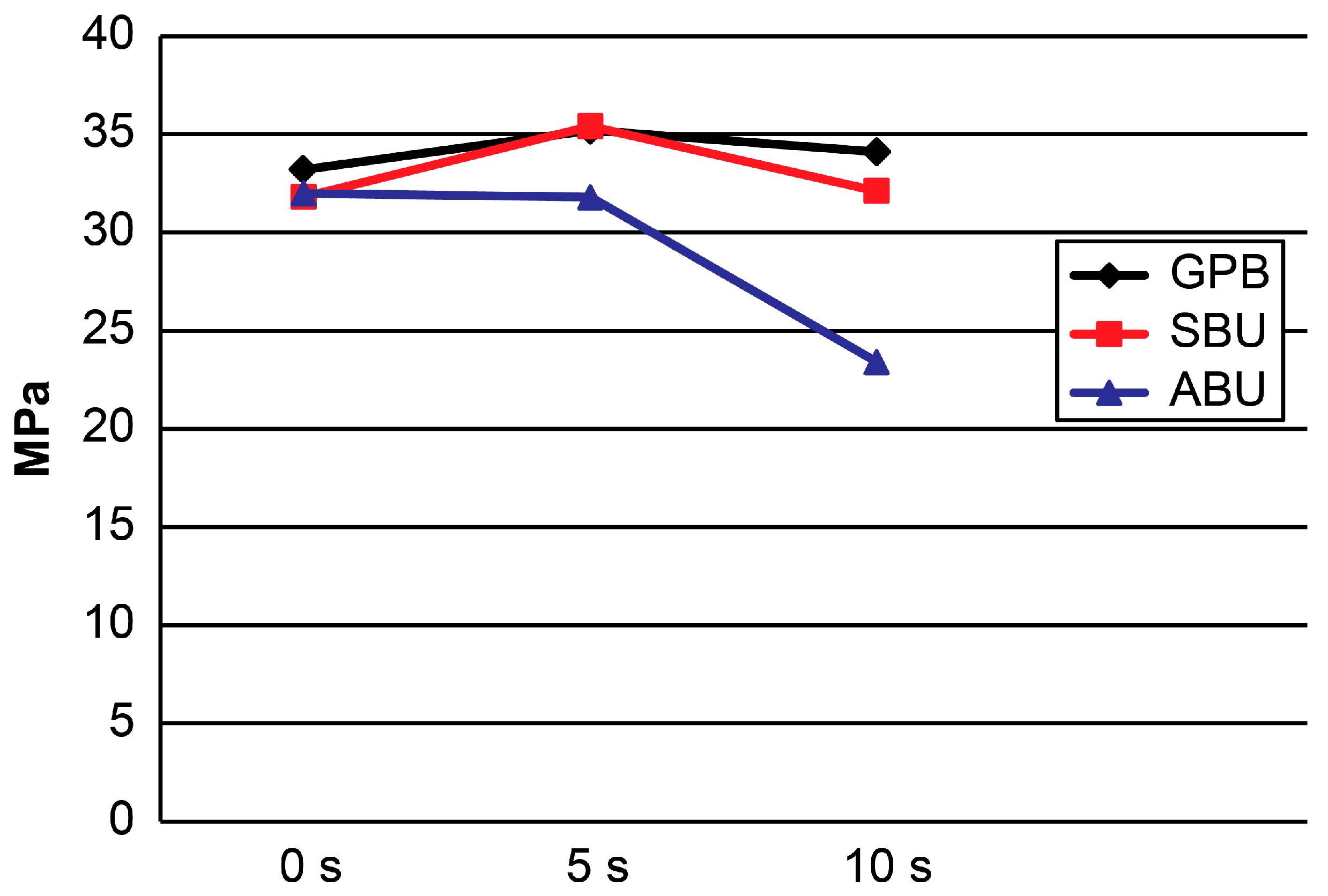
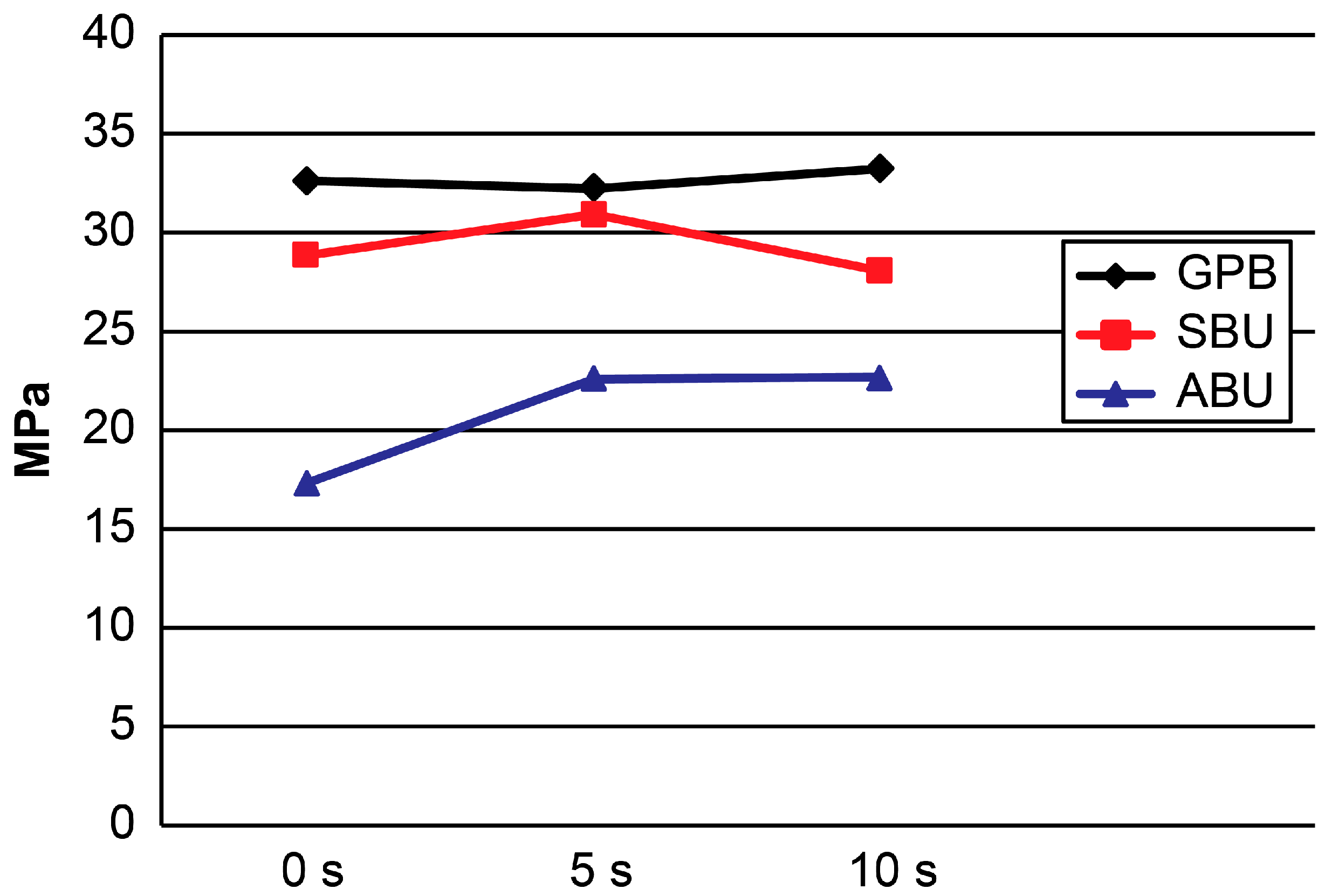
2.1. μTBS
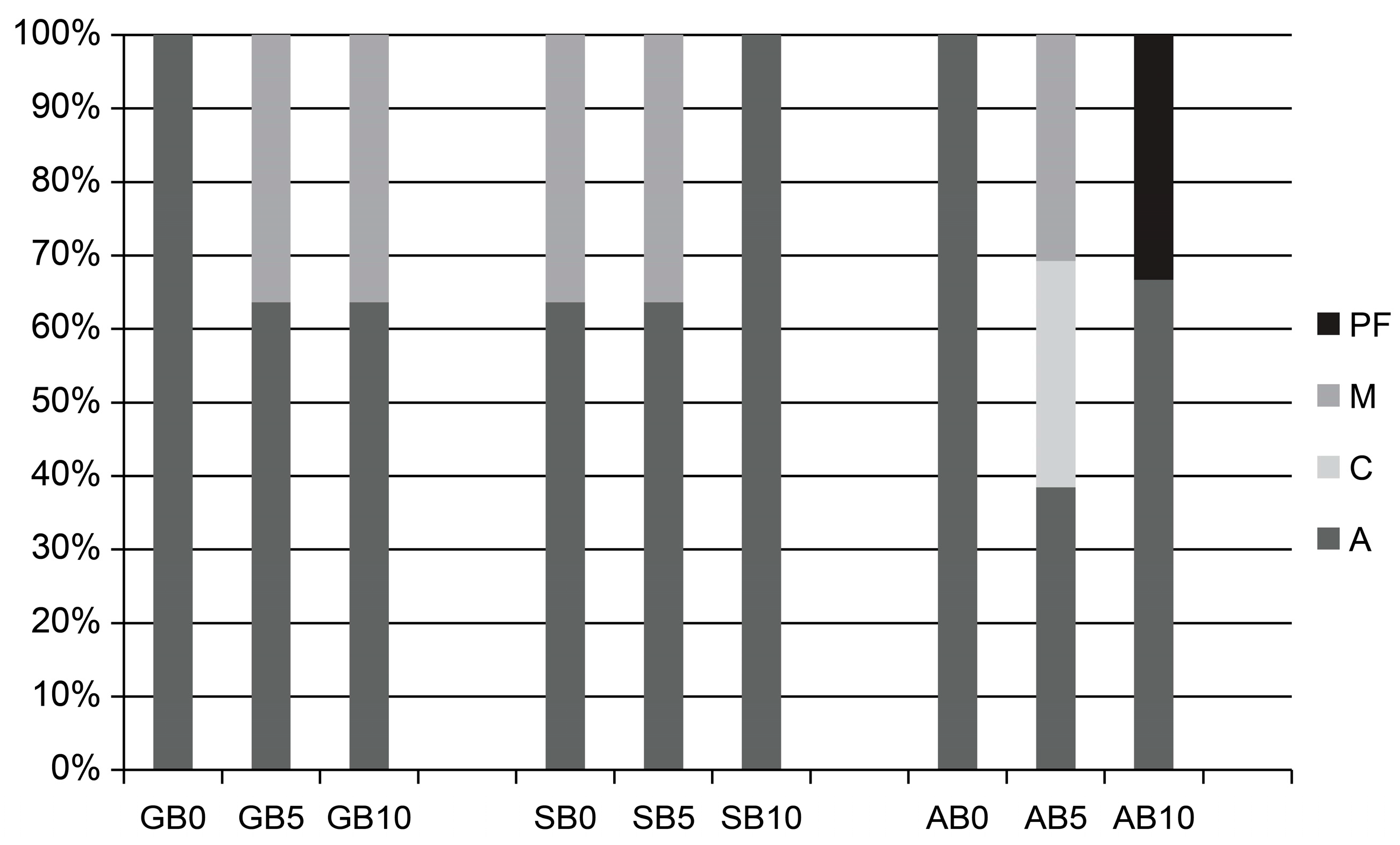
2.2. Failure Mode Analysis
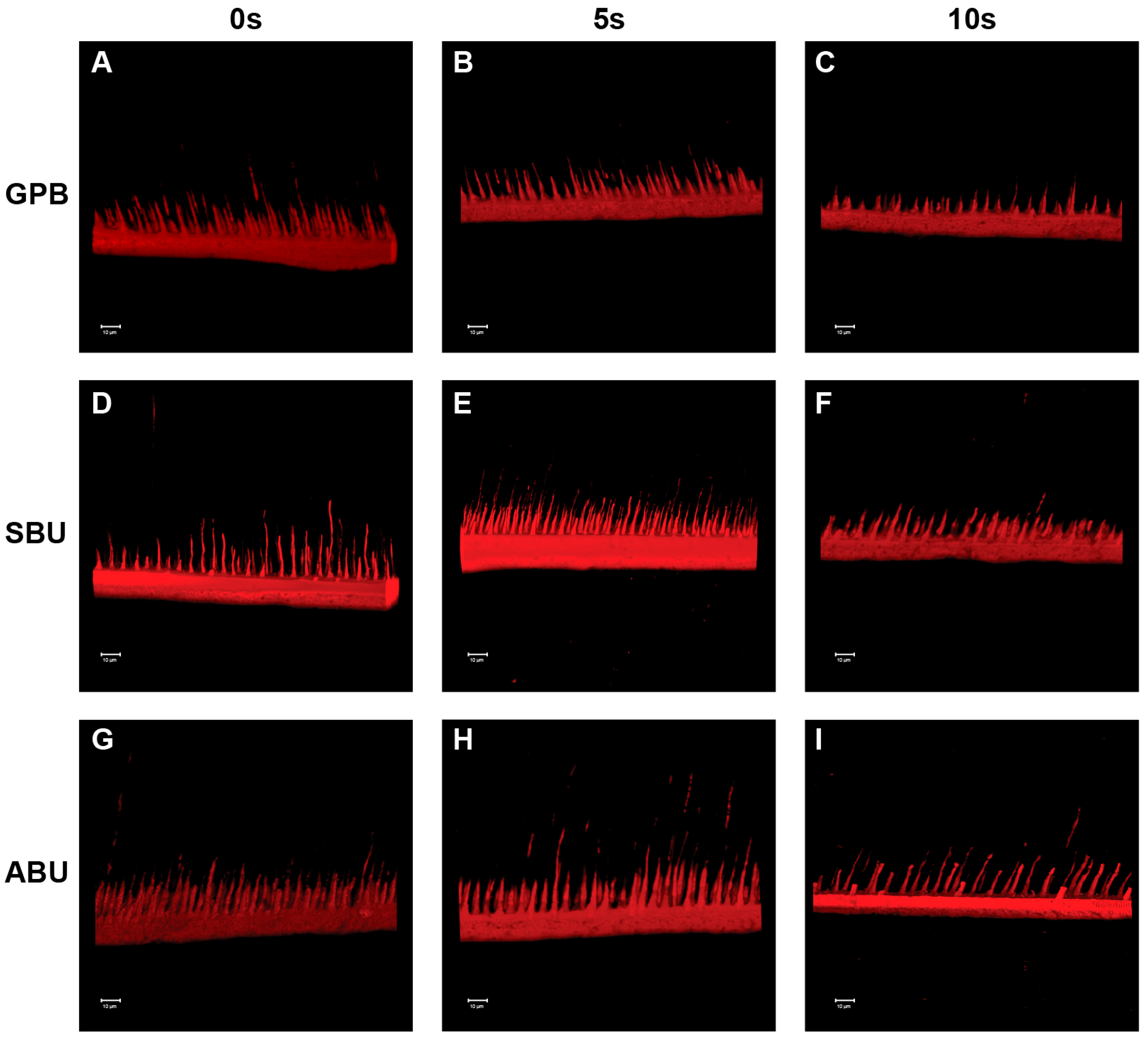
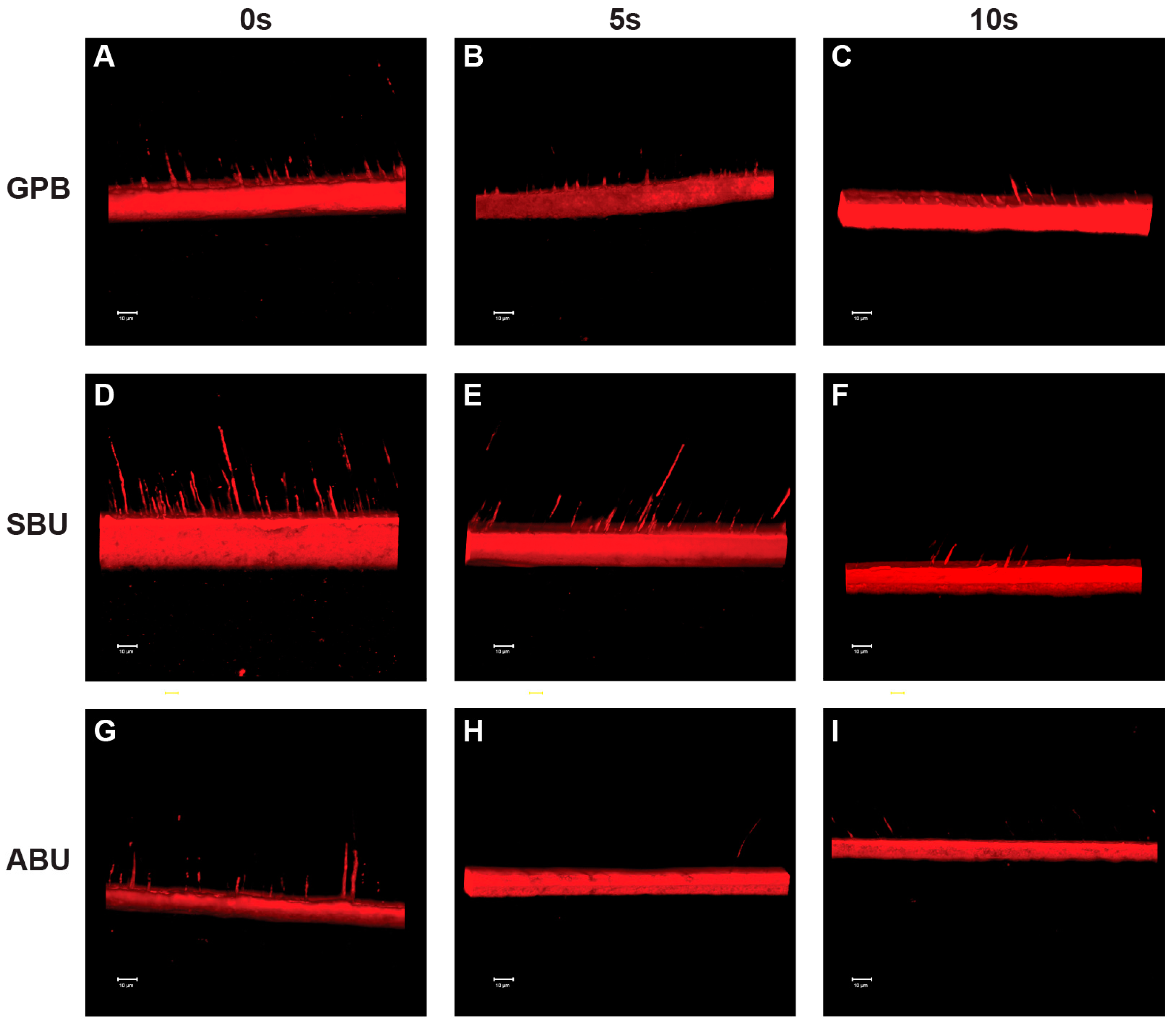
2.3. Confocal Laser Scanning Microscopy (CLSM) Analysis
3. Discussion
4. Materials and Methods
4.1. Tooth Selection and Preparation
4.2. Experimental Design and Specimen Preparation
4.3. Microtensile Bond Strength (μTBS)
4.4. Failure Mode Analysis
4.5. Confocal Laser Scanning Microscopy Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Barroso, L.P.; Grande, R.H.; Reis, A. Comparison of intra- and intertooth resin–dentin bond strength variability. J. Adhes. Dent. 2005, 7, 151–158. [Google Scholar] [PubMed]
- Pashley, D.H.; Tay, F.R.; Carvalho, R.M.; Rueggeberg, F.A.; Agee, K.A.; Carrilho, M.; Donnelly, A.; García-Godoy, F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am. J. Dent. 2007, 20, 7–20. [Google Scholar] [PubMed]
- Gwinnett, A.J. Chemically conditioned dentin: A comparison of conventional and environmental scanning electron microscopy findings. Dent. Mater. 1994, 10, 150–155. [Google Scholar] [CrossRef]
- Maciel, K.T.; Carvalho, R.M.; Ringle, R.D.; Preston, C.D.; Russell, C.M.; Pashley, D.H. The effects of acetone, ethanol, HEMA, and air on the stiffness of human decalcified dentin matrix. J. Dent. Res. 1996, 75, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E.; Peutzfeldt, A. The influence of relative humidity on the effect of dentin bonding systems. J. Adhes. Dent. 2001, 3, 123–127. [Google Scholar] [PubMed]
- Perdigão, J.; Frankenberger, R. Effect of solvent and rewetting time on dentin adhesion. Quintessence Int. 2001, 32, 385–390. [Google Scholar] [PubMed]
- Tay, F.R.; Pashley, D.H. Have dentin adhesives become too hydrophilic? J. Can. Dent. Assoc. 2003, 69, 726–731. [Google Scholar] [PubMed]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent. Mater. 2001, 17, 296–308. [Google Scholar] [CrossRef]
- Shirban, F.; Khoroushi, M.; Shirban, M. A new solvent-free one-step self-etch adhesive: Bond strength to tooth structures. J. Contempt. Dent. Pract. 2013, 14, 269–274. [Google Scholar] [CrossRef]
- De Goes, M.F.; Shinohara, M.S.; Freitas, M.S. Performance of a new one-step multi-mode adhesive on etched vs non-etched enamel on bond strength and interfacial morphology. J. Adhes. Dent. 2014, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J. Dent. Res. 2003, 82, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Alex, G. Universal adhesives: The next evolution in adhesive dentistry. Compend. Contin. Educ. Dent. 2015, 36, 15–26. [Google Scholar] [PubMed]
- Pashley, D.H.; Carvalho, R.M. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef]
- Silva e Souza, M.H., Jr.; Carneiro, K.G.; Lobato, M.F.; Silva e Souza Pde, A.; de Góes, M.F. Adhesive systems: Important aspects related to their composition and clinical use. J. Appl. Oral Sci. 2010, 18, 207–214. [Google Scholar] [CrossRef] [PubMed]
- GC Corporation. Safety Data Sheet for GC G-Premio Bond; GC Corporation: Tokyo, Japan, 2015. [Google Scholar]
- Pongprueksa, P.; Miletic, V.; De Munck, J.; Brooks, N.R.; Meersman, F.; Nies, E.; Van Meerbeek, B.; Van Landuyt, K.L. Effect of evaporation on the shelf life of a universal adhesive. Oper. Dent. 2014, 39, 500–507. [Google Scholar] [CrossRef] [PubMed]
- American Association for Dental Research (AADR) & International Association for Dental Research (IADR). Water Content (%) of Universal Adhesives; AADR & IADR: Alexandria, VA, USA, 2015. [Google Scholar]
- Hashimoto, M.; Fujita, S.; Kaga, M.; Yawaka, Y. Effect of water on bonding of one-bottle self-etching adhesives. Dent. Mater. J. 2008, 27, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Fujita, S.; Endo, K. Bonding of self-etching adhesives on dehydrated dentin. J. Adhes. Dent. 2011, 13, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tay, F.R.; Ito, S.; Sano, H.; Kaga, M.; Pashley, D.H. Permeability of adhesive resin films. J. Biomed. Mater. Res. 2005, 74, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Ito, S.; Tay, F.R.; Svizero, N.R.; Sano, H.; Kaga, M.; Pashley, D.H. Fluid movement across the resin-dentin interface during and after bonding. Dent. Res. 2004, 83, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Pellizzaro, A.; Dal-Bianco, K.; Gones, O.M.; Patzlaff, R.; Loguercio, A.D. Impact of adhesive application to wet and dry dentin on long-term resin dentin bond strengths. Oper. Dent. 2007, 32, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P.; Yao, X.; Brenda, B. Effect of solvent content on resin hybridization in wet dentin bonding. J. Biomed. Mater. Res. A 2007, 82, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, G.; Millas, A. Microscopic evaluation of dentin interface obtained with 10 contemporary self-etching systems: Correlation with their pH. Oper. Dent. 2005, 30, 481–491. [Google Scholar] [PubMed]
- Hashimoto, M.; de Gee, A.J.; Feilzer, A.J. Polymerization contraction stress in dentin adhesives bonded to dentin and enamel. Dent. Mater. 2008, 24, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Koshiro, K.; Sidhu, S.K.; Inoue, S.; Ikeda, T.; Sano, H. New concept of resin-dentin interfacial adhesion: The nanointeraction zone. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Pde, C.; Lopes, G.C.; Vieira, L.C.; Baratieri, L.N. Effect of solvent type on microtensile bond strength of a total-etch one-bottle adhesive system to moist or dry dentin. Oper. Dent. 2005, 30, 376–381. [Google Scholar] [PubMed]
- Finger, W.J.; Balkenhol, M. Practitioner variability effects on dentin bonding with an acetone-based one-bottle adhesive. J. Adhes. Dent. 1999, 1, 311–314. [Google Scholar] [PubMed]
- Jacobsen, T.; Söderholm, K.J. Effect of primer solvent, primer agitation, and dentin dryness on shear bond strength to dentin. Am. J. Dent. 1998, 11, 225–228. [Google Scholar] [PubMed]
- Reis, A.; Loguercio, A.D.; Azevedo, C.L.; de Carvalho, R.M.; da Julio Singer, M.; Grande, R.H. Moisture spectrum of demineralized dentin for adhesive systems with different solvent bases. J. Adhes. Dent. 2003, 5, 183–192. [Google Scholar] [PubMed]
- Fundingsland, J.W.; Aasen, S.M.; Bodger, P.D.; Cernhous, J.J. The effect of high humidity on adhesion to dentine. J. Dent. Res. 1992, 71, 665. [Google Scholar]
- Sauro, S.; Pashley, D.H.; Montanari, M.; Chersoni, S.; Carvalho, R.M.; Toledano, M.; Osorio, R.; Tay, F.R.; Prati, C. Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent. Mater. 2007, 23, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.; Nakabayashi, N. The durability of adhesion to phosphoric acid etched, wet dentin substrates. Dent. Mater. 1998, 14, 347–352. [Google Scholar] [CrossRef]
- Armstrong, S.R.; Keller, J.C.; Boyer, D.B. The influence of water storage and C-factor on the dentin-resin composite microtensile bond strength and debond pathway utilizing a filled and unfilled adhesive resin. Dent. Mater. 2001, 17, 268–276. [Google Scholar] [CrossRef]
- De Munck, J.; Shirai, K.; Yoshida, Y.; Inoue, S.; Van Landuyt, K.; Lambrechts, P.; Suzuki, K.; Shintani, H.; Van Meerbeek, B. Effect of water storage on the bonding effectiveness of 6 adhesives to class I cavity dentin. Oper. Dent. 2006, 30, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water—Effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Santerre, J.P.; Shajii, L.; Leung, B.W. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit. Rev. Oral Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, G.; Dabsie, F.; Delannée, M.; Akon, B.; Sharrock, P. Water permeability, hybrid layer long-term integrity and reaction mechanism of a two-step adhesive system. J. Dent. 2010, 38, 526–533. [Google Scholar] [CrossRef] [PubMed]

| Adhesive | Etch-and-Rinse Mode | |||
| 0 s | 5 s | 10 s | p-value | |
| GPB | 33.2 ± 3.3 a | 35.2 ± 4.5 a | 34.1 ± 2.4 a | 0.868 |
| SBU | 31.8 ± 3.9 a | 35.4 ± 4.9 a | 32.1 ± 4.0 a | 0.105 |
| ABU | 32.0 ± 3.1 a | 31.8 ± 2.6 a | 23.4 ± 2.2 b | <0.001 |
| Adhesive | Self-Etch Mode | |||
| 0 s | 5 s | 10 s | p-value | |
| GPB | 32.8 ± 4.3 a | 32.4 ± 2.6 a | 33.4 ± 1.9 a | 0.760 |
| SBU | 29.0 ± 3.6 a | 31.1 ± 4.9 a | 28.2 ± 2.8 a | 0.226 |
| ABU | 17.4 ± 2.6 a | 22.7 ± 5.5 b | 22.8 ± 2.5 b | 0.003 |
| Material | pH | Composition | Application Mode | |
|---|---|---|---|---|
| Self-Etch | Etch-and-Rinse | |||
| G-Premio Bond (GPB) GC Corp. Tokyo, Japan P | 1.5 | 10-MDP, phosphoric acid ester monomer, dimethacrylate, 4-MET, MEPS, acetone, silicon dioxide, initiators | 1. The only difference is the dentin drying time: 0, 5, and 10 s 2. Apply the adhesive to the entire preparation without rubbing for 10 s 3. Direct a gentle stream of air over the liquid for about 5 s until it no longer moves and the solvent is evaporated completely | 1. Apply etchant for 15 s 2. Rinse thoroughly for 5 s 3. The only difference is the dentin drying time: 0, 5, and 10 s 4. Apply adhesive as in the self-etch mode |
| Single-bond Universal (SBU) 3M ESPE Seefeld, Germany | 2.7 | 10-MDP, phosphoric acid ester monomer, HEMA, silane, dimethacrylate, Vitrebond copolymer, filler, ethanol, water, initiators, silane | 1. The only difference is the dentin drying time: 0, 5, and 10 s 2. Apply the adhesive to the entire preparation with a microbrush and rub it in for 20 s 3. Direct a gentle stream of air over the liquid for about 5 s until it no longer moves and the solvent is evaporated completely 4. Light-cure for 10 s | 1. Apply etchant for 15 s 2. Rinse thoroughly for 10 s 3. The only difference is the dentin drying time: 0, 5, and 10 s 4. Apply adhesive as in the self-etch mode |
| All-Bond Universal (ABU) Bisco Schaumburg, USA | 3.2 | 10-MDP, phosphoric acid ester monomer, Bis-GMA, HEMA, ethanol, water, initiators | 1. The only difference is the dentin drying time: 0, 5, and 10 s 2. Apply two separate coats of adhesive, scrubbing the preparation with a microbrush for 10–15 s per coat. Do not light cure between coats 3. Evaporate excess solvent by thoroughly air-drying with an air syringe for at least 10 s, there should be no visible movement of the material. The surface should have a uniform glossy appearance 4. Light cure for 10 s | 1. Apply etchant for 15 s 2. Rinse thoroughly for 10 s 3. The only difference is the dentin drying time: 0, 5, and 10 s 4. Apply adhesive as in the self-etch mode |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, A.-N.; Lee, J.-H.; Son, S.-A.; Jung, K.-H.; Kwon, Y.H.; Park, J.-K. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials 2017, 10, 1224. https://doi.org/10.3390/ma10111224
Choi A-N, Lee J-H, Son S-A, Jung K-H, Kwon YH, Park J-K. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials. 2017; 10(11):1224. https://doi.org/10.3390/ma10111224
Chicago/Turabian StyleChoi, An-Na, Ji-Hye Lee, Sung-Ae Son, Kyoung-Hwa Jung, Yong Hoon Kwon, and Jeong-Kil Park. 2017. "Effect of Dentin Wetness on the Bond Strength of Universal Adhesives" Materials 10, no. 11: 1224. https://doi.org/10.3390/ma10111224





