Sealing Ability of Alkaline Endodontic Cements versus Resin Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Teeth
2.2. Placement of Materials
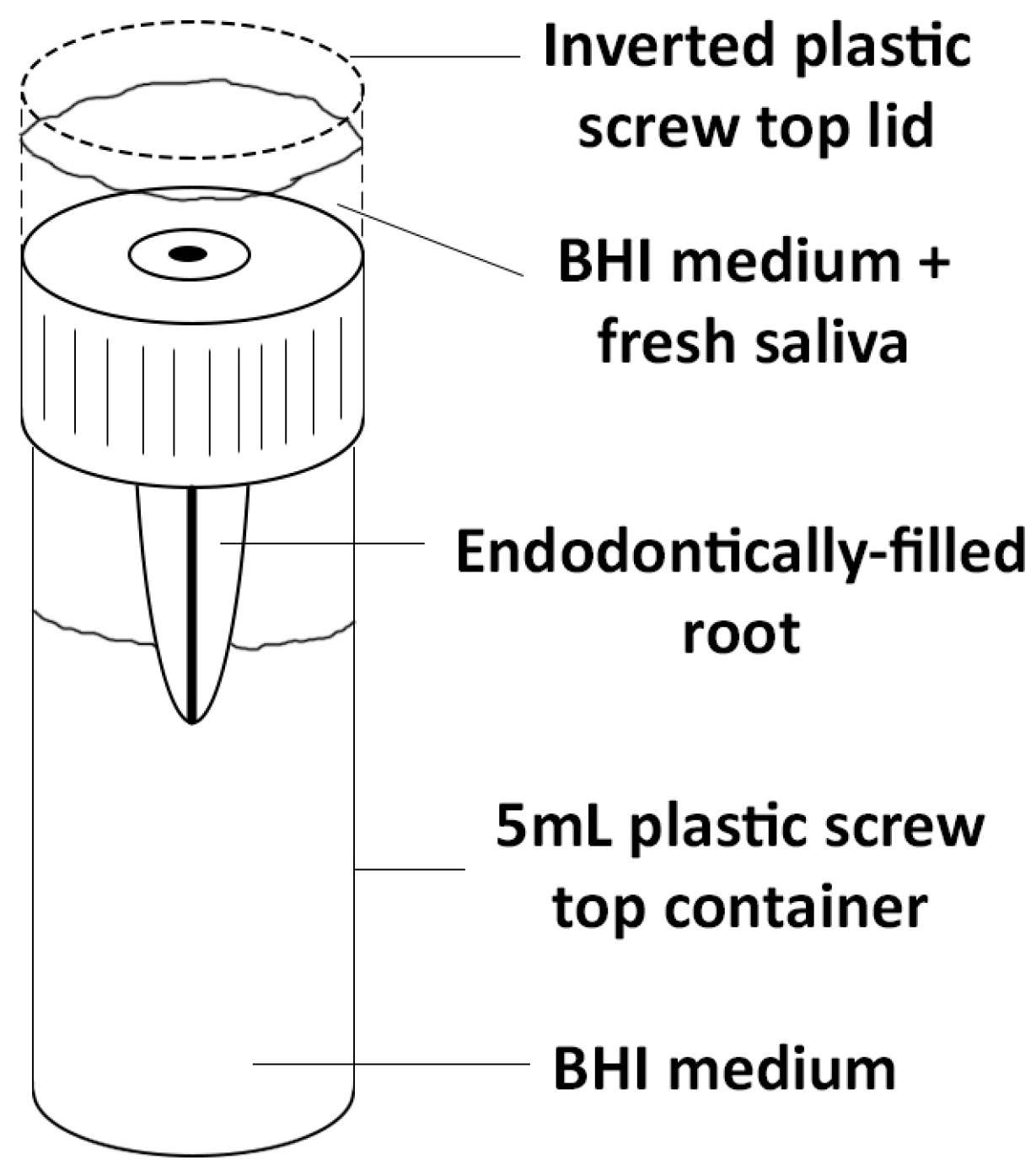
2.3 Mounting of Samples
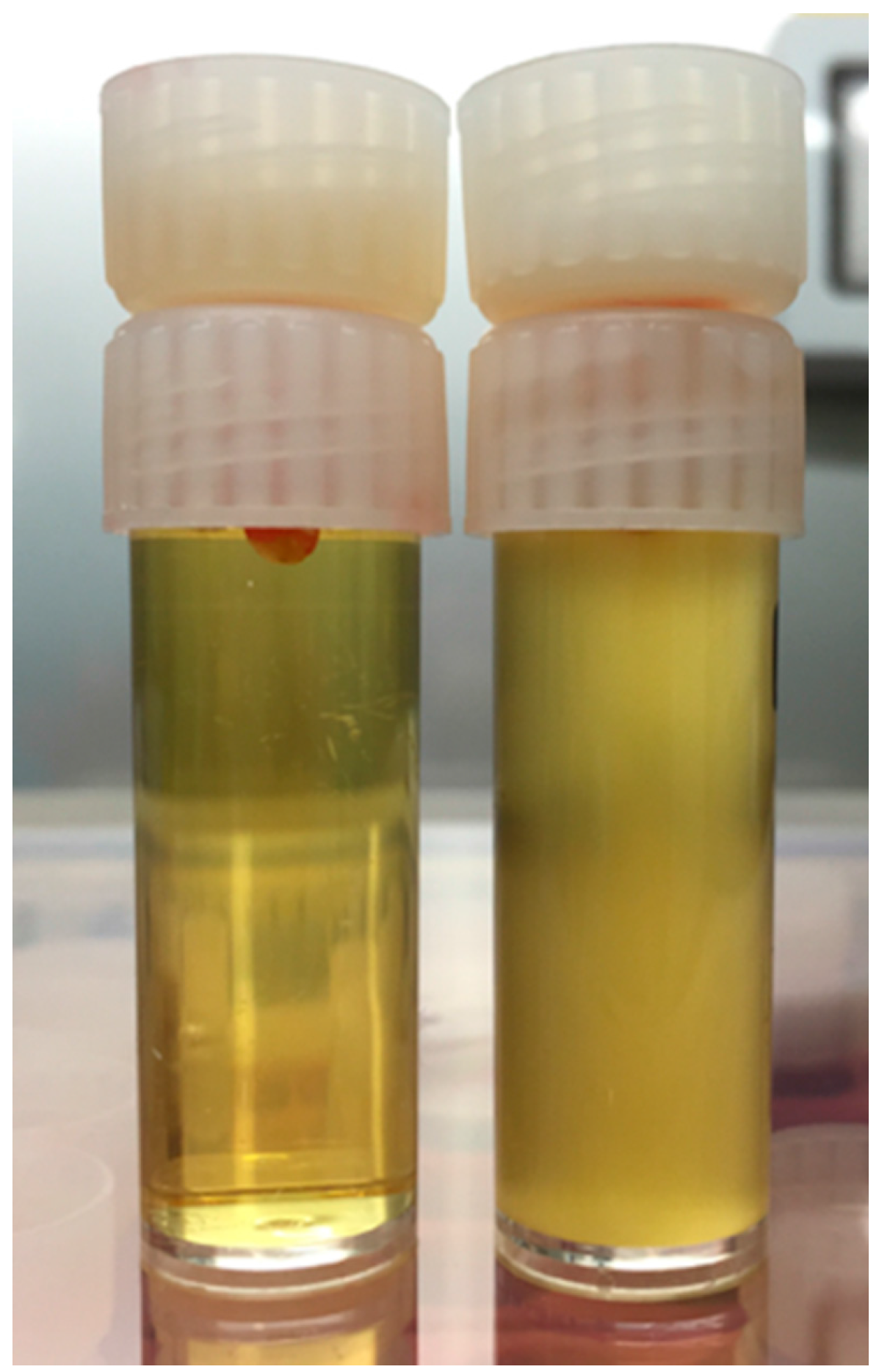
2.4 Exposure to Bacteria
2.5 Statistical Analysis
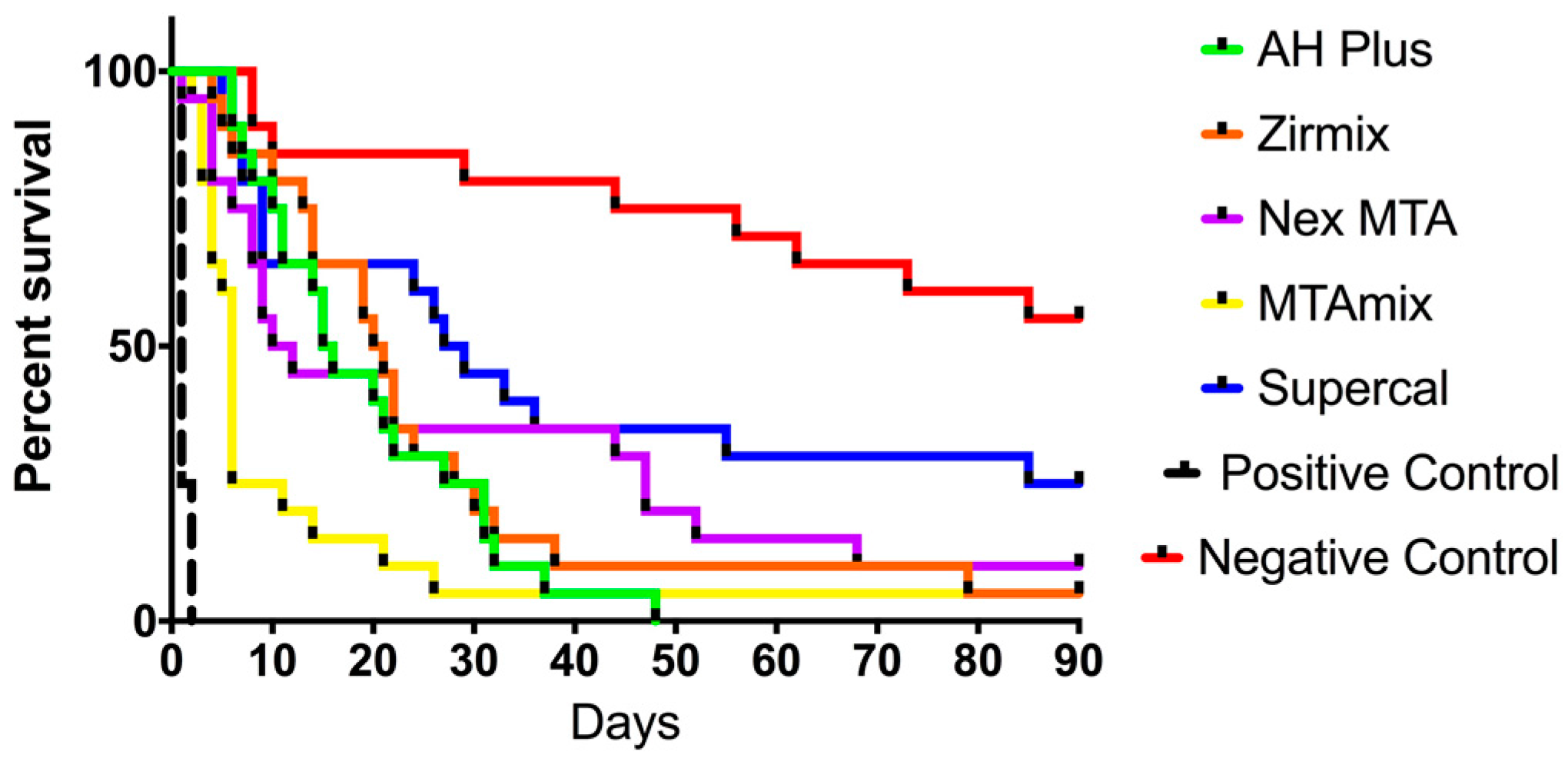
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Sundqvist, G.; Figdor, D. Endodontic Treatment of Apical Periodontitis. In Essential Endodontology: Prevention and Treatment of Apical Periodontitis; Ørstavik, D., Pitt Ford, T.R., Eds.; Blackwell Science: Malden, MA, USA, 1998; pp. 242–277. [Google Scholar]
- Kishen, A.; Peters, O.A.; Zehnder, M.; Diogenes, A.R.; Nair, M.K. Advances in endodontics: Potential applications in clinical practice. J. Conserv. Dent. 2016, 19, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Eleazer, P.D.; Gilbert, G.H.; Funkhouser, E.; Reams, G.J.; Law, A.S.; Benjamin, P.L. Techniques and materials used by general dentists during endodontic treatment procedures: Findings from the national dental practice-based research network. J. Am. Dent. Assoc. 2016, 147, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gatewood, R.S. Endodontic materials. Dent. Clin. N. Am. 2007, 51, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Schilder, H.; Goodman, A.; Aldrich, W. The thermomechanical properties of gutta-percha. Part v. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 285–296. [Google Scholar] [CrossRef]
- De Gee, A.J.; Wu, M.K.; Wesselink, P.R. Sealing properties of ketac-endo glass ionomer cement and AH26 root canal sealers. Int. Endod. J. 1994, 27, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, D.; Ritter, A.; Burkhart, A.; Fischer, J.; Mondrzyk, A.; Ritter, H. Experimental amine-epoxide sealer: A physicochemical study in comparison with AH plus and easy seal. Int. Endod. J. 2015, 48, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D.; Nordahl, I.; Tibballs, J.E. Dimensional change following setting of root canal sealer materials. Dent. Mater. 2001, 17, 512–519. [Google Scholar] [CrossRef]
- Athanassiadis, B.; Abbott, P.V.; Walsh, L.J. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust. Dent. J. 2007, 52, S64–S82. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rocas, I.N.; Loper, H.P.; Uzeda, M. Coronal leakage of two root canal sealers containing calcium hydroxide after exposure to human saliva. J. Endod. 1999, 25, 14–16. [Google Scholar] [CrossRef]
- Timpawat, S.; Cholticha, A.; Wirong-rong, T. Bacterial coronal leakage after obturation with three root canal sealers. J. Endod. 2001, 27, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Athanassiadis, B.; Walsh, L.J. Alkaline Compositions and Their Dental and Medical Use. World Patent WIPO WO2013/013275A1, 4 March 2015. [Google Scholar]
- Torabinejad, M.; Ung, B.; Kettering, J.D. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J. Endod. 1990, 16, 566–569. [Google Scholar] [CrossRef]
- Khayat, A.; Lee, S.-J.; Torabinejad, M. Human saliva penetration of coronally unsealed obturated root canals. J. Endod. 1993, 19, 458–461. [Google Scholar] [CrossRef]
- Tran, C.; Walsh, L.J. Novel models to manage biofilms on microtextured dental implant surfaces. In Microbial Biofilms—Importance and Applications; Dhanasekaran, D., Thajuddin, N., Eds.; InTech Publishers: Rijeka, Croatia, 2017; pp. 463–486. ISBN 978-953-51-2435-1. [Google Scholar]
- Zmener, O.; Spielberg, C.; Lamberghini, F.; Rucci, M. Sealing properties of a new epoxy resin-based root-canal cement. Int. Endod. J. 1997, 30, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Rechenberg, D.-K.; Thurnheer, T.; Zehnder, M. Potential systematic error in laboratory experiments on microbial leakage through filled root canals: An experimental study. Int. Endod. J. 2011, 44, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Fava, L.R.; Saunders, W.P. Calcium hydroxide pastes: Classification and clinical indications. Int. Endod. J. 1999, 32, 257–282. [Google Scholar] [CrossRef] [PubMed]
| Material | Manufacturer | Description |
|---|---|---|
| AH-Plus™ | Dentsply Maillefer, Ballaigues, Switzerland | A non-staining epoxy resin two paste sealer. |
| Zirmix™ | Ozdent, Castle Hill, Sydney, Australia | A non-staining epoxy resin power/liquid sealer |
| Nex MTA™ | GC Corporation, Tokyo, Japan | A grey MTA cement |
| MTAmix™ | Ozdent, Castle Hill, Sydney, Australia | A white MTA cement |
| Supercal™ | Ozdent, Castle Hill, Sydney, Australia | A novel calcium hydroxide alkaline cement with a glycerol-based solvent. |
| Material | Median Survival (Days) |
|---|---|
| AH Plus™ | 15.5 |
| Zirmix™ | 20.5 |
| Nex MTA™ | 11.0 |
| MTAmix™ | 6.0 |
| Supercal™ | 28.0 |
| Positive control | 1.0 |
| Material | AH Plus | Zirmix | Nex MTA | MTAmix | Supercal | Positive Control | Negative Control |
|---|---|---|---|---|---|---|---|
| AH Plus | - | 0.4136 | 0.2652 | 0.0144 | 0.0192 | 0.0001 | 0.0001 |
| Zirmix | - | - | 0.8156 | 0.0083 | 0.0771 | 0.0001 | 0.0001 |
| Nex MTA | - | - | - | 0.0269 | 0.1783 | 0.0001 | 0.0002 |
| MTAmix | - | - | - | - | 0.004 | 0.0001 | 0.0001 |
| Supercal | - | - | - | - | 0.0001 | 0.0196 | |
| Positive Control | - | - | - | - | - | - | 0.0001 |
| Negative Control | - | - | - | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teoh, Y.-Y.; Athanassiadis, B.; Walsh, L.J. Sealing Ability of Alkaline Endodontic Cements versus Resin Cements. Materials 2017, 10, 1228. https://doi.org/10.3390/ma10111228
Teoh Y-Y, Athanassiadis B, Walsh LJ. Sealing Ability of Alkaline Endodontic Cements versus Resin Cements. Materials. 2017; 10(11):1228. https://doi.org/10.3390/ma10111228
Chicago/Turabian StyleTeoh, Yu-Yao, Basil Athanassiadis, and Laurence J. Walsh. 2017. "Sealing Ability of Alkaline Endodontic Cements versus Resin Cements" Materials 10, no. 11: 1228. https://doi.org/10.3390/ma10111228






