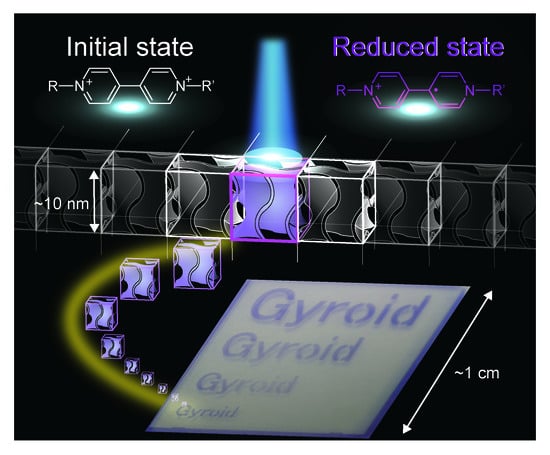
Design of Viologen-Based Liquid Crystals Exhibiting Bicontinuous Cubic Phases and Their Redox-Active Behavior
Abstract
:1. Introduction
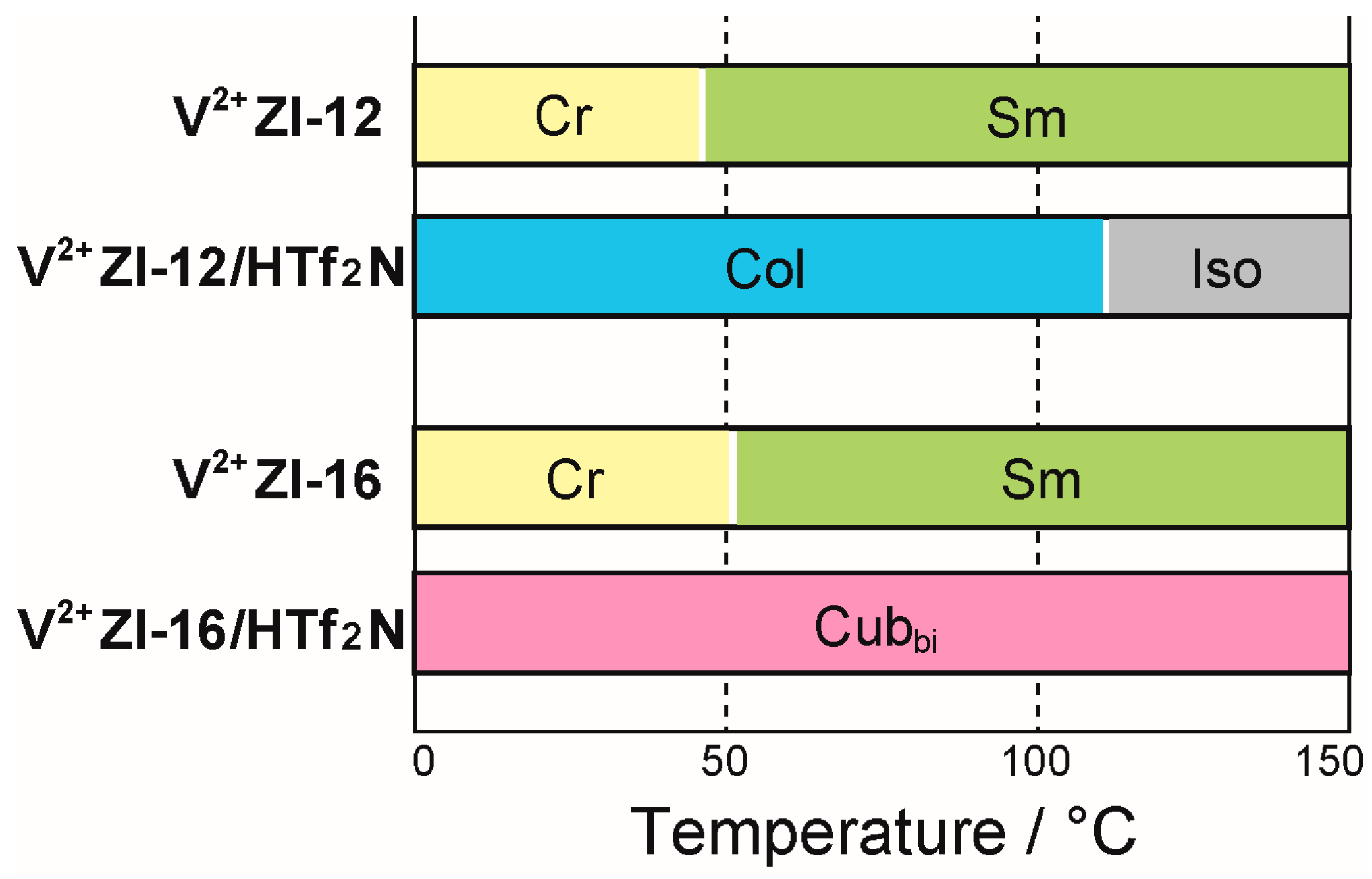
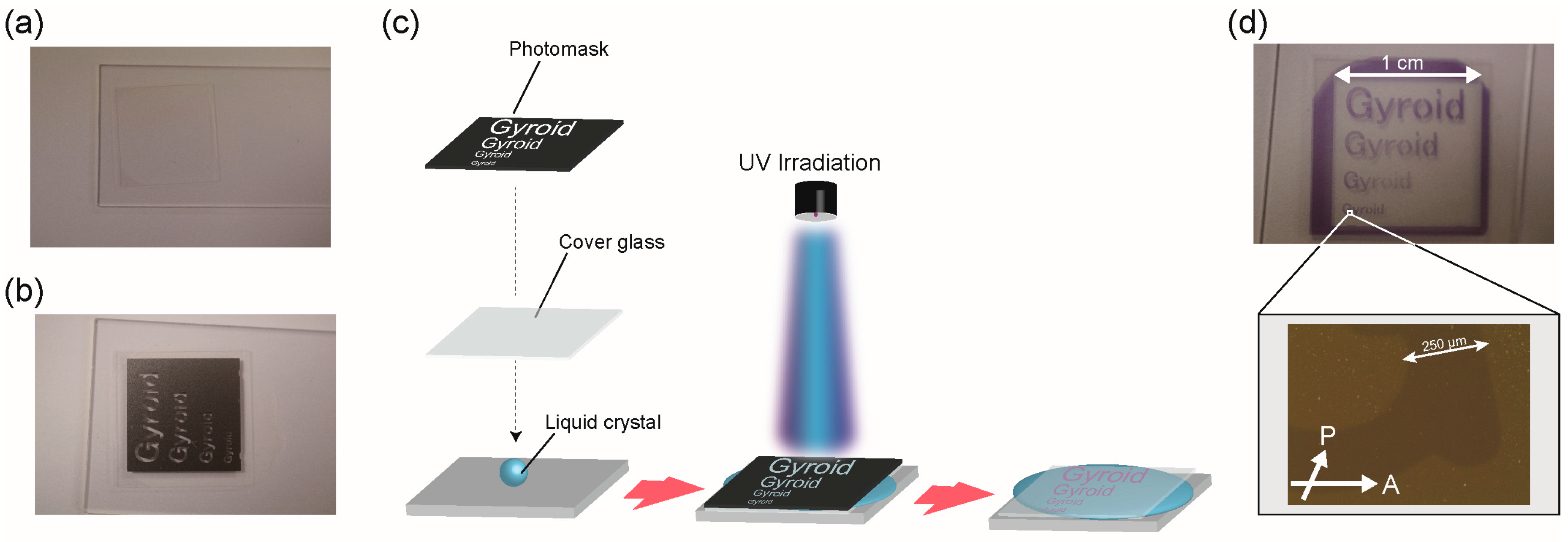
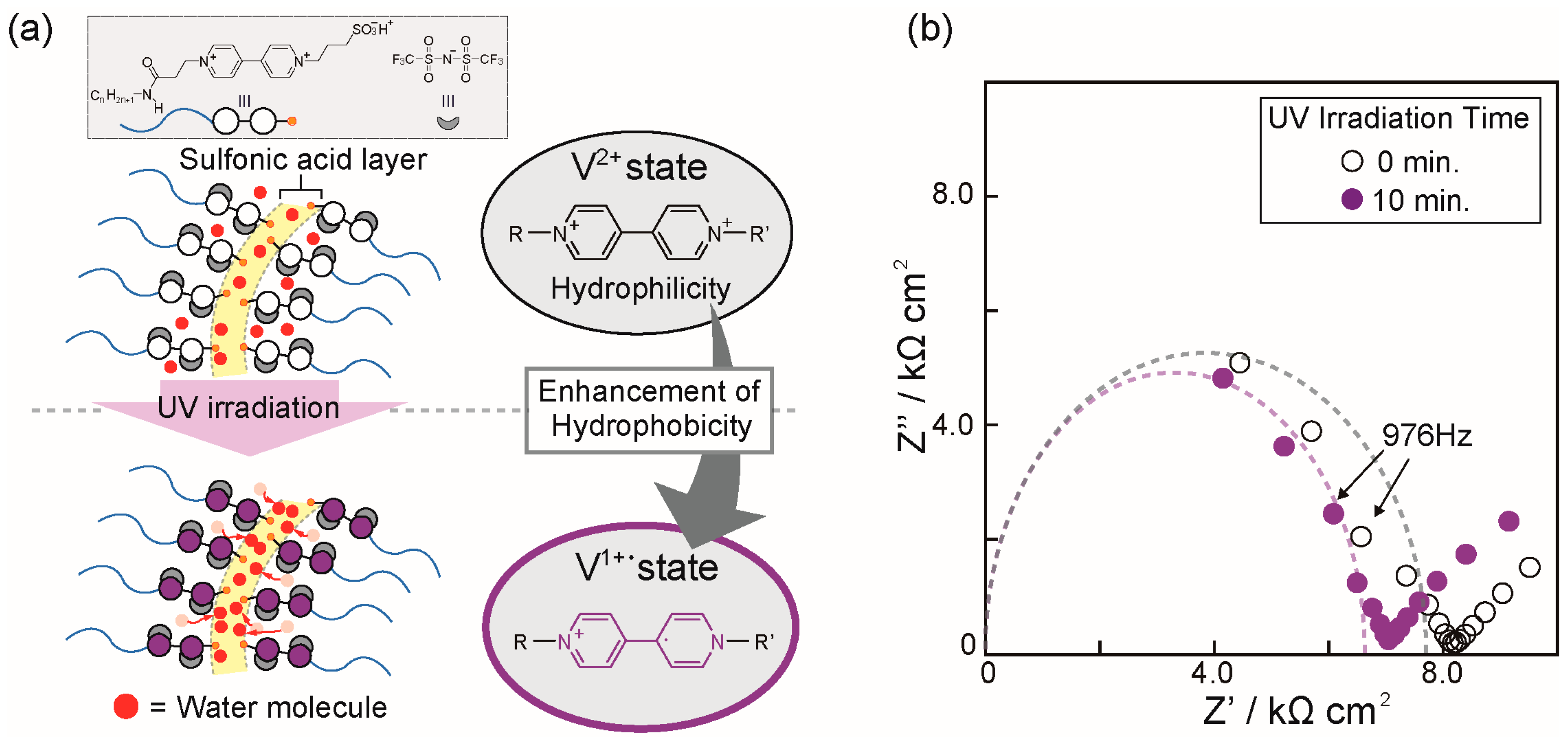
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of V2+ZI-n/H-A Mixtures
3.2. Thermotropic Liquid-Crystalline Properties
3.3. UV Irradiation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.W.; Jiao, Z. Application of nanostructures in electrochromic materials and devices: Recent progress. Materials 2010, 3, 5029–5053. [Google Scholar] [CrossRef] [PubMed]
- Bordyuh, A.B.; Garbovskiy, Y.A.; Bugaychuk, S.A.; Klimusheva, G.V.; Mirnaya, T.A.; Yaremchuk, G.G.; Polishchuk, A.P. Dynamic grating recording in lyotropic ionic smectics of metal alkanoates doped with electrochromic impurities. Opt. Mater. 2009, 31, 1109–1114. [Google Scholar] [CrossRef]
- Möller, M.; Asaftei, S.; Corr, D.; Ryan, M.; Walder, L. Switchable electrochromic images based on a combined top-down bottom-up approach. Adv. Mater. 2004, 16, 1558–1562. [Google Scholar] [CrossRef]
- Bonchio, M.; Carraro, M.; Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Thermal behaviour and electrochemical properties of bis(trifluoromethanesulfonyl)amide and dodecatungstosilicate viologen dimers. Phys. Chem. Chem. Phys. 2012, 8, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamasaki, T.; Mizuma, T.; Oyaizu, K.; Nishide, H. Dynamic switching of ionic conductivity by cooperative interaction of polyviologen and liquid crystals for efficient charge storage. J. Mater. Chem. A 2016, 4, 3249–3252. [Google Scholar] [CrossRef]
- Burgess, M.; Chénard, E.; Hernández-Burgos, K.; Nagarjuna, G.; Assary, R.S.; Hui, J.; Moore, J.S.; Rodríguez-López, J. Impact of backbone tether length and structure on the electrochemical performance of viologen redox active polymers. Chem. Mater. 2016, 28, 7362–7374. [Google Scholar] [CrossRef]
- Vermeulen, L.A.; Snover, J.L.; Sapochak, L.S.; Thompson, M.E. Efficient photoinduced charge separation in layered zirconium viologen phosphonate compounds. J. Am. Chem. Soc. 1993, 115, 11767–11774. [Google Scholar] [CrossRef]
- Dalton, E.F.; Murray, R.W. Viologen(2+/l+) and Viologen(1+/0) electron-self-exchange reactions in a redox polymer. J. Phys. Chem. 1991, 95, 6383–6389. [Google Scholar] [CrossRef]
- Kijima, M.; Setoh, K.; Shirakawa, H. Synthesis of novel ionic liquid crystalline pyrrole derivatives having a viologen moiety. Mol. Cryst. Liq. Cryst. 2001, 364, 911–918. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; Deda, M.L.; Veltri, L.; Chidichimo, G. Electrofluorochromism in π-conjugated ionic liquid crystals. Nat. Commun. 2014, 5, 3105–3157. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Ionic liquid crystals based on viologen dimers: Tuning the mesomorphism by varying the conformational freedom of the ionic layer. Liq. Cryst. 2016, 43, 1161–1173. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K.; Yasuda, T.; Yoshio, M.; Kato, T. Viologen-based redox-active ionic liquid crystals forming columnar phases. Org. Lett. 2007, 9, 4271–4274. [Google Scholar] [CrossRef] [PubMed]
- Tabushi, I.; Yamamura, K.; Kominami, K. Electric stimulus-response behavior of liquid-crystalline viologen. J. Am. Chem. Soc. 1986, 108, 6409–6410. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; Deda, M.L.; Chidichimo, G. Highly fluorescent thienoviologen-based polymer gels for single layer electrofluorochromic devices. Adv. Funct. Mater. 2015, 25, 1240–1247. [Google Scholar] [CrossRef]
- Axenov, K.V.; Laschat, S. Thermotropic ionic liquid crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Causin, V.; Rastrelli, F.; Saielli, G. Viologen-based ionic liquid crystals: Induction of a smectic A phase by dimerisation. Phys. Chem. Chem. Phys. 2014, 16, 5048–5051. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nguyen, V.; Zhou, M.; Zeng, X.; Jin, J.; Elliott, B.J.; Gin, D.L. Cross-linked bicontinuous cubic lyotropic liquid-crystal/butyl-rubber composites: Highly selective, breathable barrier materials for chemical agent protection. Adv. Mater. 2006, 18, 3294. [Google Scholar] [CrossRef]
- Cho, B.K.; Jain, A.; Gruner, S.M.; Wiesner, U. Mesophase structure-mechanical and ionic transport correlations in extended amphiphilic dendrons. Science 2004, 305, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Self-organization of room-temperature ionic liquids exhibiting liquid-crystalline bicontinuous cubic phases: Formation of nano-ion channel networks. J. Am. Chem. Soc. 2007, 129, 10662–10663. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, W.; Zhang, D. Engineering gyroid-structured functional materials via templates discovered in nature and in the Lab. Small 2015, 11, 5004–5022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Möller, M.; Zhu, X.; Rueda, J.J.H.; Rosenthal, M.; Ivanov, D.A. From channel-forming ionic liquid crystals exhibiting humidity-induced phase transitions to nanostructured ion-conducting polymer membranes. Adv. Mater. 2013, 25, 3543–3548. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Fujimura, K.; Yoshio, M.; Kato, T.; Ohno, H. Designer lyotropic liquid-crystalline systems containing amino acid ionic liquids as self-organisation media of amphiphiles. Chem. Commun. 2013, 49, 11746–11748. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Kato, T.; Ohno, H. 3D Continuous Water nanosheet as a gyroid minimal surface formed by bicontinuous cubic liquid-crystalline zwitterions. J. Am. Chem. Soc. 2012, 134, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ono, A.; Ichikawa, T.; Kato, T.; Ohno, H. Construction of gyroid-structured matrices through the design of geminized amphiphilic zwitterions and their self-organization. Chem. Commun. 2016, 52, 12167–12170. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ichikawa, T.; Kato, T.; Ohno, H. Development of glassy bicontinuous cubic liquid crystals for solid proton-conductive materials. Adv. Mater. 2017, 29, 1604429. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Okafuji, A.; Kato, T.; Ohno, H. Induction of an infinite periodic minimal surface by endowing an amphiphilic zwitterion with halogen-bond ability. ChemistryOpen 2016, 5, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic liquid crystals: Versatile materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Nalwa, H. Molecular and supramolecular nanomachines. In Nanostructured Materials and Nanotechnology, 1st ed.; Gómez-López, M., Stoddart, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; p. 665. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Ichikawa, T. Design of Viologen-Based Liquid Crystals Exhibiting Bicontinuous Cubic Phases and Their Redox-Active Behavior. Materials 2017, 10, 1243. https://doi.org/10.3390/ma10111243
Kobayashi T, Ichikawa T. Design of Viologen-Based Liquid Crystals Exhibiting Bicontinuous Cubic Phases and Their Redox-Active Behavior. Materials. 2017; 10(11):1243. https://doi.org/10.3390/ma10111243
Chicago/Turabian StyleKobayashi, Tsubasa, and Takahiro Ichikawa. 2017. "Design of Viologen-Based Liquid Crystals Exhibiting Bicontinuous Cubic Phases and Their Redox-Active Behavior" Materials 10, no. 11: 1243. https://doi.org/10.3390/ma10111243