Thermal Degradation Kinetics of Sugarcane Bagasse and Soft Wood Cellulose
Abstract
:1. Introduction
2. Material and methods
2.1. Materials
2.2. Chemical Composition
2.3. Extraction of SCB and SW Cellulose
2.4. Characterization Methods
2.5. Degradation Kinetics
2.5.1. Flynn Wall Ozawa (OFW) Model
2.5.2. Kissinger Akahira Sunose (KAS) Model
2.5.3. Kissinger (KGR) Model
3. Results and Discussion
3.1. Chemical Compositions of SCB and SW
3.2. Structure Characterization
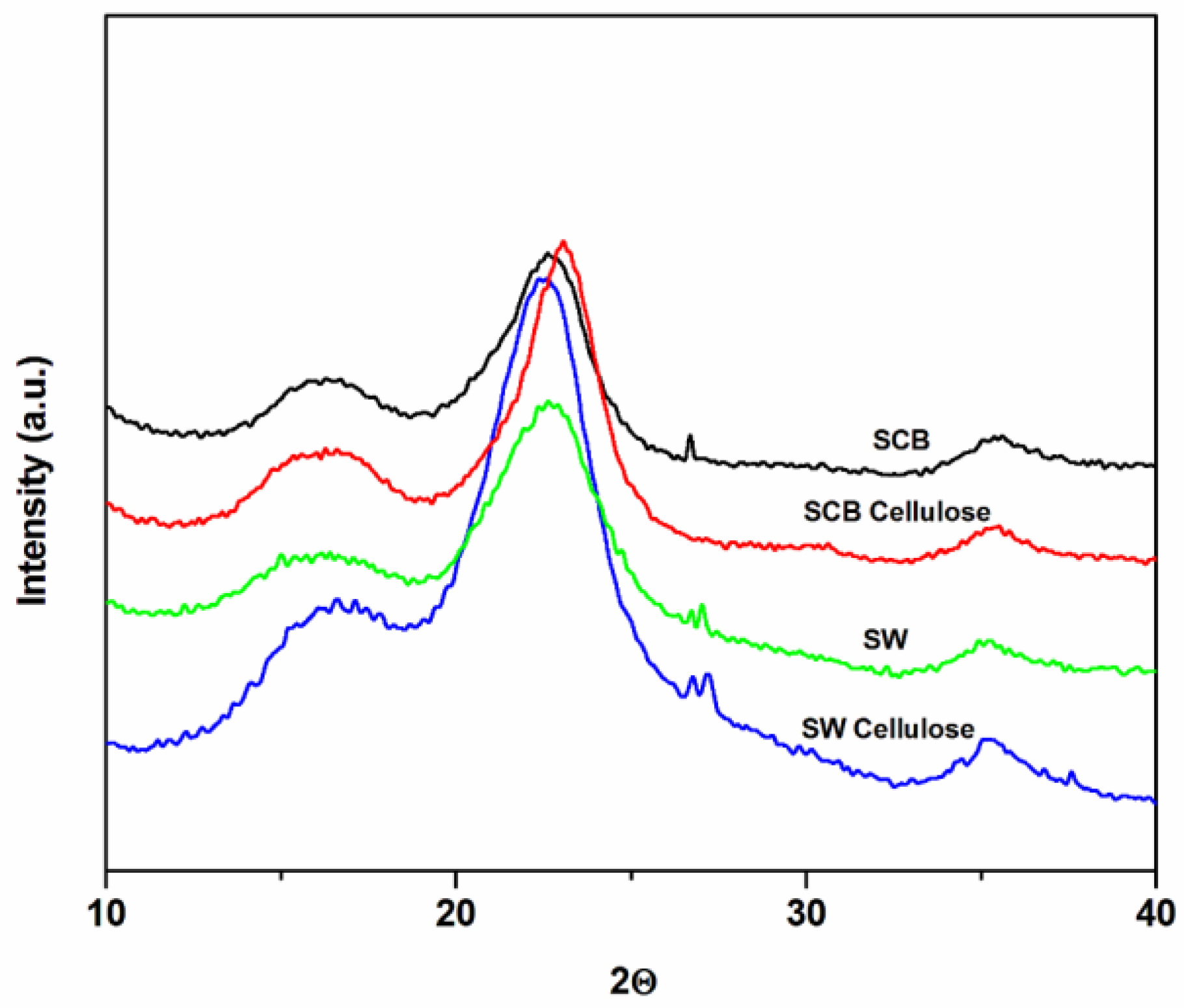
3.3. Crystallinity Characterization
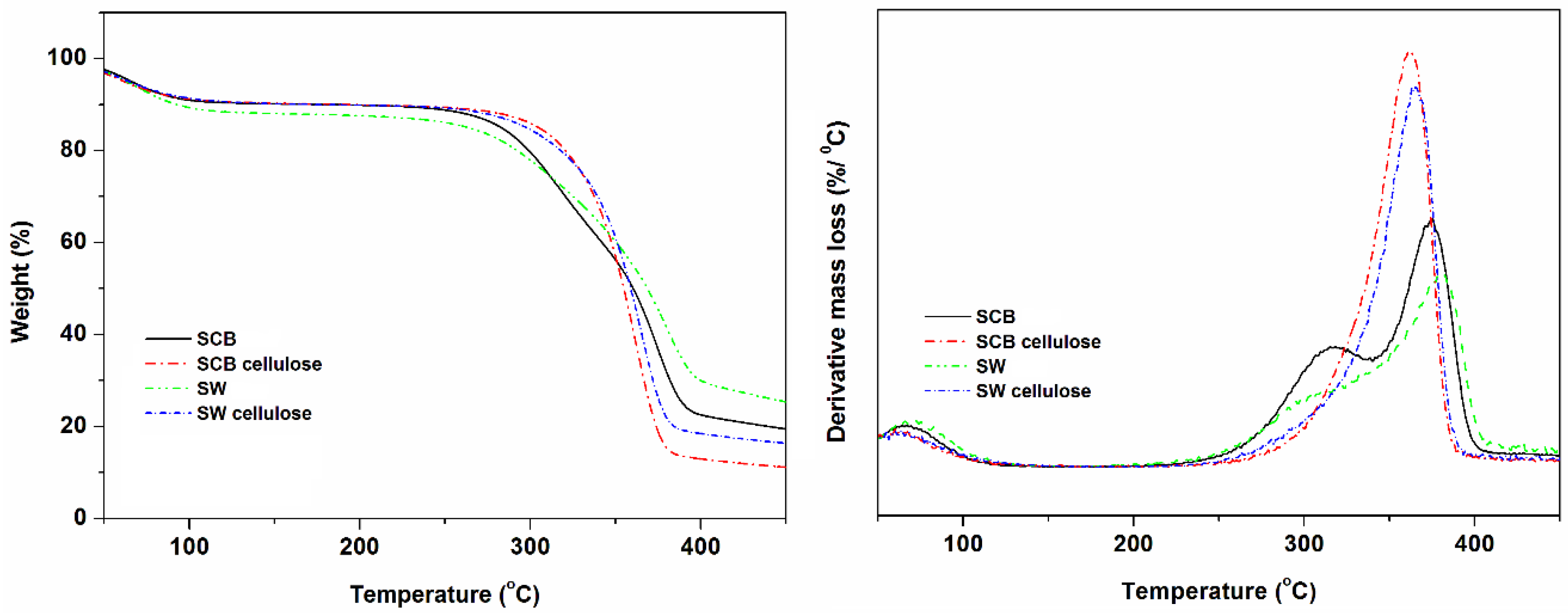
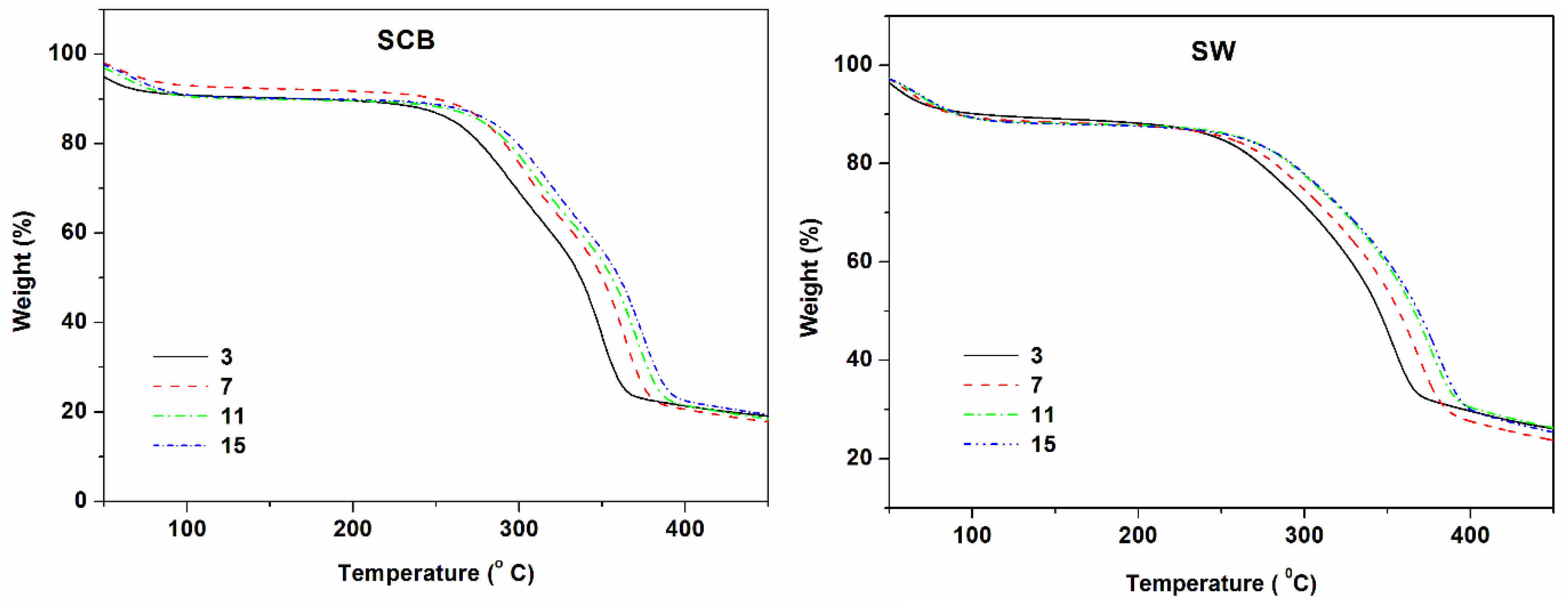
3.4. Thermal Stability
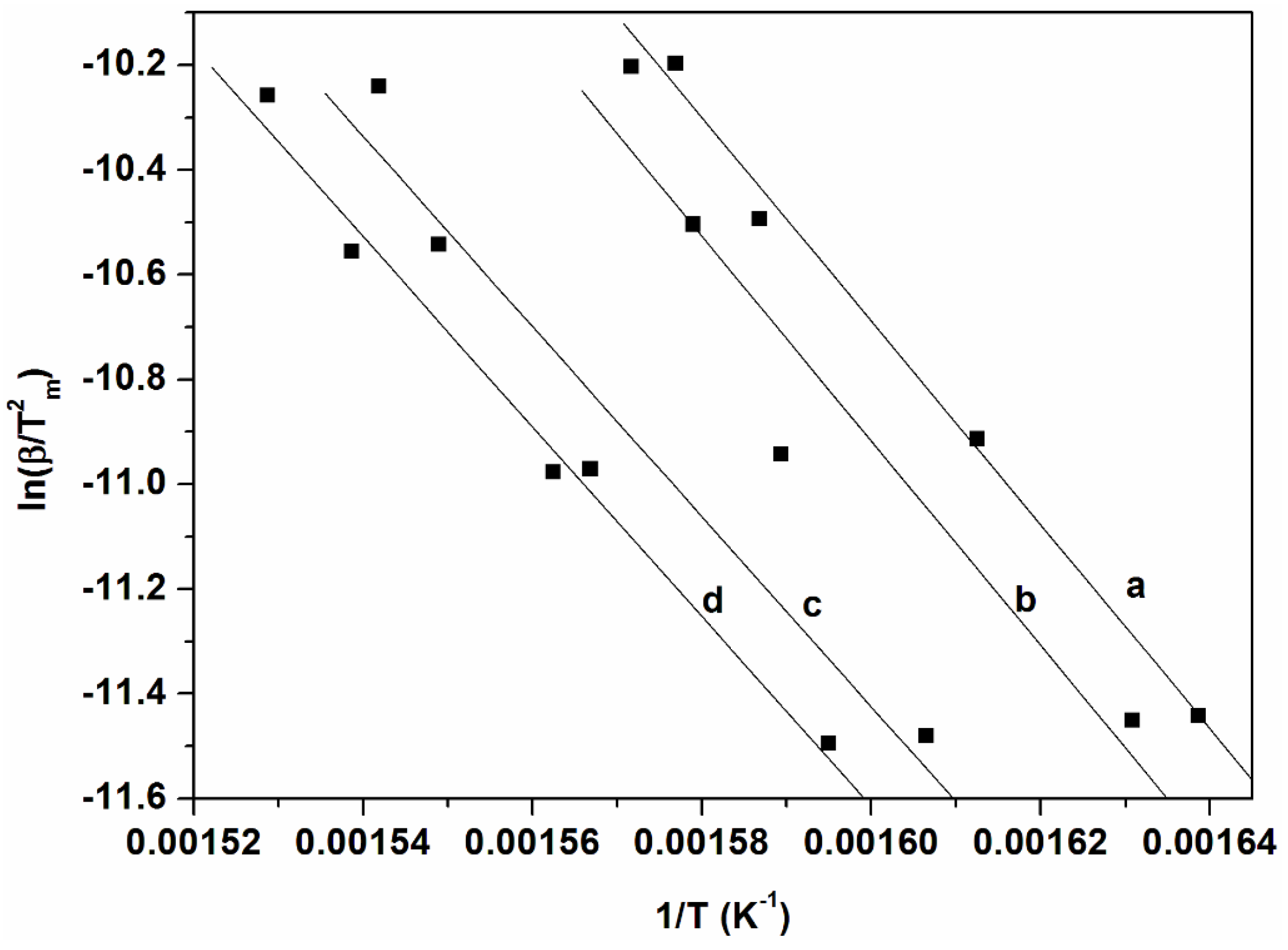
3.5. Kinetics of Degradation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Anbukarasi, K.; Kalaiselvam, S. Study of effect of fiber volume and dimension on mechanical, thermal, and water absorption behaviour of luffa reinforced epoxy composites. Mater. Des. 2015, 66, 321–330. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Corrales, R.C.N.R.; Mendes, F.M.T.; Perrone, C.C.; Sant’Anna, C.; de Souza, W.; Abud, Y.; da Silva Bon, E.P.; Ferreira-Leitão, V. Structural evaluation of sugar cane bagasse steam pretreated in the presence of CO2 and SO2. Biotechnol. Biofuels 2012, 5, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Edreis, E.M.A.; Luo, G.; Yao, H. Investigations of the structure and thermal kinetic analysis of sugarcane bagasse char during non-isothermal CO2 gasification. J. Anal. Appl. Pyrolysis 2014, 107, 107–115. [Google Scholar] [CrossRef]
- Li, M.Y.; Cheng, S.C.; Li, D.; Wang, S.N.; Huang, A.N.; Sun, S.Q. Structural characterization of steam-heat treated Tectona grandis wood analyzed by FT-IR and 2D-IR correlation spectroscopy. Chin. Chem. Lett. 2015, 26, 221–225. [Google Scholar] [CrossRef]
- Emmanuel, V.; Odile, B.; Celine, R. FTIR spectroscopy of woods: A new approach to study the weathering of the carving face of a sculpture. Spectrochim. Acta 2015, 136, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2016, 127, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Saffe, A.; Pereyra, R.; Mazza, G.; Rodriguez, R. Kinetic study of regional agro-industrial wastes pyrolysis using non-isothermal TGA analysis. Appl. Therm. Eng. 2016, 106, 1157–1164. [Google Scholar] [CrossRef]
- Ramajo-Escalera, B.; Espina, A.; Garcıa, J.R.; Sosa-Arnao, J.H.; Nebra, S.A. Model-free kinetics applied to sugarcane bagasse combustion. Thermochim. Acta 2006, 448, 111–116. [Google Scholar] [CrossRef]
- Motaung, T.E.; Anandjiwala, R.D. Effect of alkali and acid treatment on thermal degradation kinetics of sugar cane bagasse. Ind. Crop. Prod. 2015, 74, 472–477. [Google Scholar] [CrossRef]
- Edreis, E.M.A.; Yao, H. Kinetic thermal behaviour and evaluation of physical structure of sugar cane bagasse char during non-isothermal steam gasification. J. Mater. Res. Technol. 2016, 5, 317–326. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Thermal decomposition of wood: Kinetics and degradation mechanism. Bioresour. Technol. 2012, 126, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Gu, S.; Luo, K.H.; Bridgwater, A.V.; Fang, M.X. Kinetic study on thermal decomposition of woods in oxidative environment. Fuel 2009, 88, 1024–1030. [Google Scholar] [CrossRef]
- Grioui, N.; Halouani, K.; Zoulalian, A.; Halouani, F. Thermogravimetric analysis and kinetics modeling of isothermal carbonization of olive wood in inert atmosphere. Thermochim. Acta 2006, 440, 23–30. [Google Scholar] [CrossRef]
- TranVan, L.; Legrand, V.; Jacquemin, F. Thermal decomposition kinetics of balsa wood: Kinetics and degradation mechanisms comparison between dry and moisturized materials. Polym. Degrad. Stab. 2014, 110, 208–215. [Google Scholar] [CrossRef]
- Patachia, S.F.; Nistor, M.T.; Vasile, C. Thermal behavior of some wood species treated with ionic liquid. Ind. Crop. Prod. 2013, 44, 511–519. [Google Scholar] [CrossRef]
- Ge, J.; Wang, R.Q.; Liu, L. Study on the thermal degradation kinetics of the common wooden boards. Procedia Eng. 2016, 135, 72–82. [Google Scholar] [CrossRef]
- Costa, L.A.D.; Fonêsca, A.F.; Pereira, F.V.; Druzian, J.I. Extraction and characterization of cellulose nanocrystals from corn stover. Cell. Chem. Technol. 2015, 49, 127–133. [Google Scholar]
- Dhar, P.; Vangala, S.P.K.; Tiwari, P.; Kumar, A.; Katiyar, V. Thermal degradation kinetics of poly (3-hydroxybutyrate)/cellulose nanocrystals based nanobiocomposite. J. Thermodyn. Catal. 2014, 5, 127–134. [Google Scholar]
- Henrique, M.A.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; da Silva Barud, H.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crop. Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef]
- Sittisun, P.; Tippayawong, N.; Wattanasiriwech, D. Thermal degradation characteristics and kinetics of oxy combustion of corn residues. Adv. Mater. Sci. Eng. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Cortés, A.M.; Bridgwater, A.V. Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process. Technol. 2015, 38, 184–193. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Otero, M.; Gomez, X.; Moran, A. Thermogravimetric kinetic analysis of the combustion of biowastes. Renew. Energy 2009, 34, 1622–1627. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; Martínez-Abad, A.; Evtuguin, D.V.; Colodette, J.L.; Lindström, M.E.; Vilaplana, F.; Sevastyanov, O. Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw. Carbohydr. Polym. 2017, 156, 223–234. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.B.; Bras, J.; Pimenta, M.T.B.; Curvelo, A.A.S.; Belgacem, M.N. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind. Crop. Prod. 2016, 93, 48–57. [Google Scholar] [CrossRef]
- Do Nascimento, D.M.; Almeida, J.S.; Vale, M.S.; Leitão, R.C.; Muniz, C.R.; de Figueirêdo, M.C.B.; Morais, J.P.S.; Rosa, M.F. A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part I: Proposition of technological pathways. Ind. Crop. Prod. 2016, 93, 66–75. [Google Scholar] [CrossRef]
- Guilherme, A.A.; Dantas, P.V.F.; Santos, E.S.; Fernandes, F.A.N.; Macedo, G.R. Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Braz. J. Chem. Eng. 2015, 32, 23–33. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.; Monteiro, P.A.S.; Braz, C.E.M.; Seleghin, P., Jr.; Polikarpov, I.; Crnkovic, P.M. Thermal and morphological evaluation of chemically pretreated sugarcane bagasse. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2013, 7, 435–440. [Google Scholar]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species. Thermochim. Acta 2008, 47, 43–50. [Google Scholar] [CrossRef]
- Mortari, D.A.; Britto, M.C.; Crnkovic, P.M. Correlation between activation energy and thermal decomposition yield of sugar cane bagasse under CO2/O2 and N2/O2. Chem. Eng. Trans. 2014, 37, 31–36. [Google Scholar]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Gorgens, J.H. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Haameem, J.A.M.; Majid, M.S.A.; Afendi, M.; Marzuki, H.F.A.; Hilmi, E.A.; Fahmi, I.; Gibson, A.G. Effects of water absorption on Napier grass fiber/polyester composites. Compos. Struct. 2016, 144, 138–146. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.M.; Gibbs, B.M. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmosphere. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef] [PubMed]



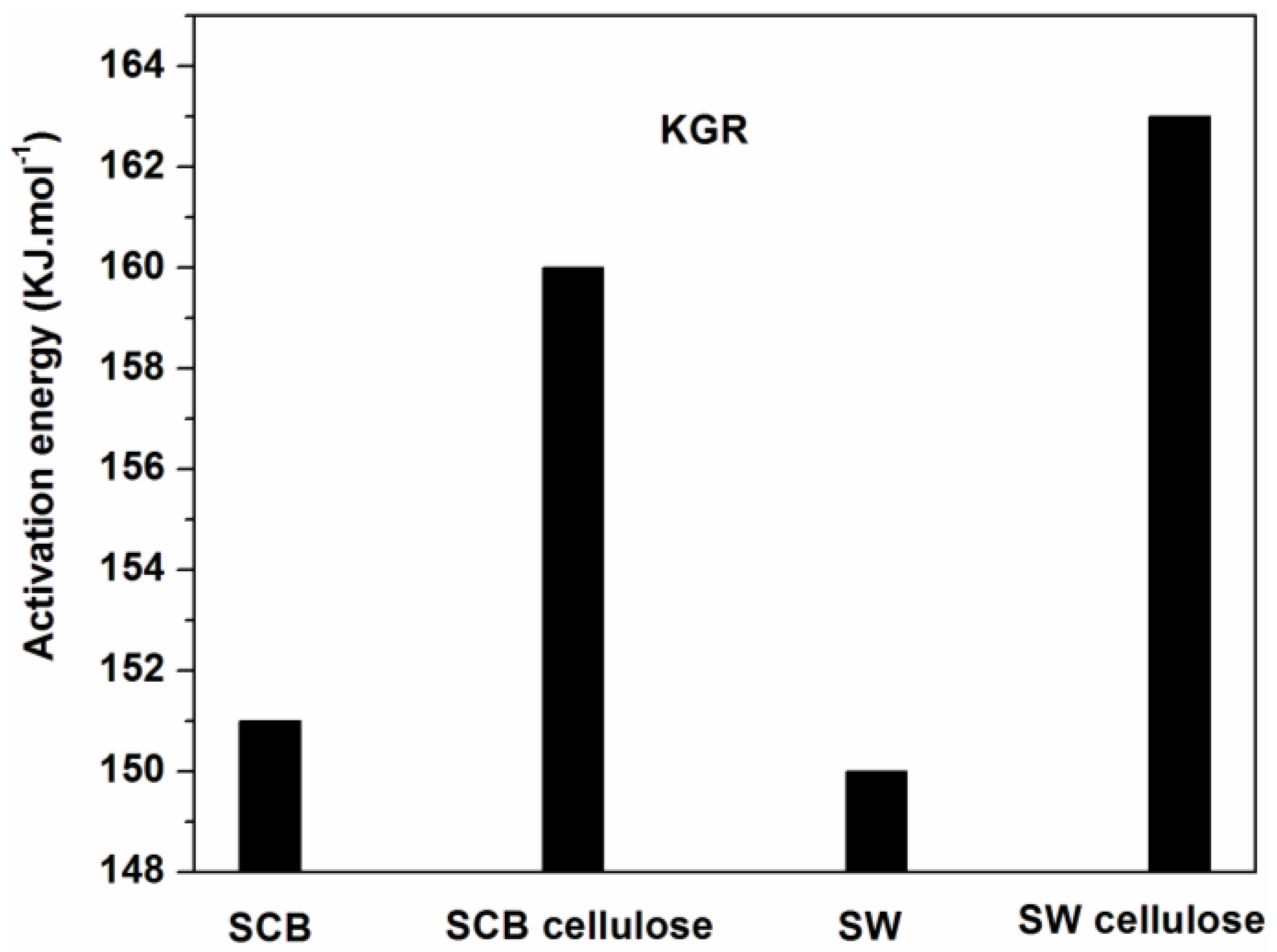

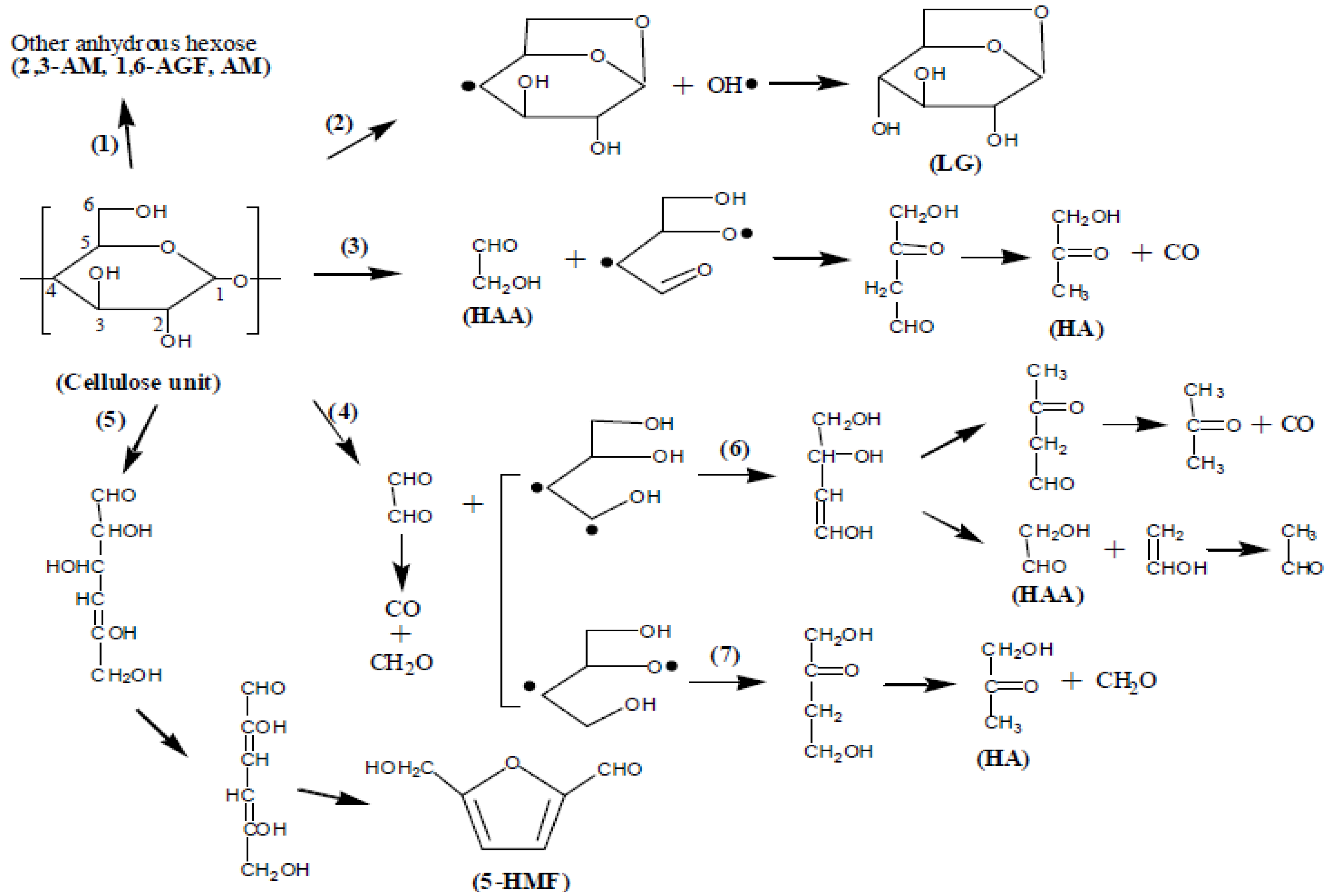
| Sample | Lignin (%) | Cellulose (%) | Hemicellulose (%) |
|---|---|---|---|
| SCB | 18.8 ± 0.1 | 42.9 ± 0.99 | 38.2 ± 1.08 |
| SW | 37.2 ± 0.01 | 39.2 ± 0.01 | 23.6 ± 0.01 |
| Samples | Segal Method | Deconvolution Method |
|---|---|---|
| SCB | 53.9 | 33.5 |
| SW | 52.8 | 32.3 |
| SCB cellulose | 61.3 | 43.9 |
| SW cellulose | 88.7 | 57.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohomane, S.M.; Motaung, T.E.; Revaprasadu, N. Thermal Degradation Kinetics of Sugarcane Bagasse and Soft Wood Cellulose. Materials 2017, 10, 1246. https://doi.org/10.3390/ma10111246
Mohomane SM, Motaung TE, Revaprasadu N. Thermal Degradation Kinetics of Sugarcane Bagasse and Soft Wood Cellulose. Materials. 2017; 10(11):1246. https://doi.org/10.3390/ma10111246
Chicago/Turabian StyleMohomane, Samson M., Tshwafo E. Motaung, and Neerish Revaprasadu. 2017. "Thermal Degradation Kinetics of Sugarcane Bagasse and Soft Wood Cellulose" Materials 10, no. 11: 1246. https://doi.org/10.3390/ma10111246




