Waterborne Polyurethane Coatings with Covalently Linked Black Dye Sudan Black B
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
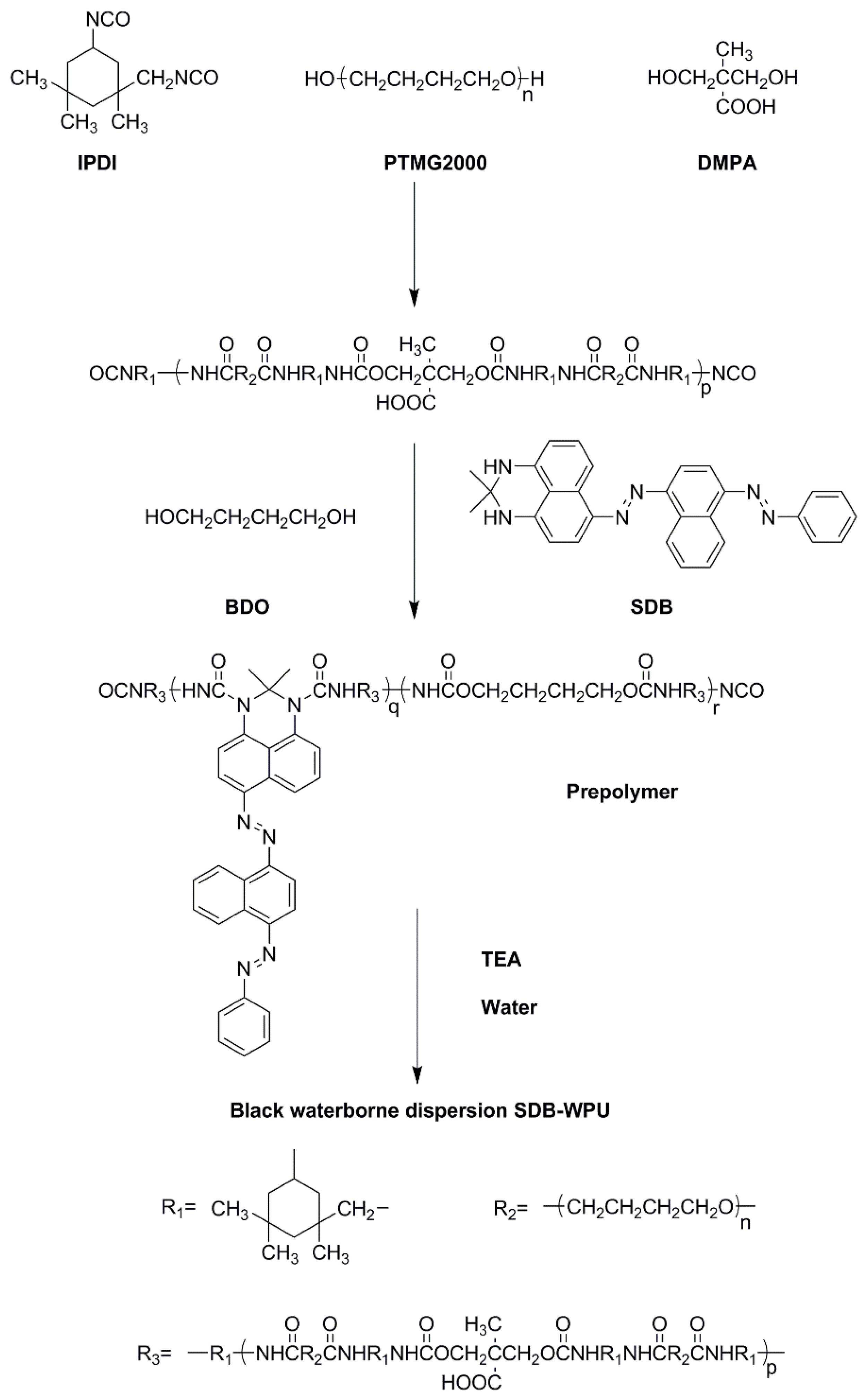
2.3. Synthesis of SDB-WPUs
3. Results and Discussion
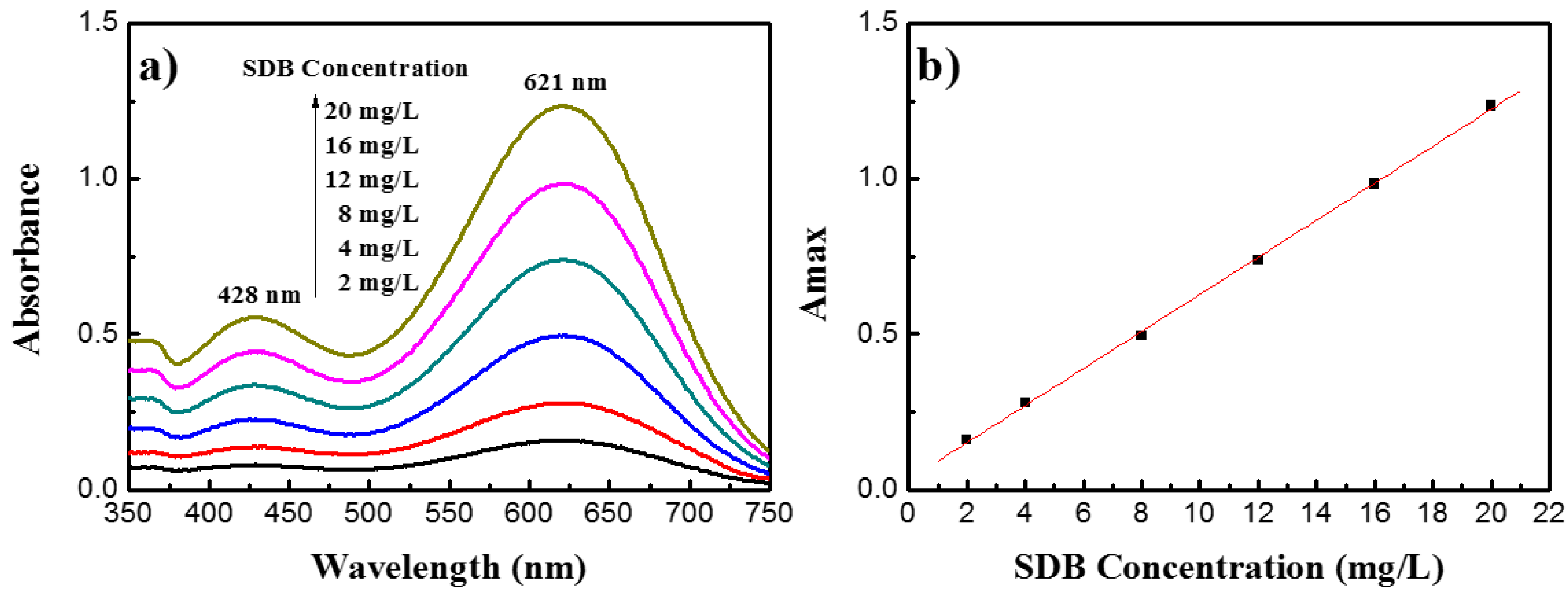
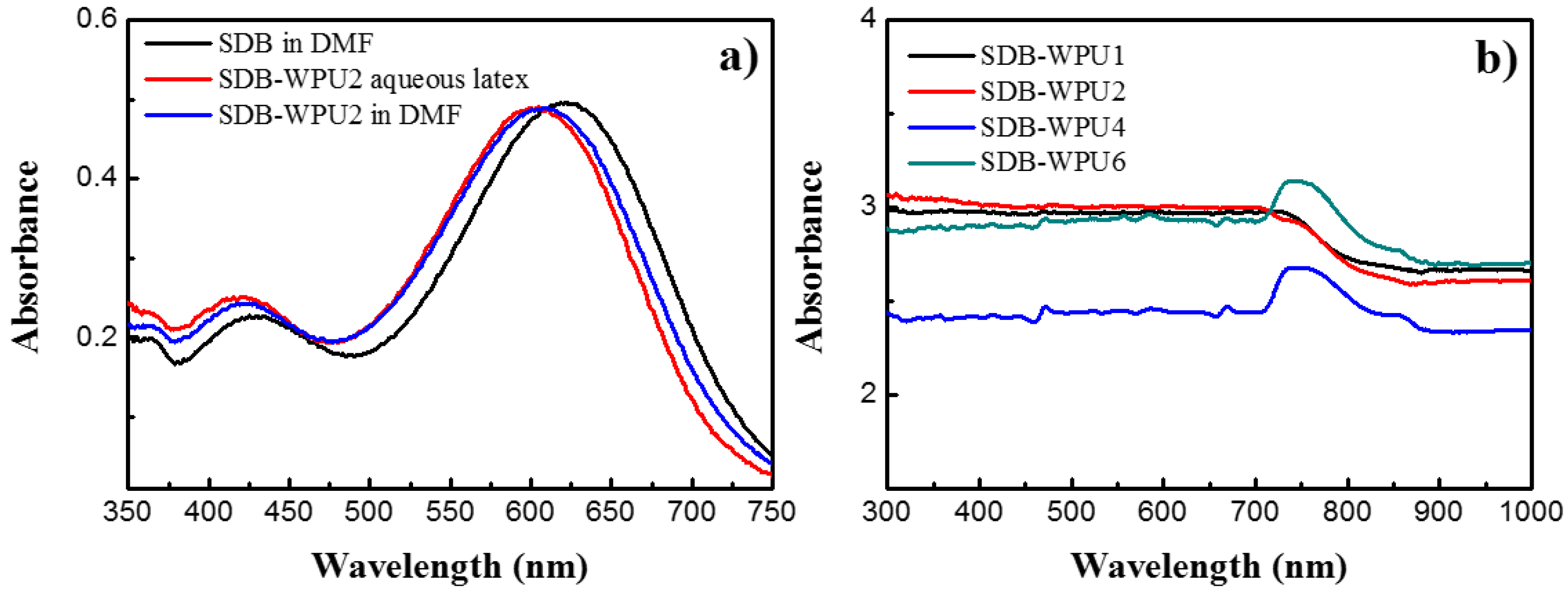
3.1. UV-Vis Spectra of SDB
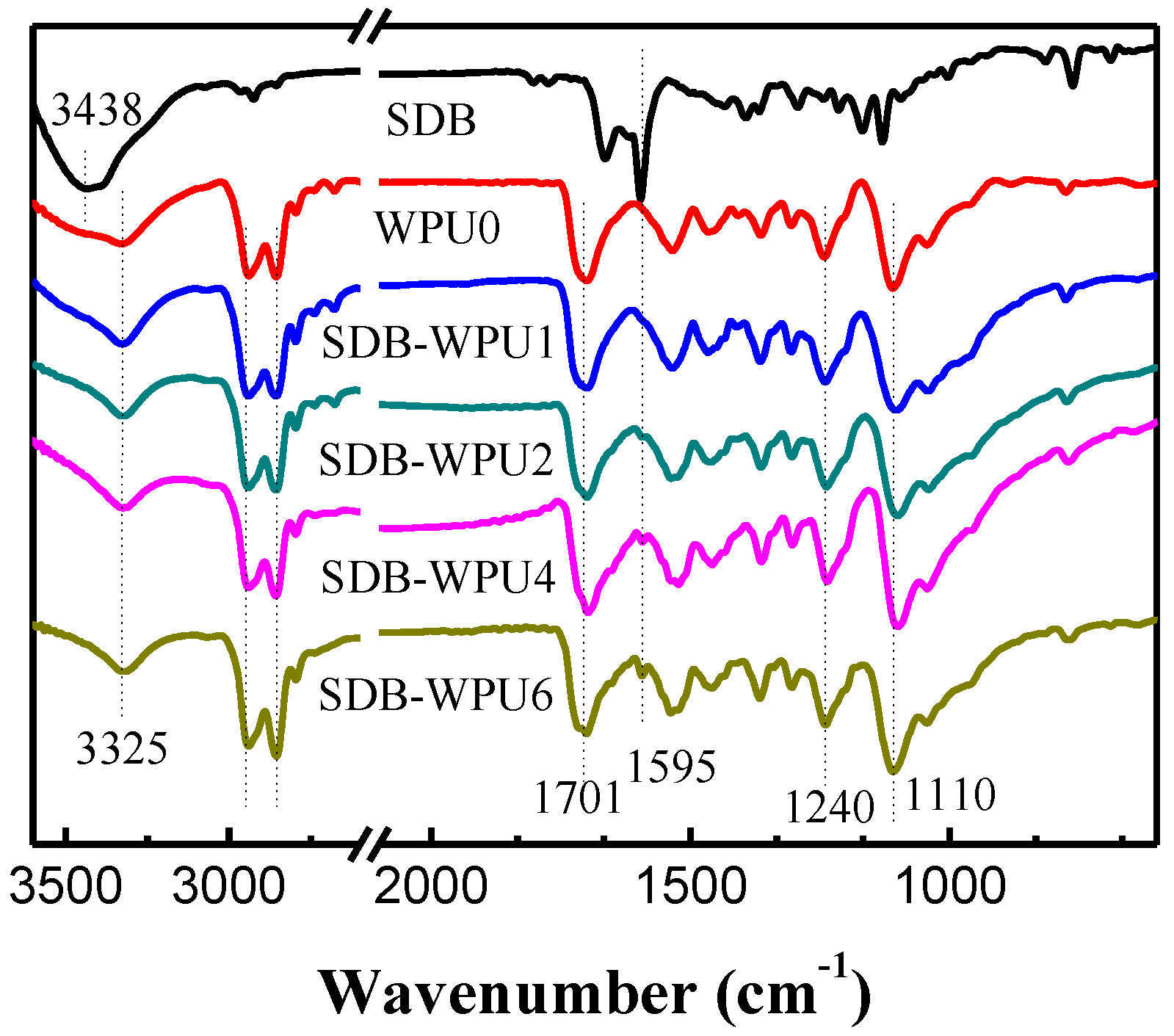
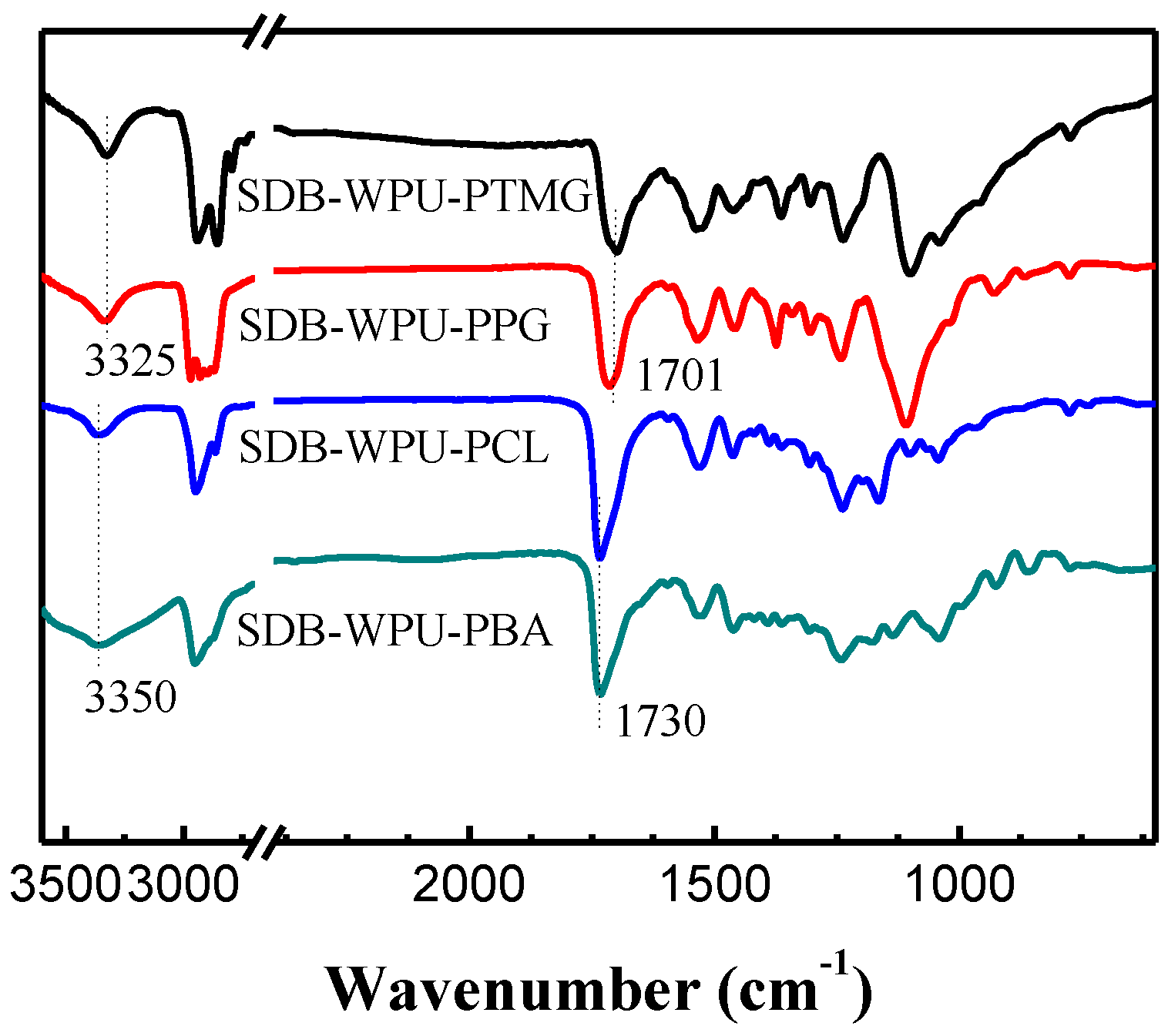
3.2. Structural Analysis of SDB-WPUs
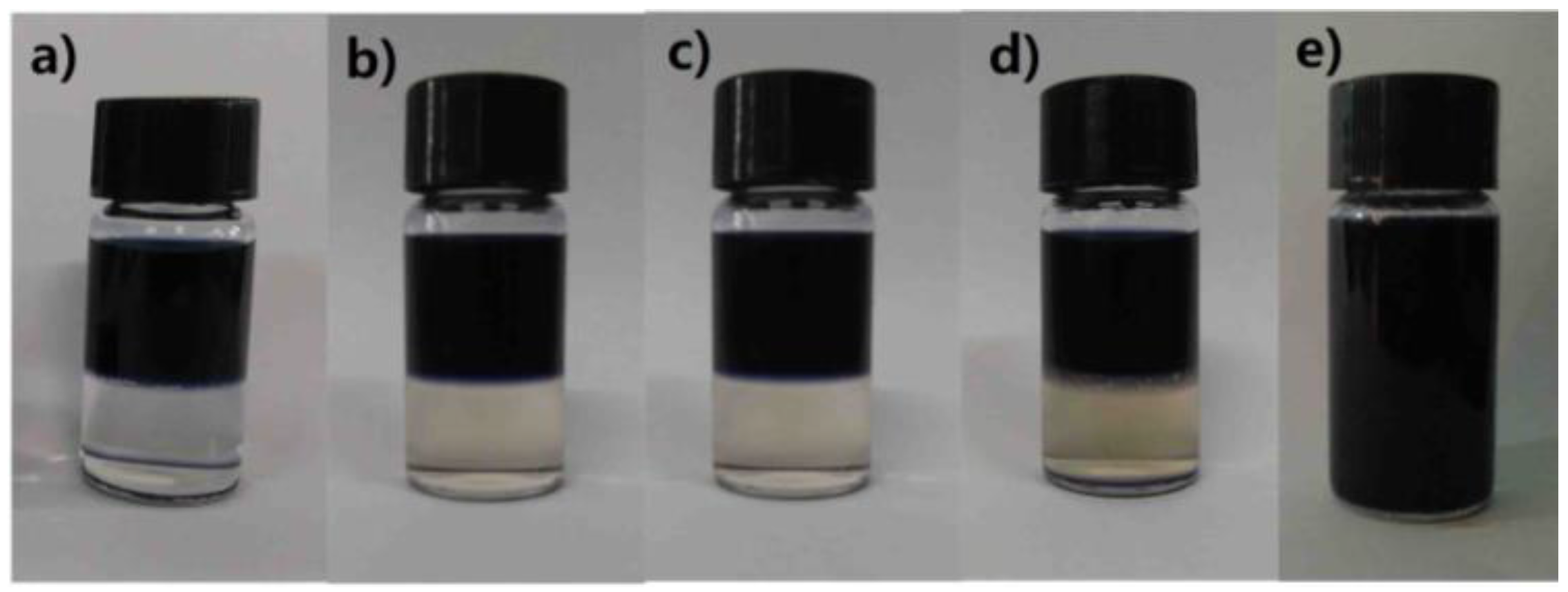
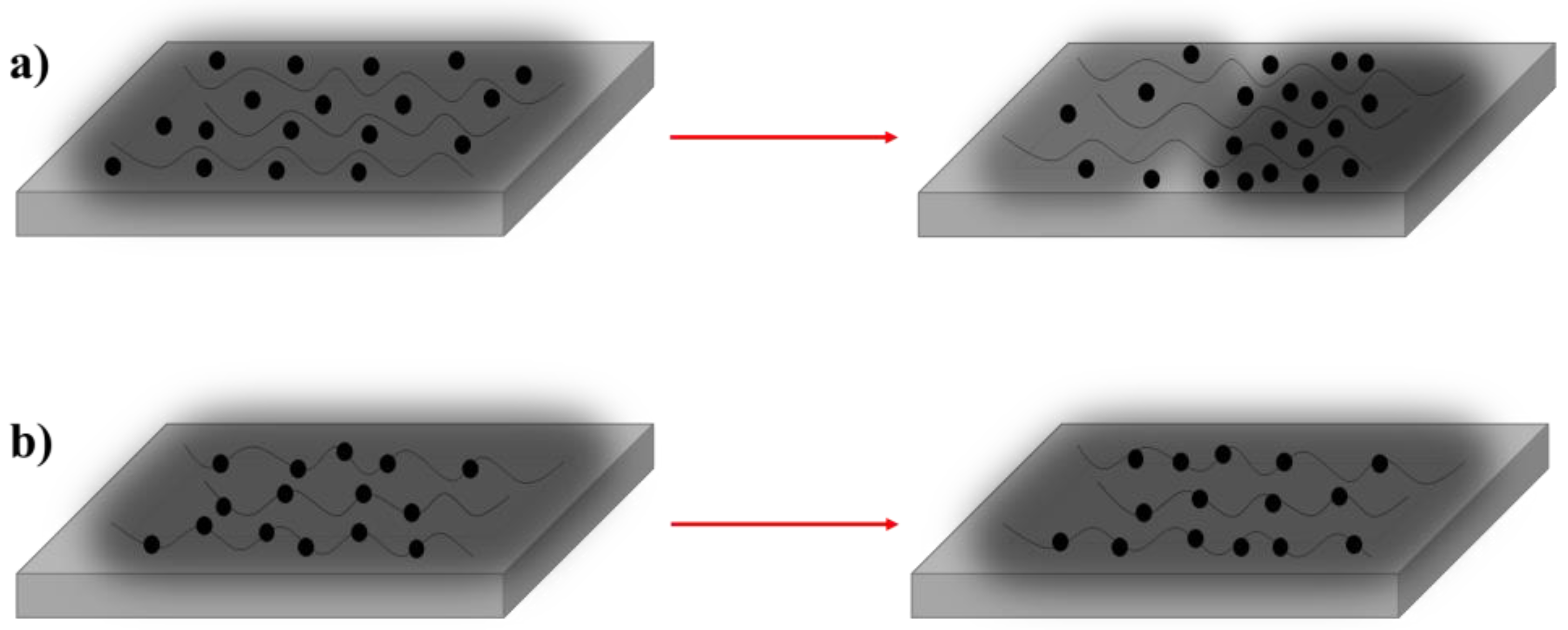
3.3. Statistical Properties of SDB-WPUs
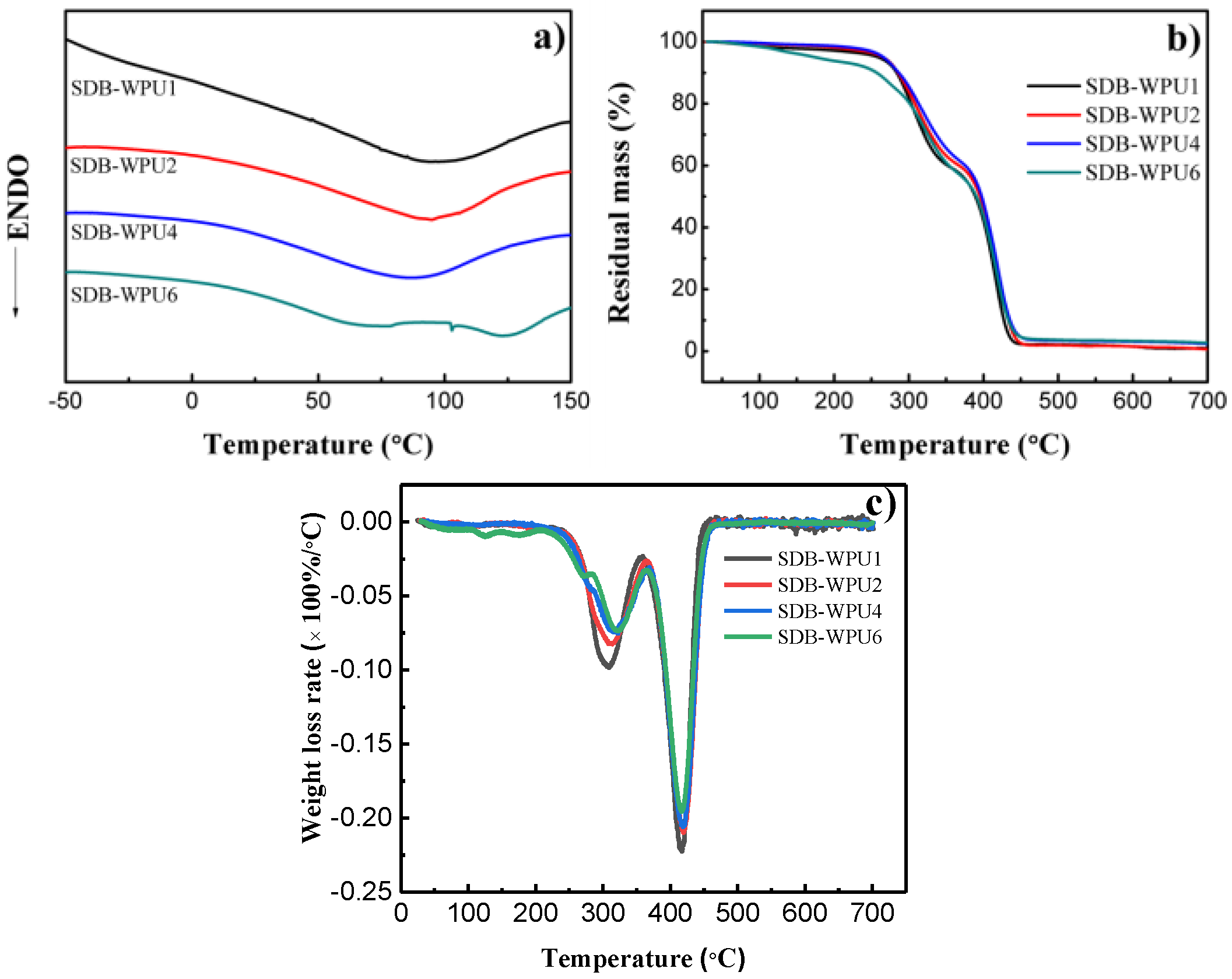
3.4. DSC and TG Analyses
3.5. UV-Vis Spectra Analysis of SDB-WPUs
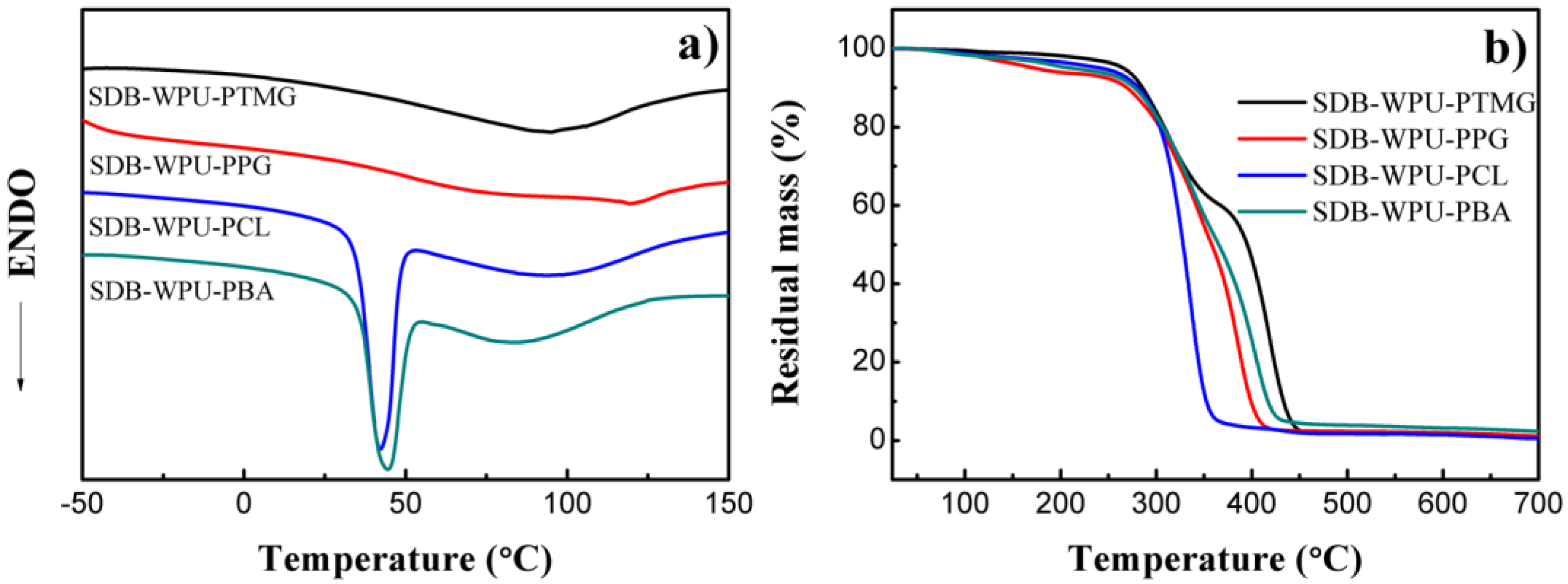
3.6. Analyses of SDB-WPUs with Different Polymeric Diols
3.7. Application in Metal Coatings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ventura-Camargo, B.C.; De Angelis, D.F.; Marin-Morales, M.A. Assessment of the cytotoxic, genotoxic and mutagenic effects of the commercial black dye in Allium cepa cells before and after bacterial biodegradation treatment. Chemosphere 2016, 161, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nabil, G.M.; El-Mallah, N.M.; Mahmoud, M.E. Enhanced decolorization of reactive black 5 dye by active carbon sorbent-immobilized-cationic surfactant (AC-CS). J. Ind. Eng. Chem. 2014, 20, 994–1002. [Google Scholar] [CrossRef]
- Zhuge, J.; Yu, Z.; Gao, J.; Zheng, D. Influence of color coatings on aircraft surface ice detection based on multi-wavelength imaging. Optoelectron. Lett. 2016, 12, 144–147. [Google Scholar] [CrossRef]
- Haroun, A. Evaluation of modified leather dyeing technique using black dyestuffs from the economical view. Dyes Pigments 2005, 67, 215–221. [Google Scholar] [CrossRef]
- Han, L.; Islam, A.; Chen, H.; Malapaka, C.; Chiranjeevi, B.; Zhang, S.; Yang, X.; Yanagida, M. High-efficiency dye-sensitized solar cell with a novel co-adsorbent. Energ. Environ. Sci. 2012, 5, 6057–6060. [Google Scholar] [CrossRef]
- Ozawa, H.; Shimizu, R.; Arakawa, H. Significant improvement in the conversion efficiency of black-dye-based dye-sensitized solar cells by cosensitization with organic dye. RSC Adv. 2012, 2, 3198–3200. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Wang, F.; Yang, L.; Liu, L. Structure–properties relationships investigation on the azo dyes derived from benzene sulfonamide intermediates. Dyes Pigments 2008, 76, 636–645. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Kyu, J.K.; Mukherjee, A. In vitro genotoxicity evaluation of 4-carboxyl-2, 6-dinitrophenylazohydroxynaphthalenes using human lymphocytes. Food. Chem. Toxicol. 2012, 50, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Thakur, V.; Gajbe, V. Adsorptive removal of toxic azo dye amido black 10b by hen feather. Environ. Sci. Pollut. R. 2013, 20, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroglu, F.E.; Alexander, S.C.; Ji, D.; Prusty, D.K.; Borsch, M.; Herrmann, A. Poly (bodipy) s: A new class of tunable polymeric dyes. Macromolecules 2009, 42, 6529–6536. [Google Scholar] [CrossRef]
- Miltsov, S.; Karavan, V.; Goikhman, M.; Podeshvo, I.; Gómez-de Pedro, S.; Puyol, M.; Alonso-Chamarro, J. Synthesis of bis-aminosubstituted indocyanine dyes for their use in polymeric compositions. Dyes Pigments 2014, 109, 34–41. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, S.; Yang, J.; Liu, F. Synthesis of a novel water-soluble crosslinking polymeric dye with good dyeing properties. Dyes Pigments 2006, 68, 69–73. [Google Scholar] [CrossRef]
- Marechal, E. Polymeric dyes—synthesis, properties and uses. Prog. Org. Coat. 1982, 10, 251–287. [Google Scholar] [CrossRef]
- Marechal, E. Macromolecular dyes. Oligomeric and unsaturated dyes for UV curing. Pure Appl. Chem. 1980, 52, 1923–1928. [Google Scholar] [CrossRef]
- Libert, C.; Marechal, E. Synthesis of macromolecules containing color-forming monomers. Eur. Poly. J. 1980, 16, 951–956. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, B.; Zhao, H.; Xu, J.; Xiao, J.; Zhang, X.; Xu, H.; Zhang, S. Dyeing method and properties of polymaleic acid dyes on cotton. Color. Technol. 2013, 129, 144–149. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, B.; Zhang, S. A facile synthesis of poly (acrylanilide-co-acrylic acid). Asian J. Chem. 2013, 25, 3247. [Google Scholar]
- Xu, H.; Tang, B.T.; Zhang, S.F. Synthesis and dyeing performance of a novel polycarboxylic acid azo dye. Chin. Chem. Lett. 2011, 22, 424–426. [Google Scholar] [CrossRef]
- Zamanloo, M.R.; nasser Shamkhali, A.; Alizadeh, M.; Mansoori, Y.; Imanzadeh, G. A novel barbituric acid-based azo dye and its derived polyamides: Synthesis, spectroscopic investigation and computational calculations. Dyes Pigments 2012, 95, 587–599. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, T.; Zhou, R.; Trindle, C.O.; Tikman, Y.; Zhang, X.; Zhang, G. Waterborne polyurethanes with tunable fluorescence and room-temperature phosphorescence. ACS Appl. Mater. Interfaces 2015, 7, 17209–17216. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, S.A.; Xia, Y.; Kessler, M.R. Rheological behavior of environmentally friendly castor oil-based waterborne polyurethane dispersions. Macromolecules 2013, 46, 4606–4616. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.C. Waterborne polyurethanes and their properties. J. Poly. Sci. Poly. Chem. 1996, 34, 1095–1104. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Wang, Y. Synthesis of polymeric dyes based on waterborne polyurethane for improved color stability. New J. Chem. 2015, 39, 3543–3550. [Google Scholar] [CrossRef]
- Mao, H.; Wang, Y.; Yao, D.; Wang, C.; Sun, S. Synthesis of blocked waterborne polyurethane polymeric dyes with tailored molecular weight: Thermal, rheological and printing properties. RSC Adv. 2016, 6, 56831–56838. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Liu, J.; Dai, J. Synthesis, characterization and fluorescence performance of a waterborne polyurethane-based polymeric dye. J. Lumin. 2013, 142, 23–27. [Google Scholar]
- Mao, H.; Yang, F.; Wang, C.; Wang, Y.; Yao, D.; Yin, Y. Anthraquinone chromophore covalently bonded blocked waterborne polyurethanes: Synthesis and application. RSC Adv. 2015, 5, 30631–30639. [Google Scholar] [CrossRef]
- ASTM International. Available online: https://www.astm.org/Standards/D2572.htm (accessed on 16 September 2017).
- Bai, C.Y.; Zhang, X.Y.; Dai, J.B.; Li, W.H. A new UV curable waterborne polyurethane: Effect of C=C content on the film properties. Prog. Org. Coat. 2006, 55, 291–295. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Deng, Y.; Sun, W.; Wang, Q.; Xu, F.; Huang, X. Dual-emissive waterborne polyurethanes prepared from naphthalimide derivative. Polymers 2017, 9, 411. [Google Scholar] [CrossRef]
- Seymour, R.W.; Cooper, S.L. Thermal analysis of polyurethane block polymers. Macromolecules 1973, 6, 48–53. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, L.; Liu, Z.; Yuan, Y.; Yan, P.; Zhou, C.; Lei, J. Effect of phase separation on the crystallization of soft segments of green waterborne polyurethanes. Polym. Test. 2017, 60, 160–165. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, G.; Zhang, H. Effects of the reagent molar ratio on the phase separation and properties of waterborne polyurethane for application in a water-based ink binder. J. Appl. Polym. Sci. 2017, 134, 45406. [Google Scholar] [CrossRef]
- Čulin, J.; Andreis, M.; Šmit, I.; Veksli, Z.; Anžlovar, A.; Žigon, M. Motional heterogeneity and phase separation of functionalized polyester polyurethanes. Eur. Polym. J. 2004, 40, 1857–1866. [Google Scholar] [CrossRef]


| Sample | Polymeric Diol | Polymeric Diol /g | IPDI /g | DMPA /g | SDB /g | BDO /g | SDB (wt %) |
|---|---|---|---|---|---|---|---|
| SDB-WPU1 | PTMG | 25 | 12 | 2.3 | 0.42 | 1.8 | 1% |
| SDB-WPU2 | PTMG | 25 | 12 | 2.3 | 0.83 | 1.7 | 2% |
| SDB-WPU4 | PTMG | 25 | 12 | 2.3 | 1.7 | 1.55 | 4% |
| SDB-WPU6 | PTMG | 25 | 12 | 2.3 | 2.6 | 1.4 | 6% |
| SDB-WPU-PTMG | PTMG | 25 | 12 | 2.3 | 0.83 | 1.7 | 2% |
| SDB-WPU-PPG | PPG | 25 | 12 | 2.3 | 0.83 | 1.7 | 2% |
| SDB-WPU-PCL | PCL | 25 | 12 | 2.3 | 0.83 | 1.7 | 2% |
| SDB-WPU-PBA | PBA | 25 | 12 | 2.3 | 0.83 | 1.7 | 2% |
| Sample | Average Particle Size | Storage Stability | Mechanical Stability | Mn a | PDI b |
|---|---|---|---|---|---|
| SDB-WPU1 | 19.79 nm | Unchanged | Unchanged | 16,100 | 3.61 |
| SDB-WPU2 | 21.38 nm | Unchanged | Unchanged | 16,300 | 3.70 |
| SDB-WPU4 | 41.69 nm | Unchanged | Unchanged | 14,400 | 3.63 |
| SDB-WPU6 | 43.32 nm | Unchanged | Unchanged | 13,200 | 3.17 |
| SDB-WPU-PTMG | 21.38 nm | Unchanged | Unchanged | 16,300 | 3.70 |
| SDB-WPU-PPG | 19.21 nm | Unchanged | Unchanged | 16,000 | 2.79 |
| SDB-WPU-PCL | 13.40 nm | Unchanged | Unchanged | 18,900 | 2.85 |
| SDB-WPU-PBA | 13.97 nm | Unchanged | Unchanged | 17,400 | 2.67 |
| Samples | A1 a | A2 b | Mp (%) c |
|---|---|---|---|
| WPU+SDB | 0.332 | 0.435 | 31.0% |
| SDB-WPU-PTMG | 0.309 | 0.332 | 7.4% |
| SDB-WPU-PPG | 0.276 | 0.298 | 8.0% |
| SDB-WPU-PCL | 0.327 | 0.351 | 7.3% |
| SDB-WPU-PBA | 0.252 | 0.271 | 7.5% |
| Entry | Function | wt % |
|---|---|---|
| SDB-WPU | Resin | 95 |
| BYK-025 | Defoamer | 0.5 |
| BYK-331 | Leveling additive | 0.3 |
| ACRYSOLTM RM-8W | Thickener | 1.2 |
| Deionized water | - | 3 |
| Total | - | 100 |
| Property | SDB-WPU-PTMG | SDB-WPU-PPG | SDB-WPU-PCL | SDB-WPU-PBA |
|---|---|---|---|---|
| Appearance | Smooth, No bubbles | Smooth, No bubbles | Smooth, No bubbles | Smooth, No bubbles |
| Gloss (60°) | 83 | 83 | 86 | 87 |
| Adhesion force (grade) | 0 | 1 | 0 | 0 |
| Pencil hardness | 2B | 3B | B | 2B |
| Impact strength (kg/cm) | 50 | 50 | 50 | 50 |
| Water resistance (24 h) | Unchanged | Unchanged | Unchanged | Unchanged |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Sun, W.; Zhang, X.; Xu, H.; Xu, F. Waterborne Polyurethane Coatings with Covalently Linked Black Dye Sudan Black B. Materials 2017, 10, 1247. https://doi.org/10.3390/ma10111247
Wang T, Sun W, Zhang X, Xu H, Xu F. Waterborne Polyurethane Coatings with Covalently Linked Black Dye Sudan Black B. Materials. 2017; 10(11):1247. https://doi.org/10.3390/ma10111247
Chicago/Turabian StyleWang, Tao, Wei Sun, Xingyuan Zhang, Haiyan Xu, and Fei Xu. 2017. "Waterborne Polyurethane Coatings with Covalently Linked Black Dye Sudan Black B" Materials 10, no. 11: 1247. https://doi.org/10.3390/ma10111247





