Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding
Abstract
:1. Introduction
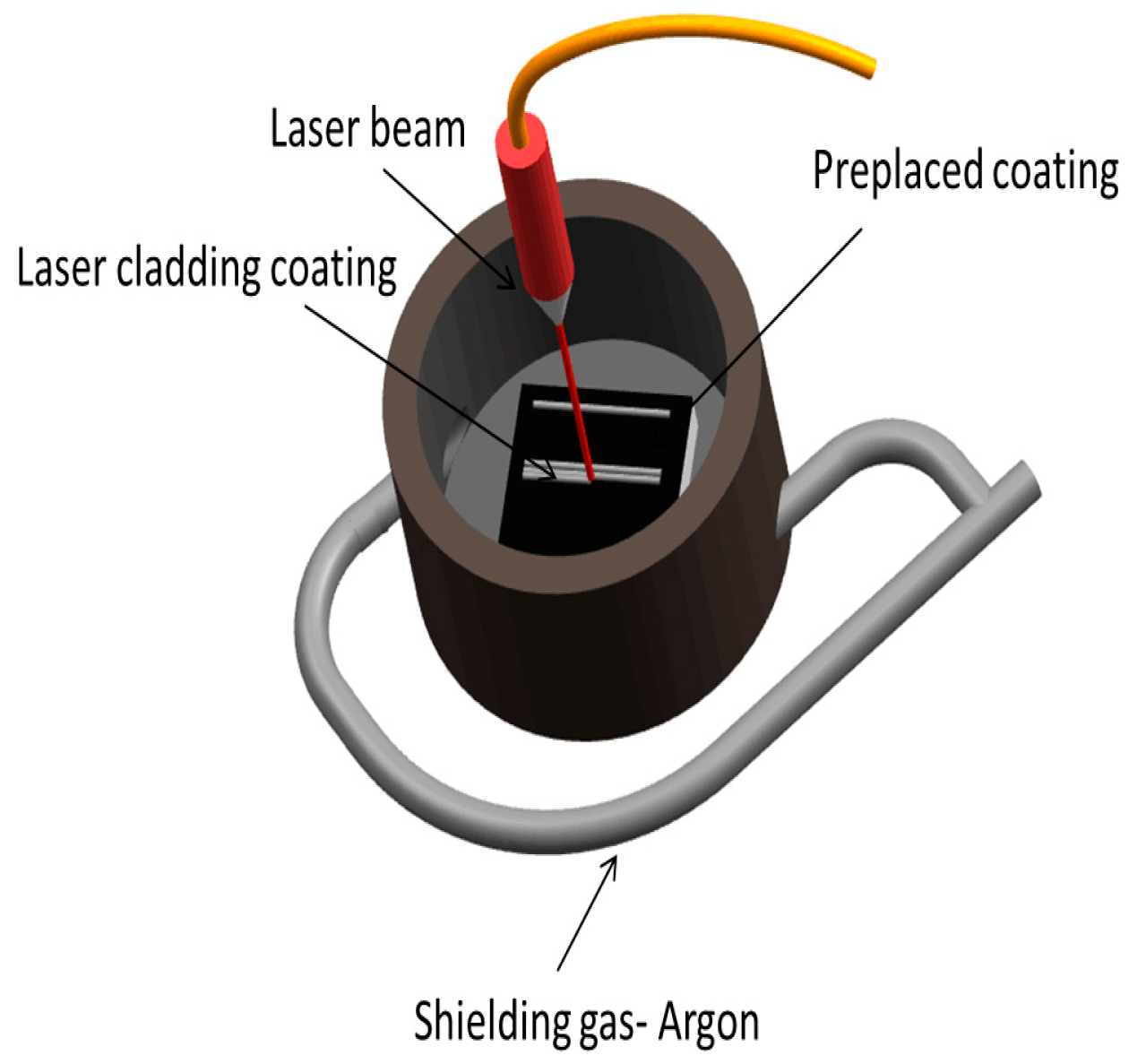
2. Materials and Methods
3. Results and Discussion
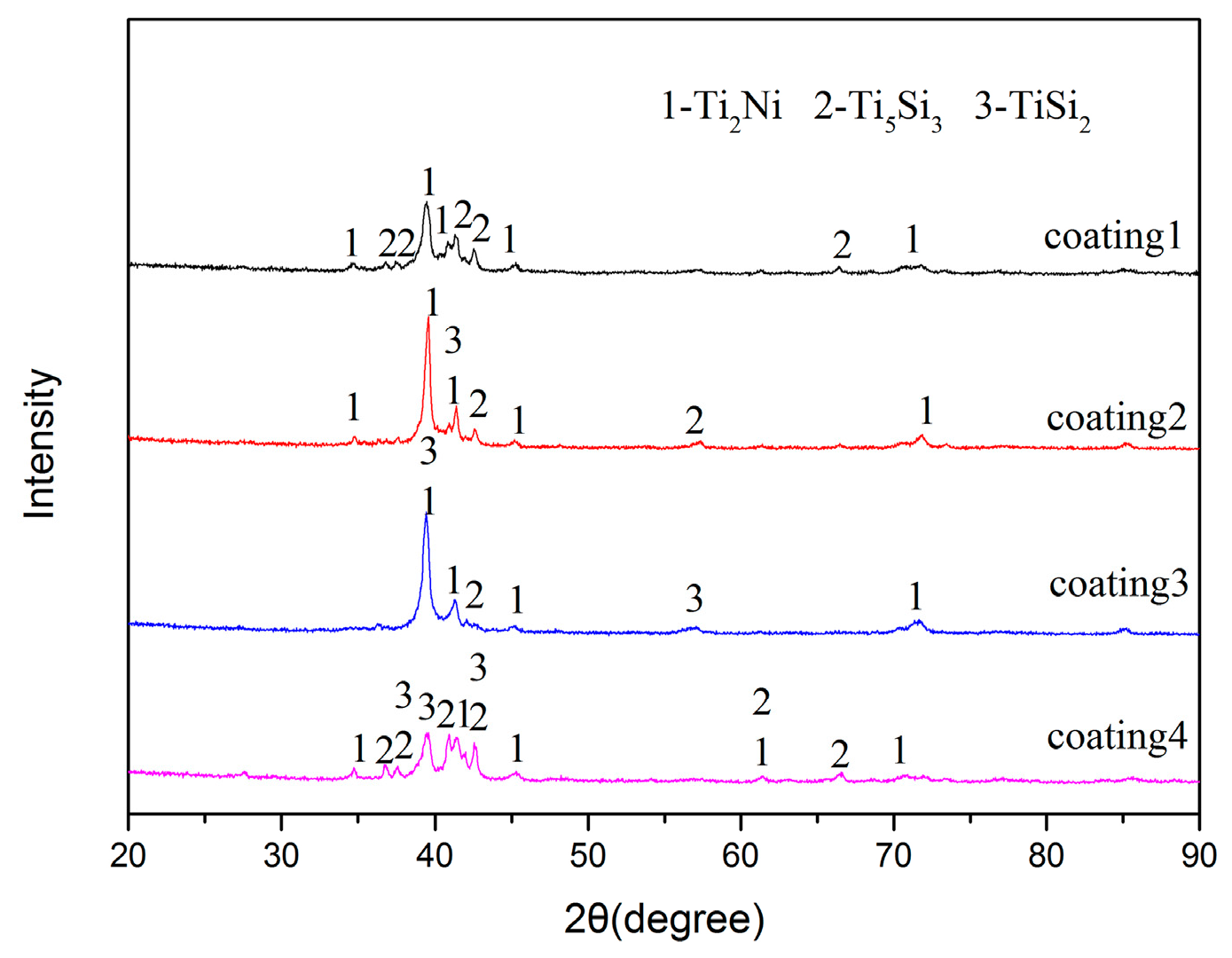
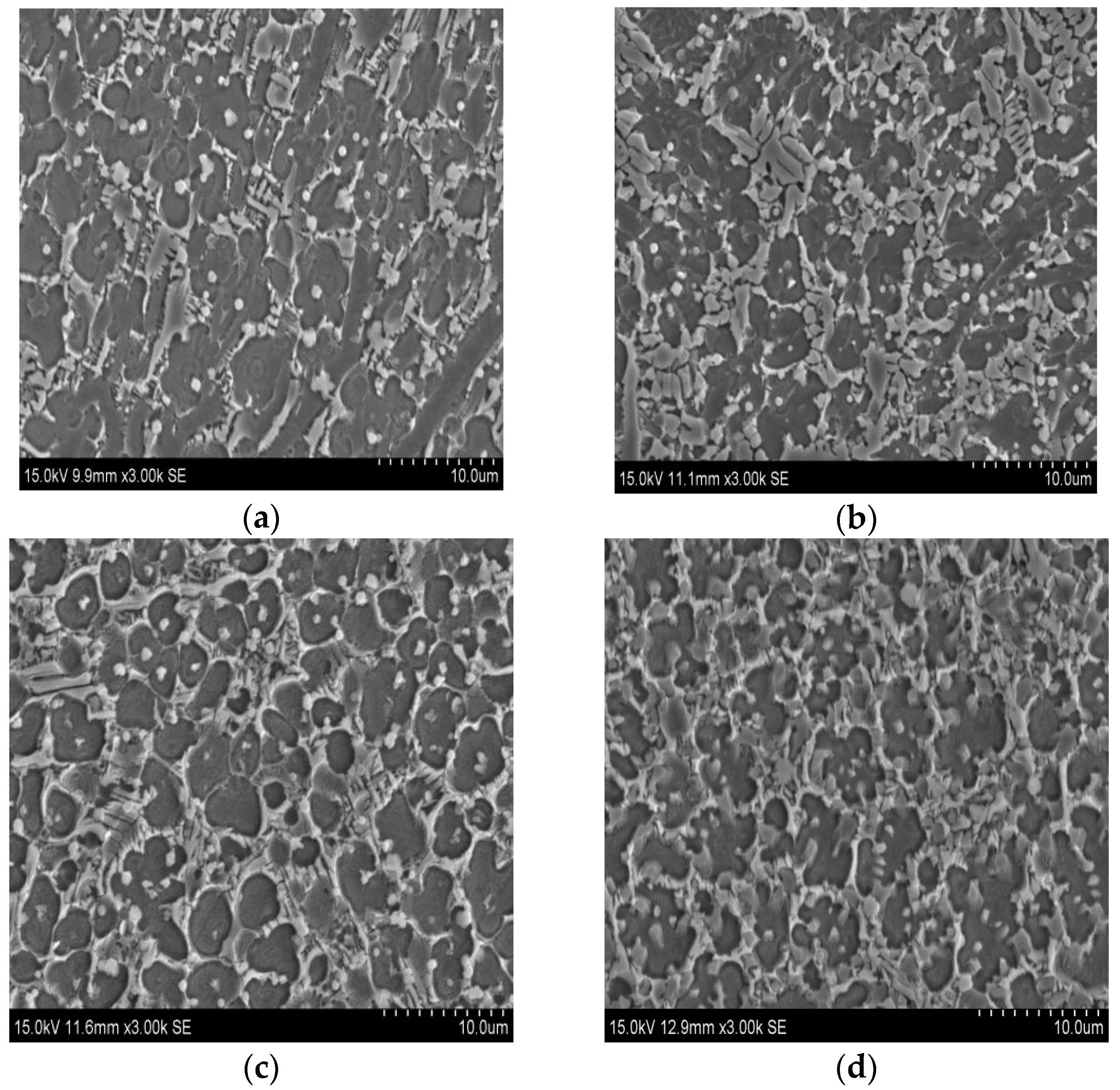
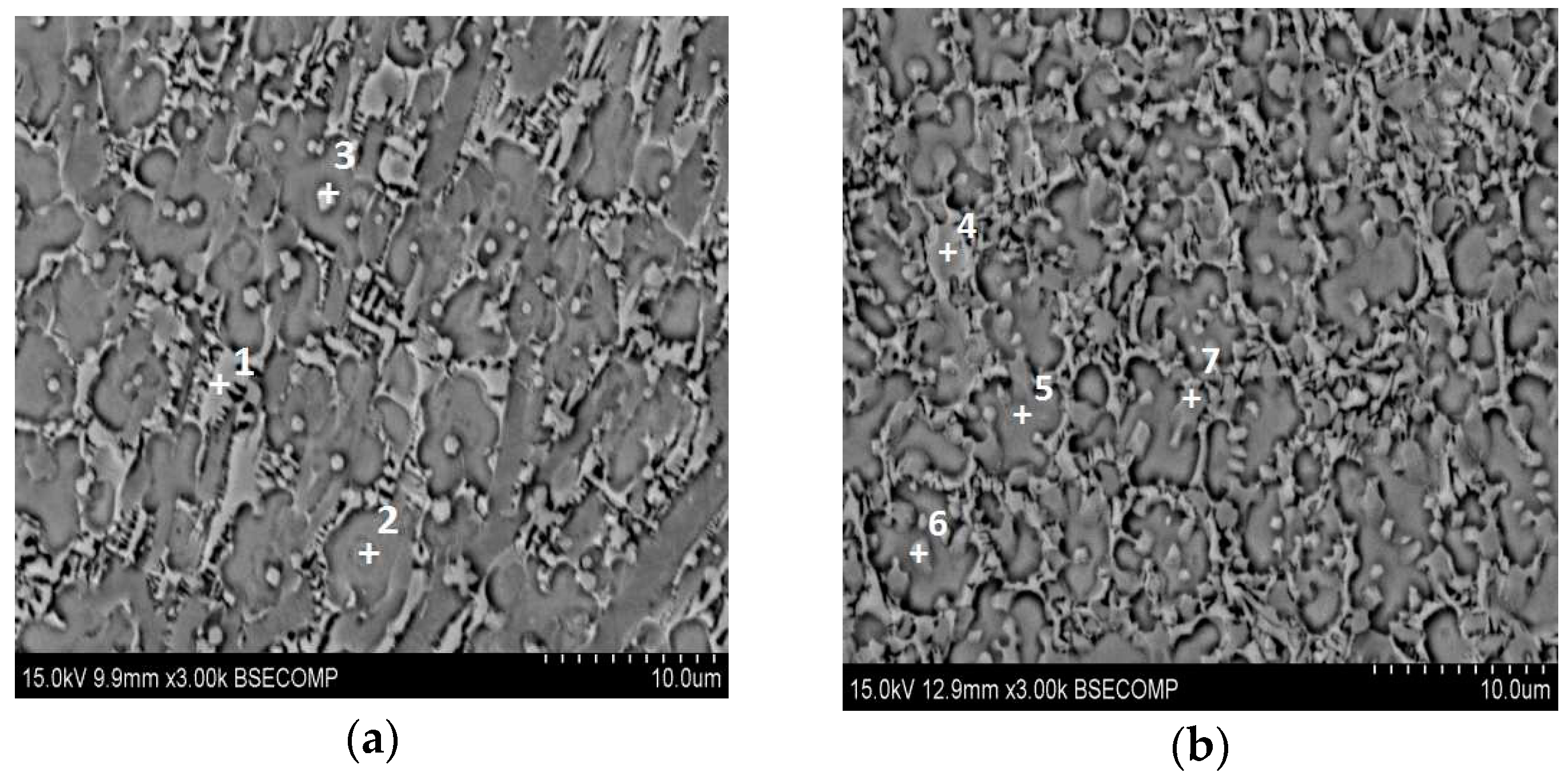
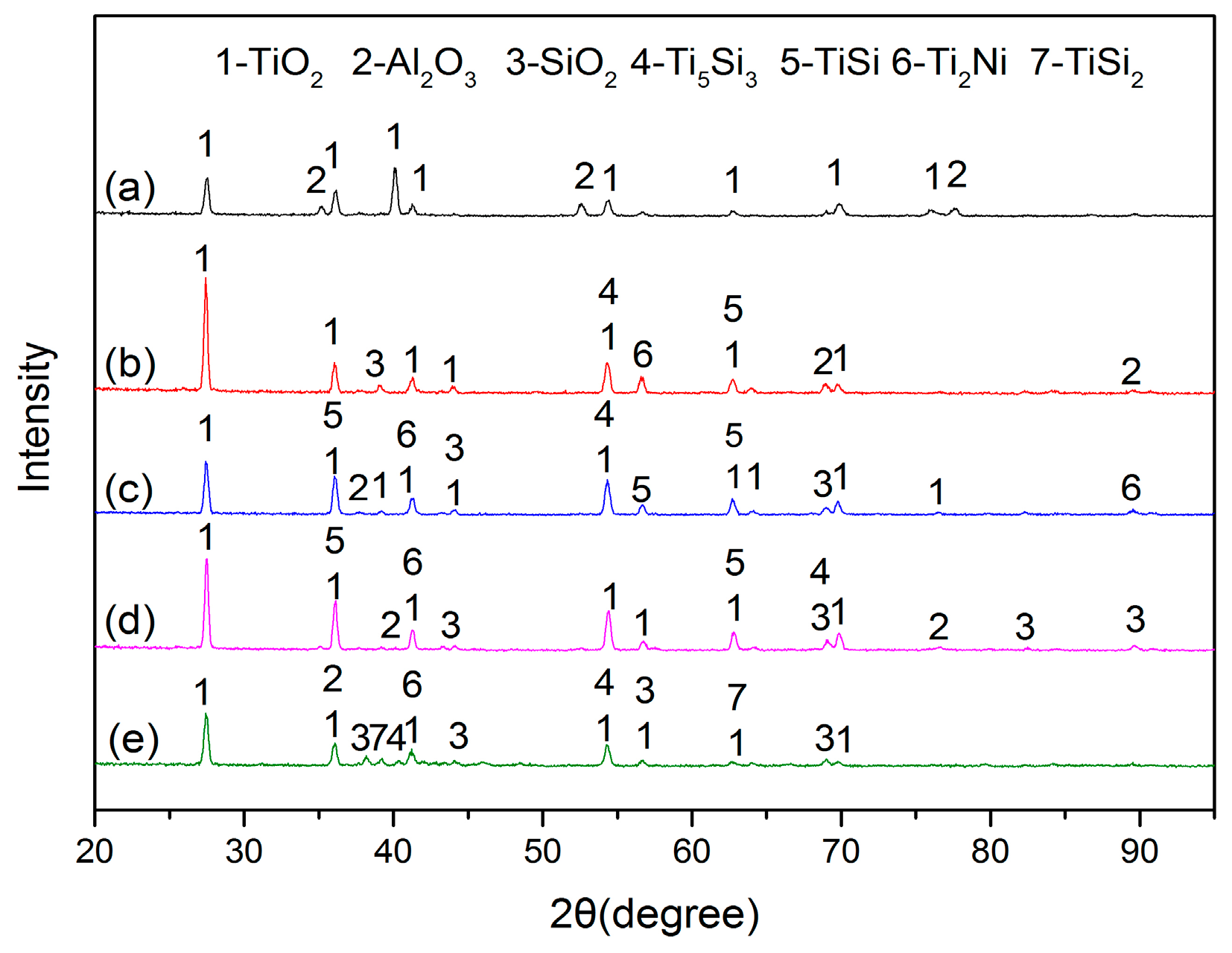
3.1. XRD Results and Microstructures
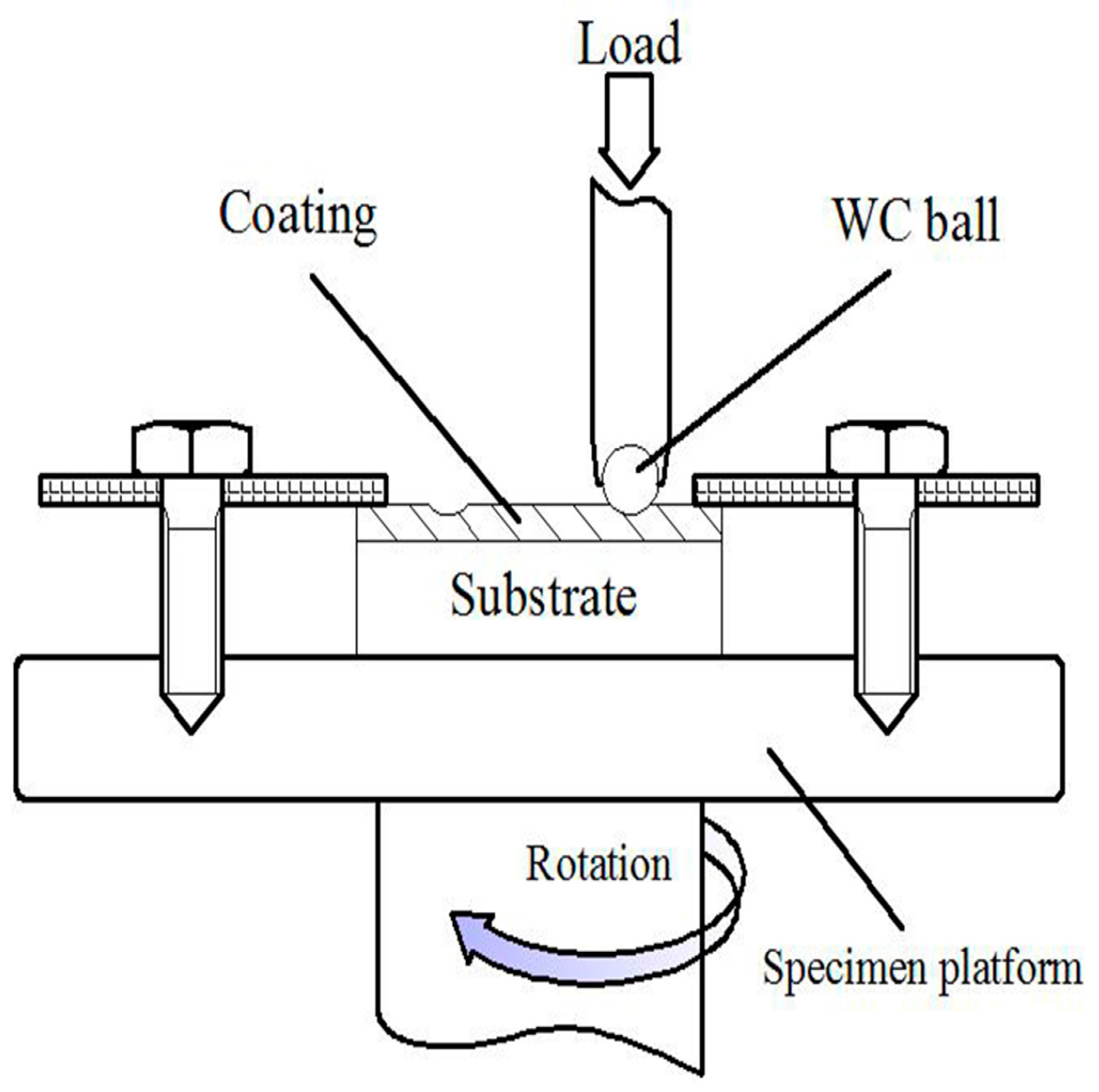
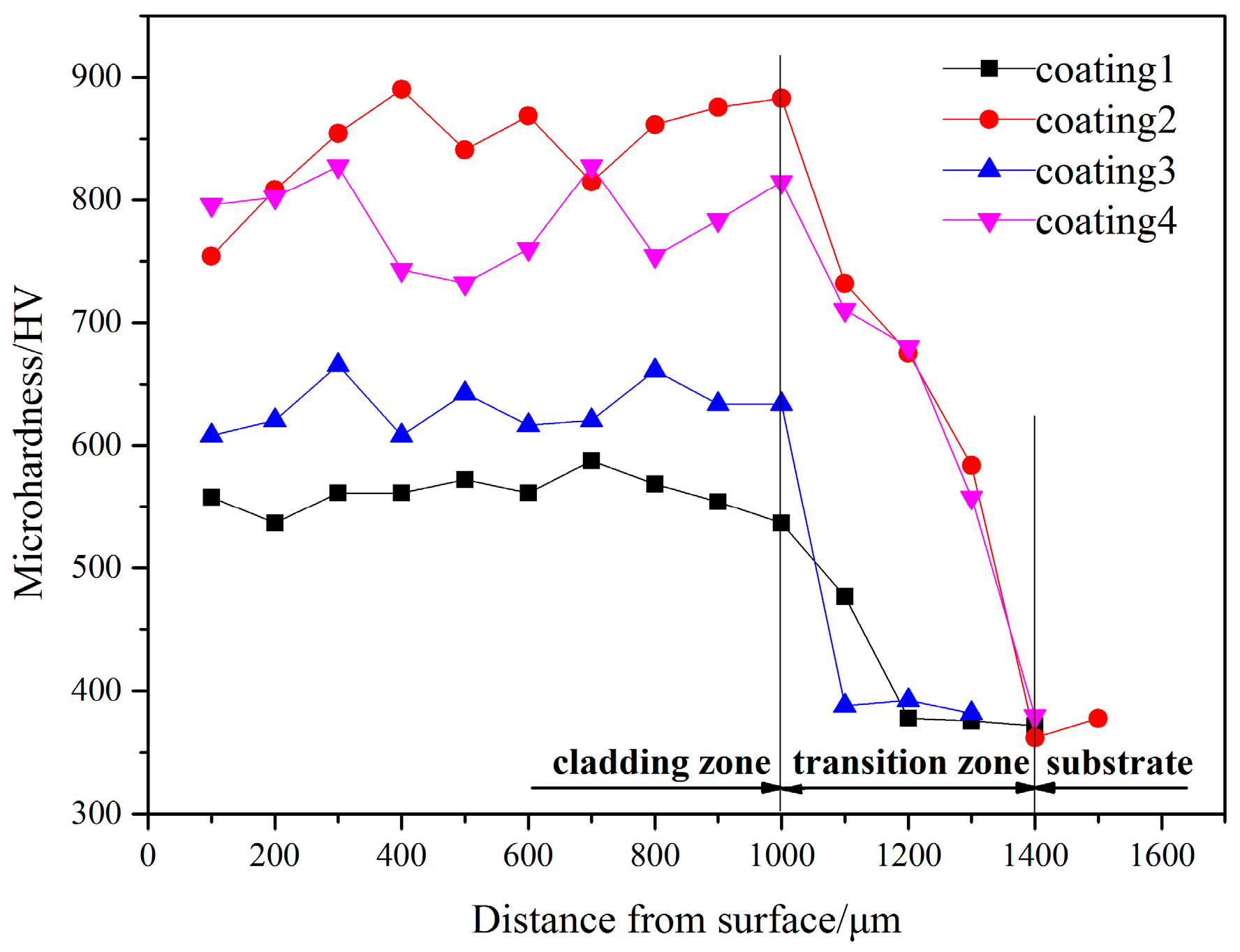
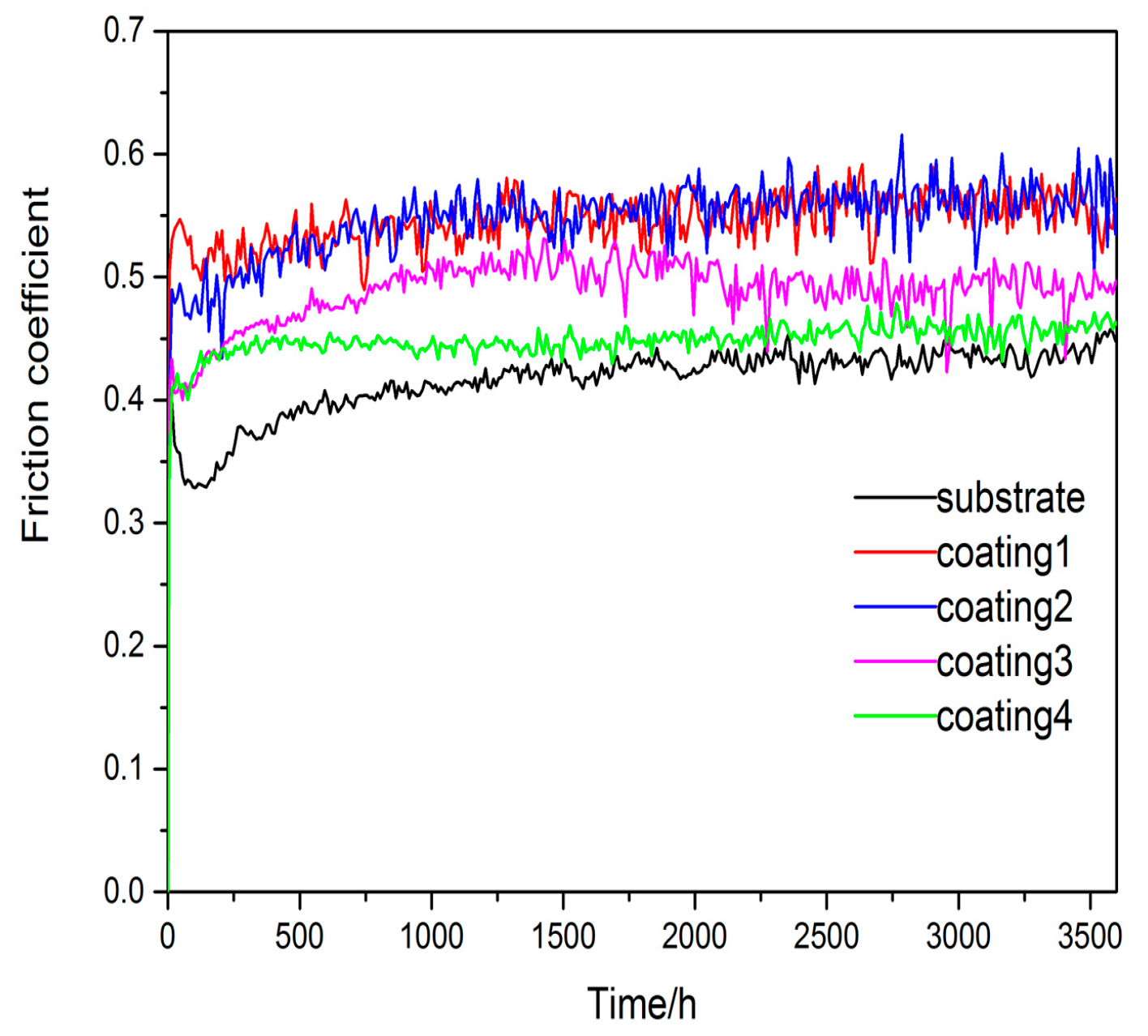
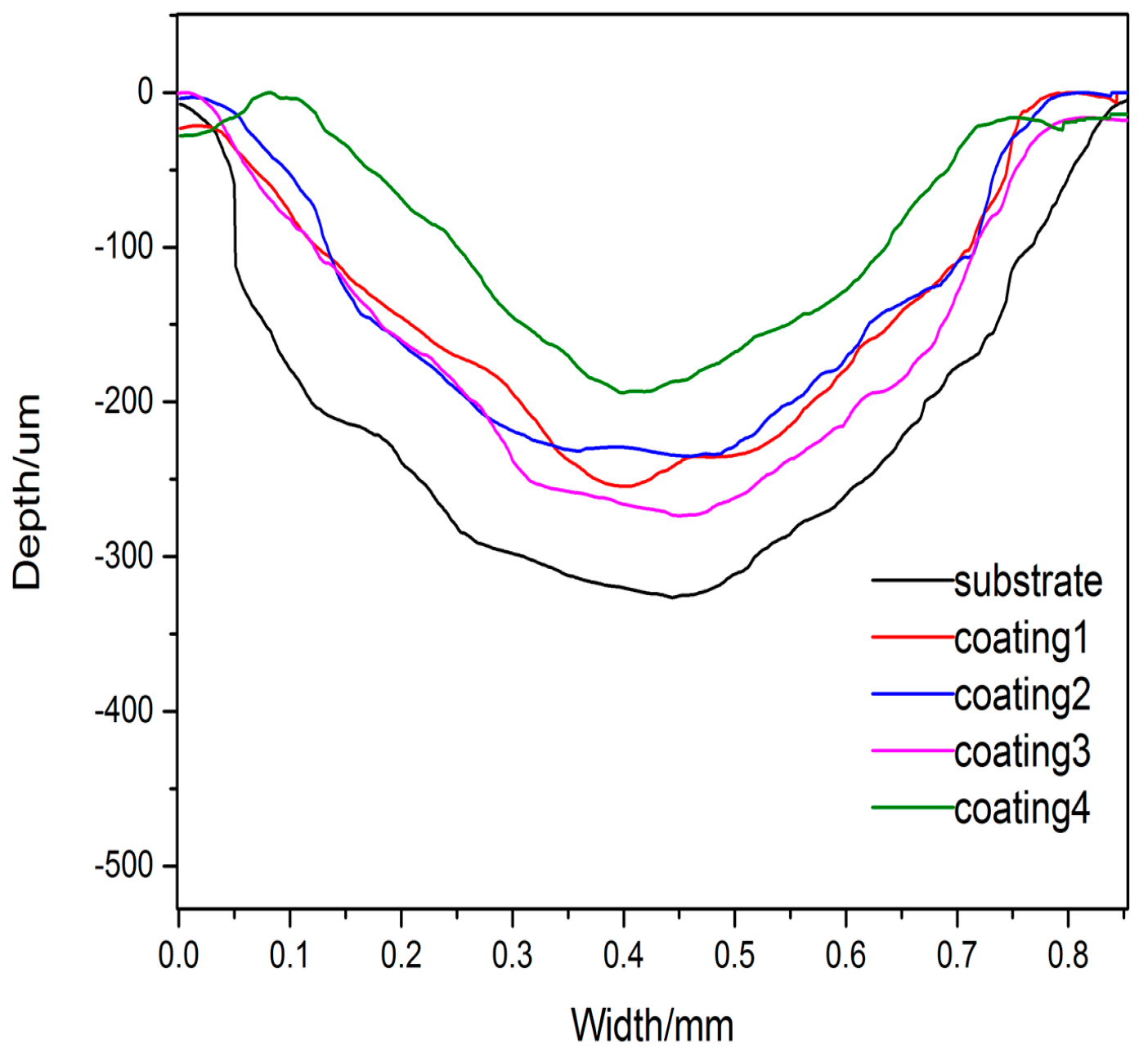
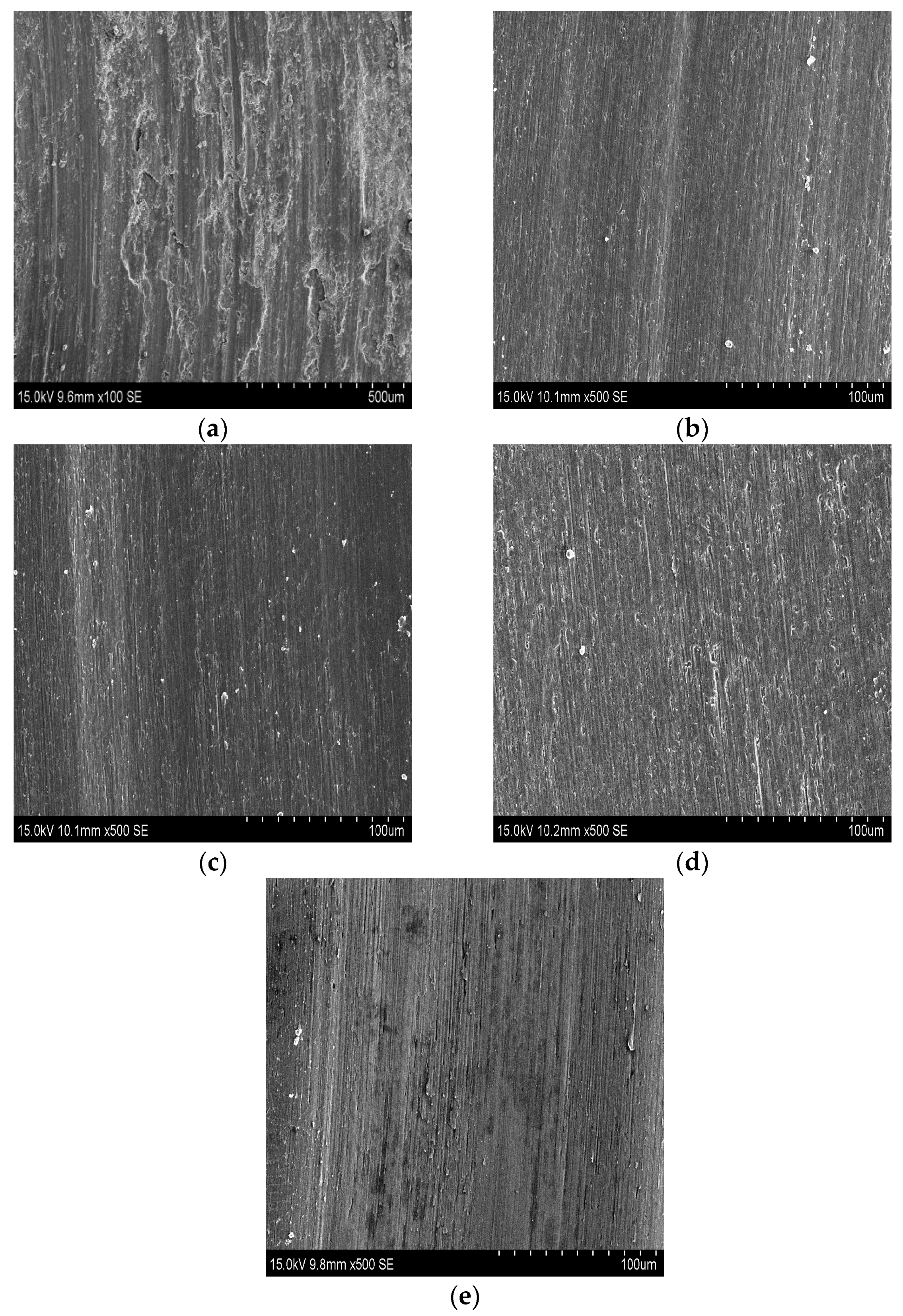
3.2. Microhardness and Wear Resistance
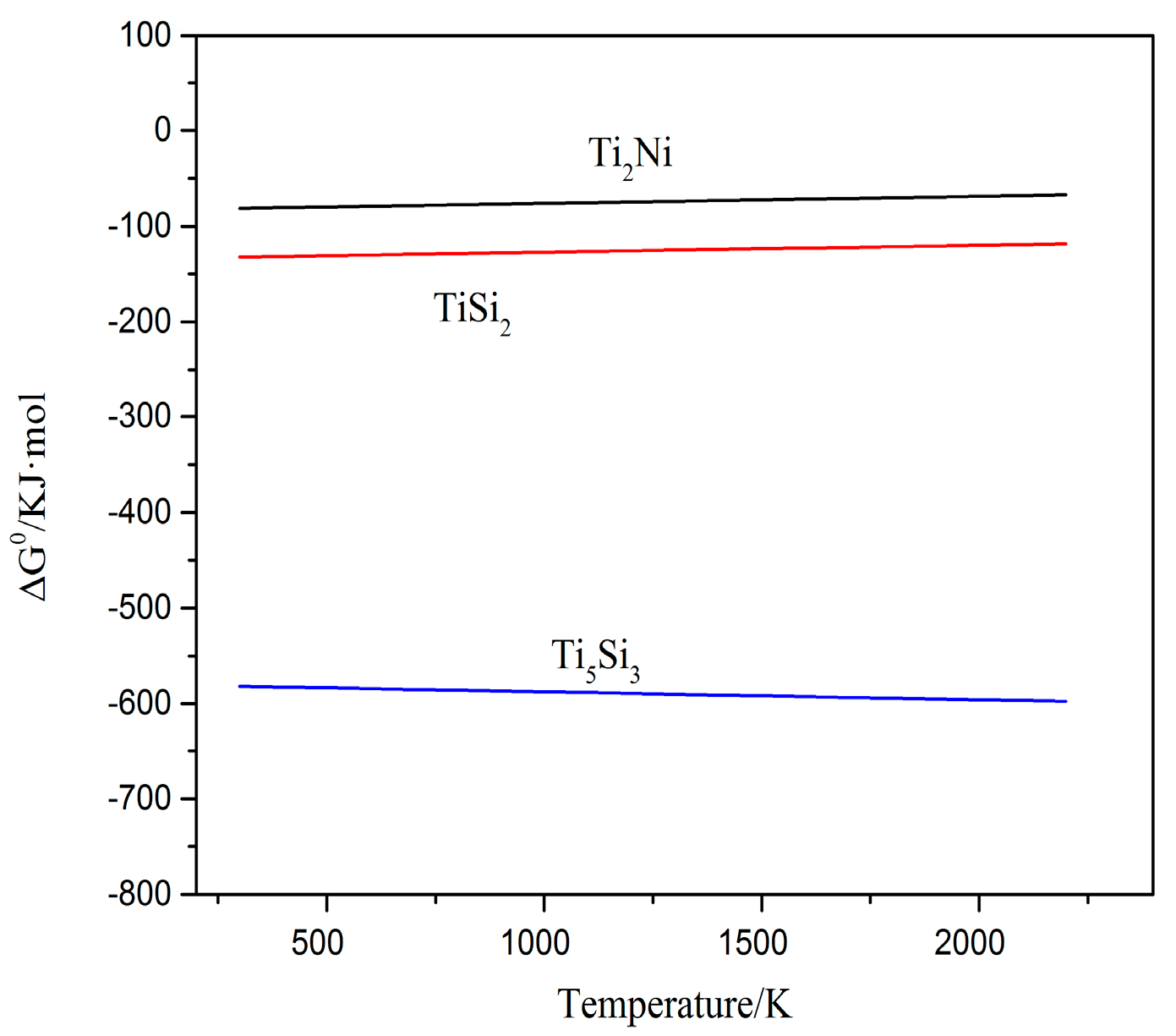
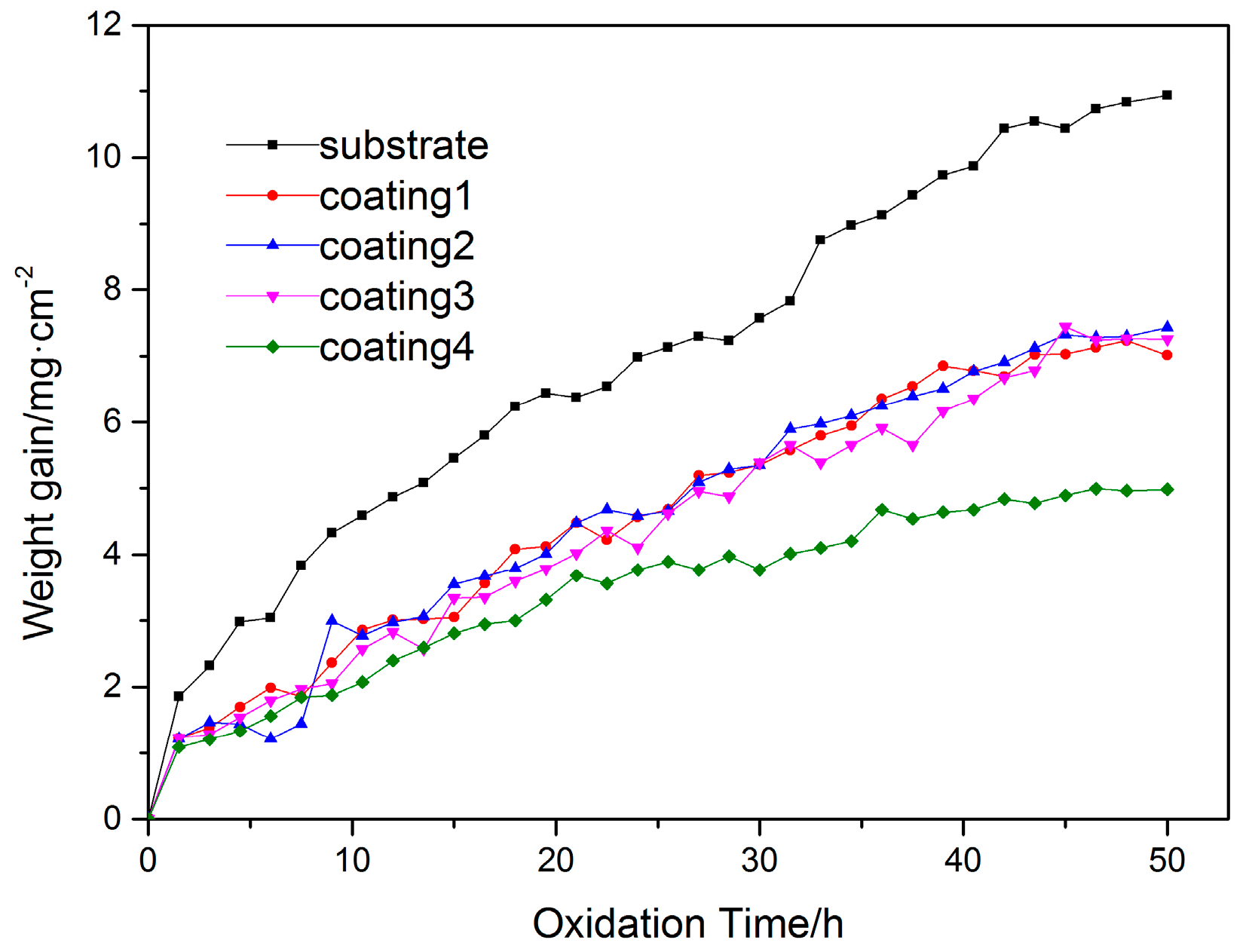
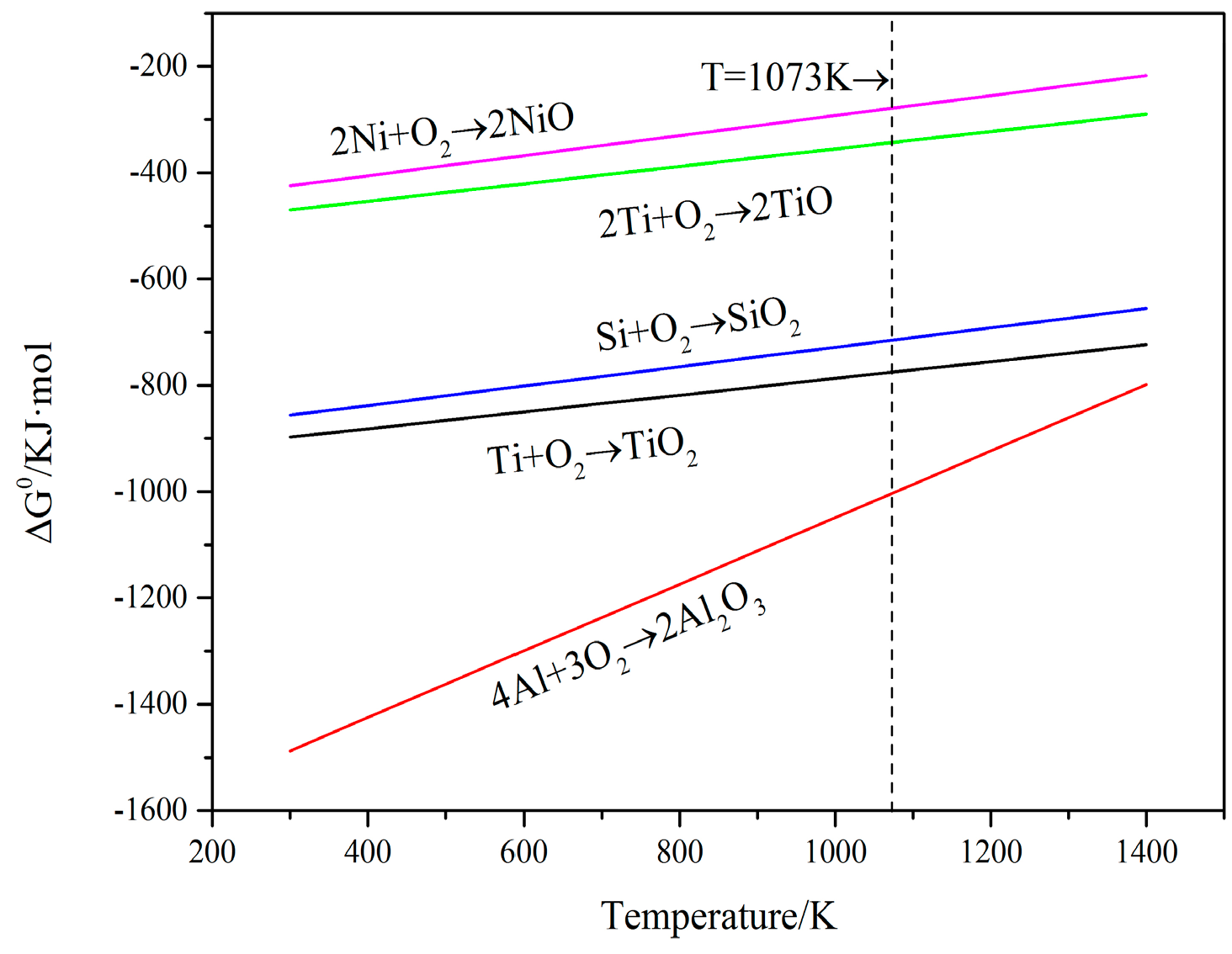
3.3. High Temperature Oxidation Resistance
4. Conclusions
- Coatings constituted of Ti5Si3, Ti2Ni and TiSi2 phases were obtained by laser cladding with different contents of Ni, Ti and Si powders. Because of the distribution of the inherent high hardness of these phases, the coatings have a higher microhardness than the substrate. While the proportion of the Ti content is 40 at %, the coating has the highest microhardness, which is 826 HV.
- By means of combining the reinforcement phases of Ti5Si3 and TiSi2 with the relatively ductile phase of Ti2Ni, the coatings present good wear resistance. Coating 4 shows the better wear resistance.
- The oxidation process can be divided into two stages, the rapid and the slow oxidation sections. The oxidation rate of the coatings is lower than that of the substrate during the process. With the decrease of Ti content, the oxidation rate gradually reduces. Moreover, coating 4 has the lowest oxidation weight gain rate. It can demonstrate that coating 4 presents the best oxidation resistance at high temperature.
- In the oxidation process, the oxides of coatings are mainly TiO2, Al2O3 and SiO2 compared with oxides of the substrate, which is only TiO2, Al2O3. Furthermore, the Ti5Si3, Ti2Ni, TiSi and TiSi2 phases are also found in coatings. It can be ascribed to the good oxidation resistance of coatings.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sheng, W.; Liu, D.; Wang, H.M. Microstructure and high-temperature wear behavior of laser clad Ni-Ti-Si ternary metal silicide coatings. Surf. Coat. Technol. 2008, 202, 2871–2877. [Google Scholar] [CrossRef]
- Lv, Y.H.; Li, J.; Tao, Y.F.; Hu, L.F. Oxidation behaviors of the TiNi/Ti2Ni matrix composite coatings with different contents of TaC addition fabricated on Ti6Al4V by laser cladding. J. Alloys Compd. 2016, 679, 202–212. [Google Scholar] [CrossRef]
- Altus, E.; Konstantino, E. Optimum laser surface treatment of fatigue damaged Ti-6Al-4V alloy. Mater. Sci. Eng. A 2001, 302, 100–105. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, F.Y.; Wang, A.; Yu, H.J.; Chen, C. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley and Sons, Ltd.: New York, NY, USA, 2003; ISBN 3-527-30534-3. [Google Scholar]
- Zhang, P.L.; Liu, X.P.; Lu, Y.L.; Yan, H.; Yu, Z.; Li, C.; Lu, Q.H. Microstructure and wear behavior of Cu-Mo-Si coatings by laser cladding. Appl. Surf. Sci. 2014, 311, 709–714. [Google Scholar] [CrossRef]
- Maliutina, I.N.; Si-Mohand, H.; Sijobert, J.; Bertrand, Ph.; Lazurenko, D.V.; Bataev, I.A. Structure and oxidation behavior of γ-TiAl coating produced by laser cladding on titanium alloy. Surf. Coat. Technol. 2017, 319, 136–144. [Google Scholar] [CrossRef]
- Miranda, R. Laser Surface Modification of Alloys for Corrosion and Erosion Resistance; Woodhead Publishing Limited: Cambridge, UK, 2012; ISBN 978-0-85709-015-7. [Google Scholar]
- Kathuria, Y.P. Some aspects of laser surface cladding in the turbine industry. Surf. Coat. Technol. 2000, 132, 262–269. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.Z.; Vilar, R.; Shen, J.Y. Dry sliding wear behavior of laser clad TiVCrAlSi high entropy alloy coatings on Ti-6Al-4V substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.J.; Chen, C.Z.; Dai, J.J. Microstructures and wear properties of laser cladding Co-based composite coatings on Ti-6Al-4V. Mater. Des. 2015, 80, 174–181. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wang, H.M. Microstructure and dry sliding wear resistance of laser clad TiC reinforced Ti-Ni-Si intermetallic composite coating. Surf. Coat. Technol. 2009, 204, 731–735. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhang, X.W.; Jiang, Y.H.; Zhou, R. Microstructure and high temperature oxidation resistance of in-situ synthesized TiN/Ti3Al intermetallic composite coatings on Ti6Al4V alloy by laser cladding process. J. Alloys Compd. 2016, 670, 268–274. [Google Scholar] [CrossRef]
- Liu, X.B.; Wang, H.M. Microstructure wear and high-temperature oxidation resistance of laser clad Ti5Si3/γ/TiSi composite coatings on γ-TiAl intermetallic alloy. Surf. Coat. Technol. 2006, 200, 4462–4470. [Google Scholar] [CrossRef]
- Lv, Y.H.; Li, J.; Tao, Y.F.; Hu, L.F. High-temperature wear and oxidation behaviors of TiNi/Ti2Ni matrix composite coatings with TaC addition prepared on Ti6Al4V by laser cladding. Appl. Surf. Sci. 2017, 402, 478–494. [Google Scholar] [CrossRef]
- Ye, D.L. Practical Inorganic Thermodynamics Manual, 2nd ed.; Metallurgy Industry Press: Beijing, China, 2002; ISBN 7-5024-3055-5. [Google Scholar]
- Liu, J.; Bai, Y.N.; Chen, P.W.; Cui, N.F.; Yin, H. Reaction synthesis of TiSi2 and Ti5Si3 by ball-milling and shock loading and their photocatalytic activities. J. Alloys Compd. 2013, 555, 375–380. [Google Scholar] [CrossRef]
- Callister, W.D.J.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach, 4th ed.; Wiley-VCH: Hoboken, NJ, USA, 2012; ISBN 978-1-118-06160-2. [Google Scholar]
- Zhang, L.; Xie, C.Y.; Wu, J.S. Oxidation behavior of sputter-deposited Ti-Ni thin films at elevated temperature. Mater. Charact. 2007, 58, 471–478. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.M.; Shen, Y.F. Oxidation behavior of a high Si content Al-Si composite coating fabricates on Ti-6Al-4V substrate by mechanical alloying method. J. Alloys Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.Y.; Chen, C.Z.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Liu, G.L.; Li, Y. The electronic theory study on high-temperature oxidation mechanism of TiAl alloy. Acta Phys. Sin. 2012, 61, 1–5. [Google Scholar] [CrossRef]



| Al | V | Fe | Si | C | N | H | O | Ti |
|---|---|---|---|---|---|---|---|---|
| 5.5–6.8 | 3.5–4.5 | ≤0.30 | ≤0.15 | ≤0.10 | ≤0.05 | ≤0.015 | ≤0.20 | Bal. |
| Coating | Ni | Ti | Si | Laser Power/KW | Scanning Speed/(mm/min) |
|---|---|---|---|---|---|
| 1 | 35 | 50 | 15 | 1.5 | 1000 |
| 2 | 35 | 40 | 25 | ||
| 3 | 35 | 30 | 35 | ||
| 4 | 35 | 20 | 45 |
| Phase | Ni | Ti | Si | Al | V |
|---|---|---|---|---|---|
| 1 | 23.65 | 63.28 | 2.87 | 8.25 | 1.94 |
| 2 | 3.37 | 65.04 | 34.77 | 6.39 | 3.37 |
| 3 | 22.65 | 69.86 | 2.81 | 3.41 | 1.27 |
| 4 | 27.45 | 62.06 | 3.11 | 5.93 | 1.46 |
| 5 | 3.05 | 58.26 | 22.98 | 2.53 | 1.39 |
| 6 | 28.05 | 57.73 | 3.25 | 8.92 | 2.04 |
| 7 | 8.14 | 17.13 | 64.16 | 8.38 | 2.19 |
| Regions | Ni | Ti | Si | Al | O |
|---|---|---|---|---|---|
| 8 | - | 34.59 | - | 0.83 | 64.58 |
| 9 | - | 27.03 | - | 17.86 | 55.1 |
| 10 | 1.55 | 25.12 | 0.92 | 22.89 | 49.48 |
| 11 | 0.65 | 30.38 | 1.56 | 4.36 | 63.06 |
| 12 | 0.86 | 18.85 | 8.22 | 4.01 | 68.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Q.; Zhang, P.; Li, M.; Yan, H.; Yu, Z.; Lu, Q. Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding. Materials 2017, 10, 1248. https://doi.org/10.3390/ma10111248
Zhuang Q, Zhang P, Li M, Yan H, Yu Z, Lu Q. Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding. Materials. 2017; 10(11):1248. https://doi.org/10.3390/ma10111248
Chicago/Turabian StyleZhuang, Qiaoqiao, Peilei Zhang, Mingchuan Li, Hua Yan, Zhishui Yu, and Qinghua Lu. 2017. "Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding" Materials 10, no. 11: 1248. https://doi.org/10.3390/ma10111248






