New SmAPF Mesogens Designed for Analog Electrooptics Applications
Abstract
:1. Introduction
2. Design Rationale for the New SmAPF Mesogens
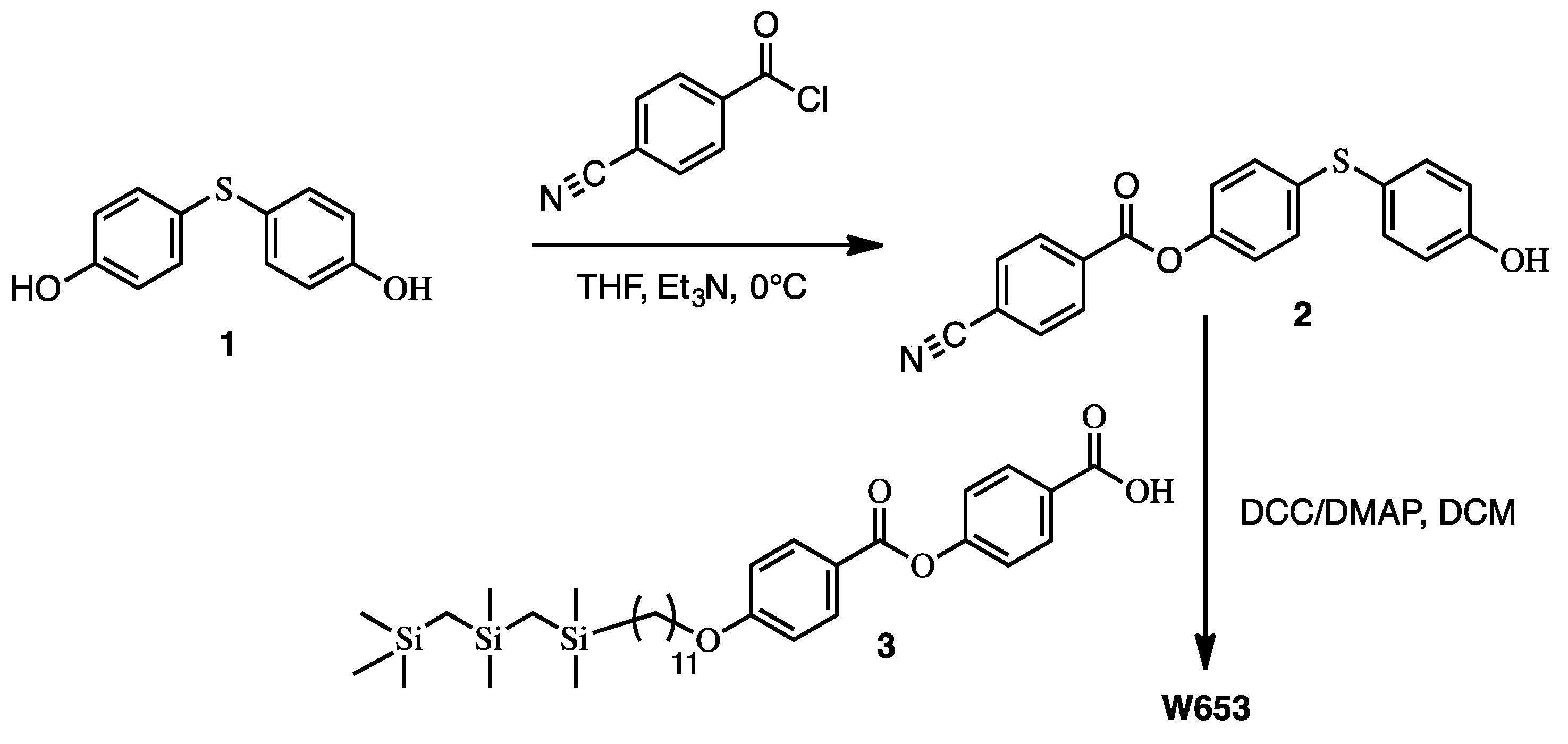
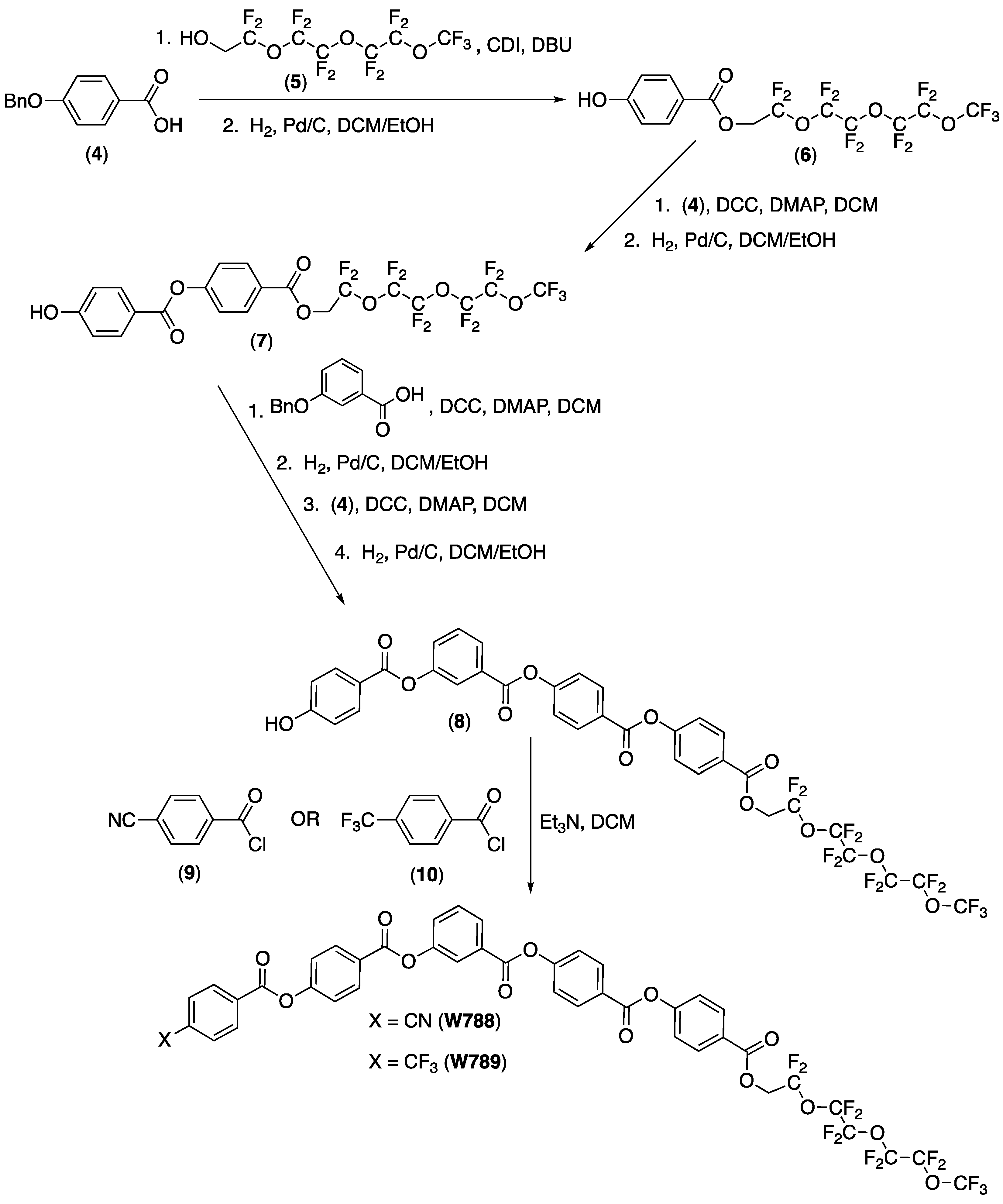
3. Synthesis of the New Mesogens
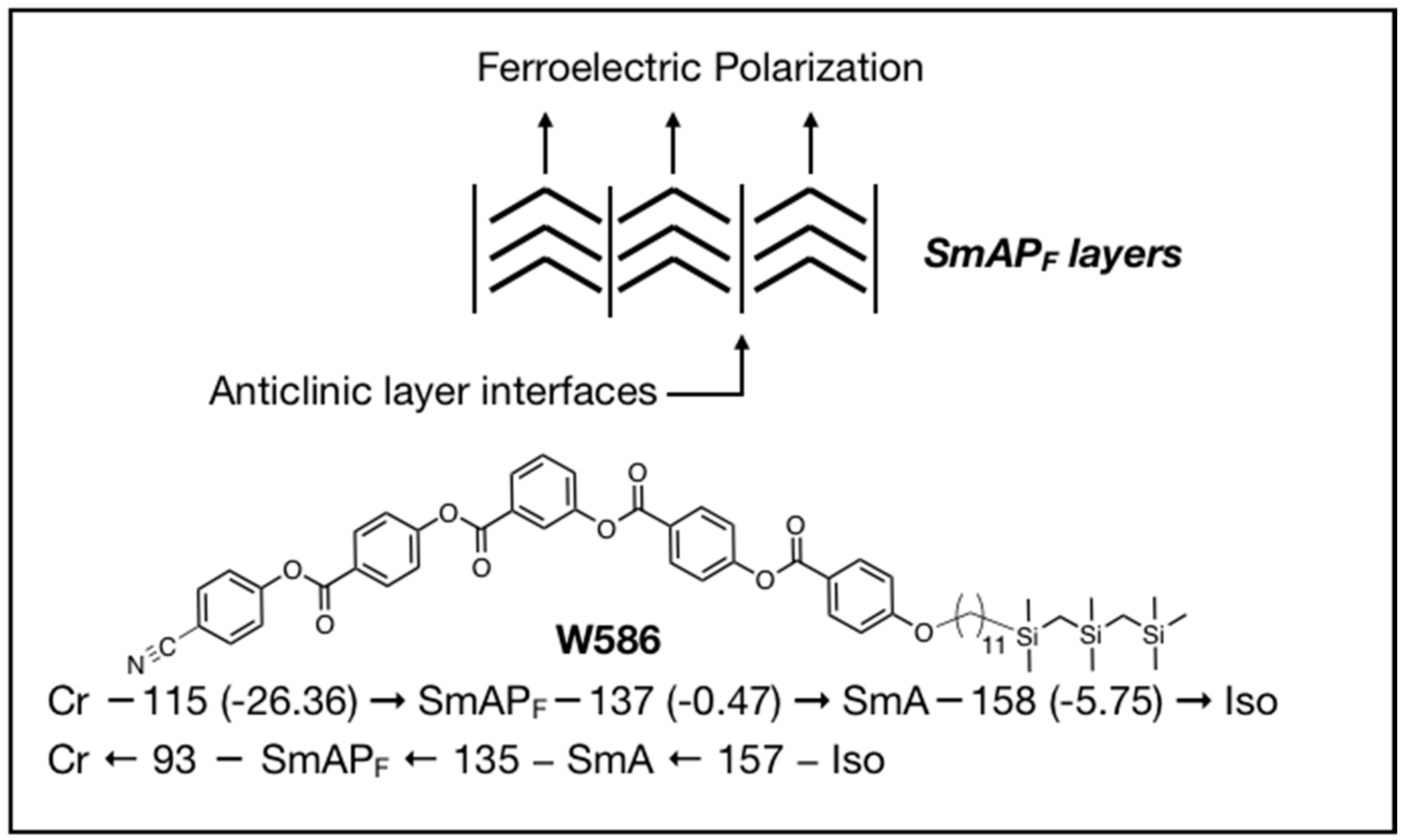
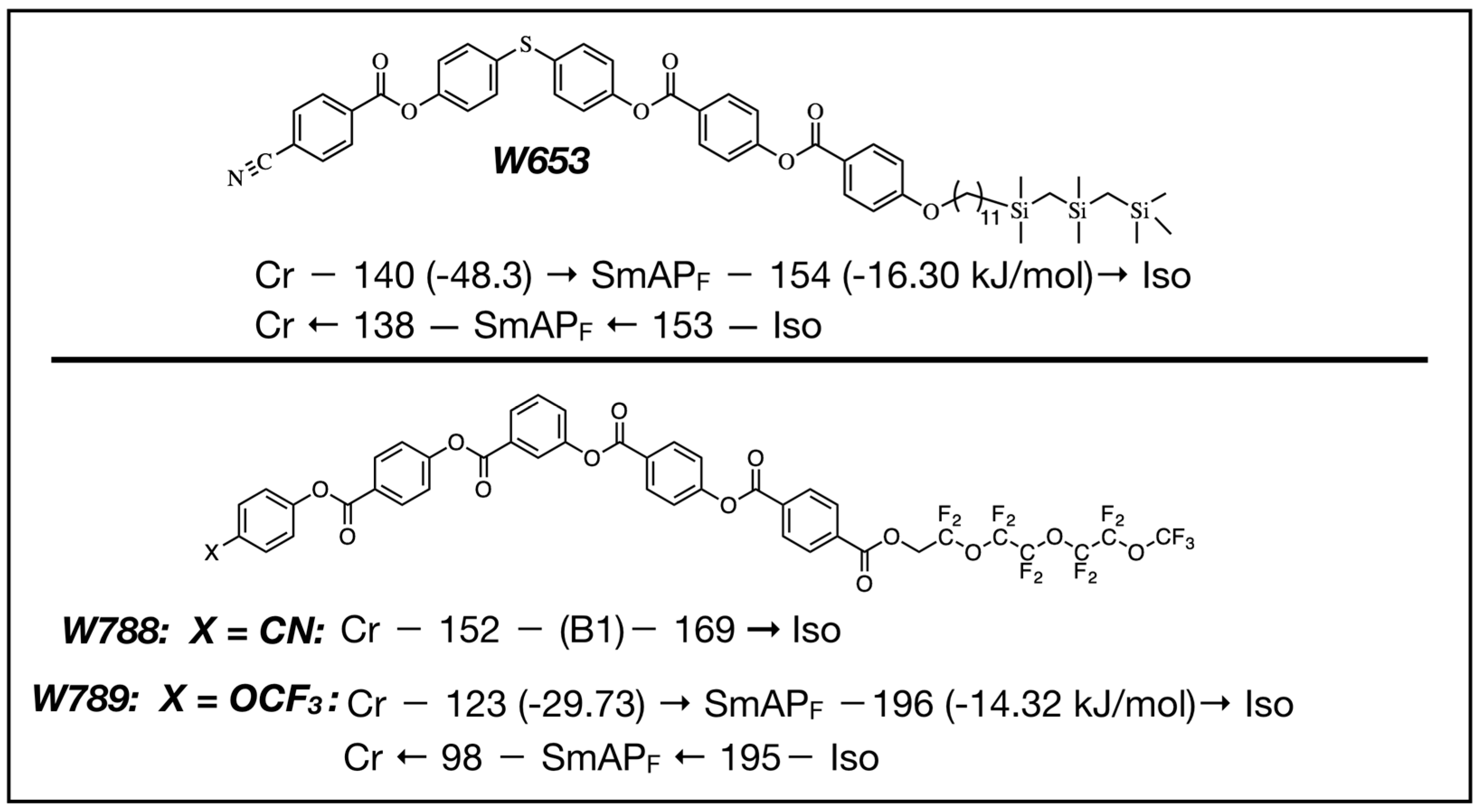
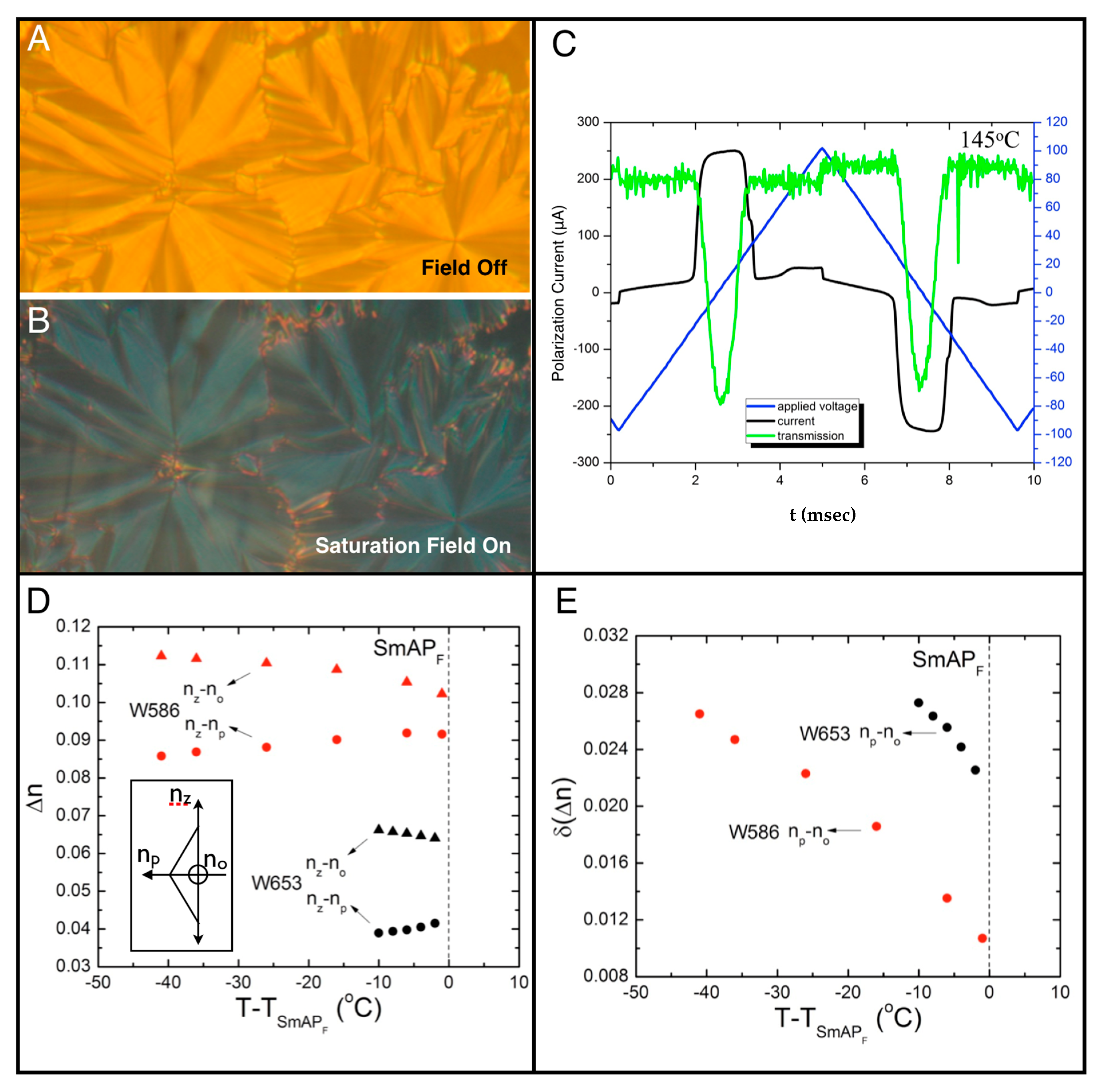
4. Properties of Diphenylthioether W653
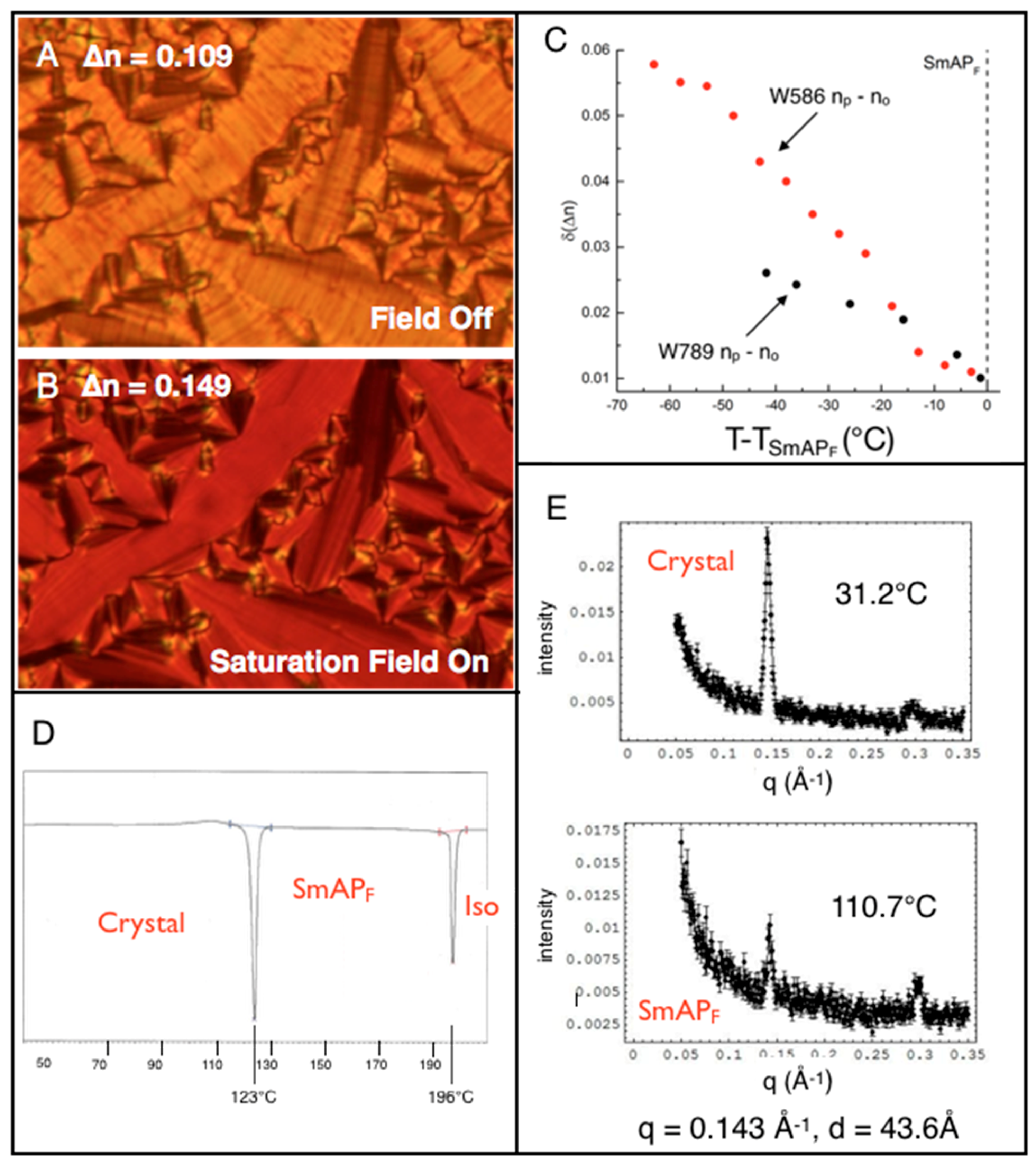
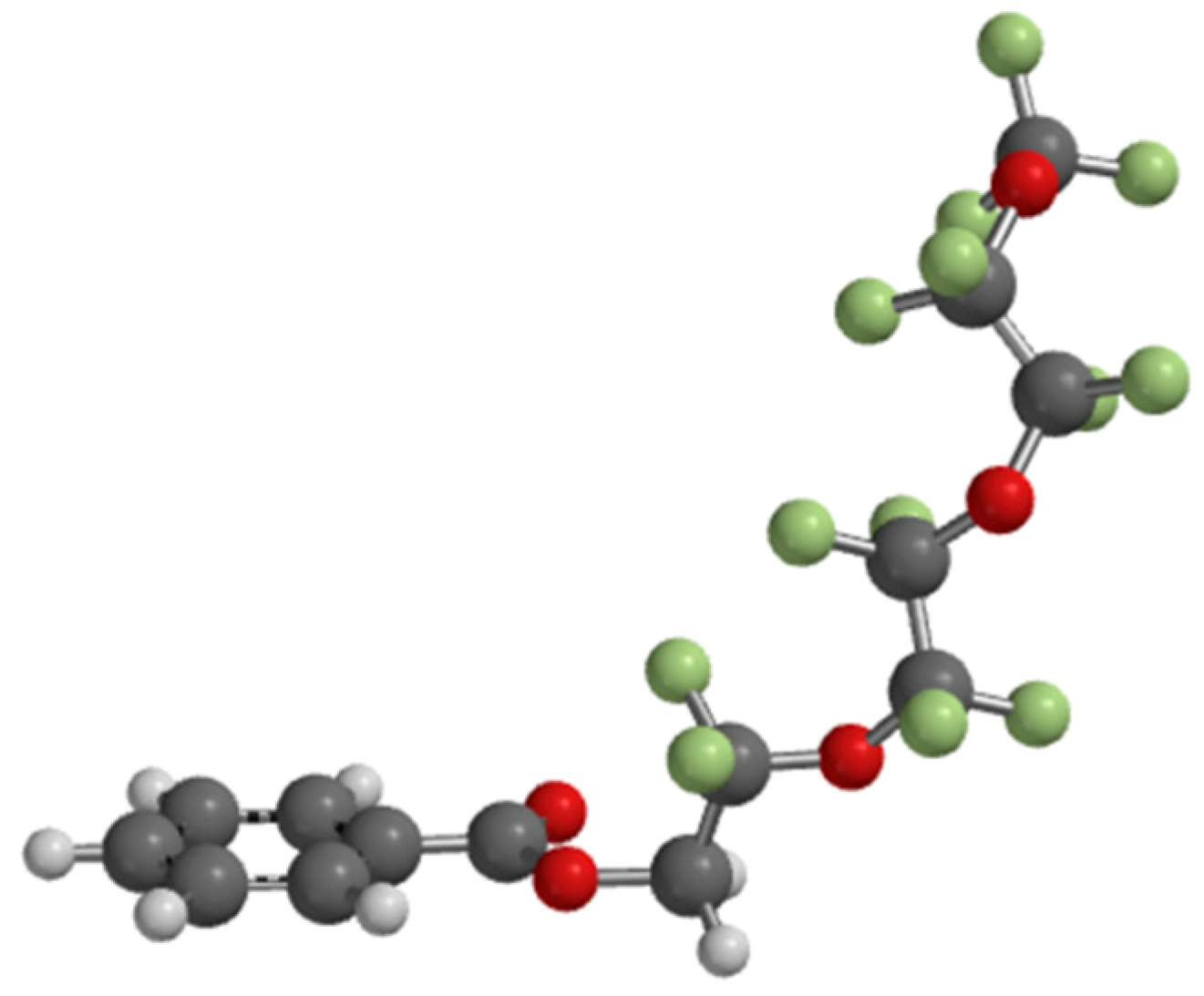
5. Properties of a New SmAPF Mesogen Possessing a PF-PEG Tail (W789)
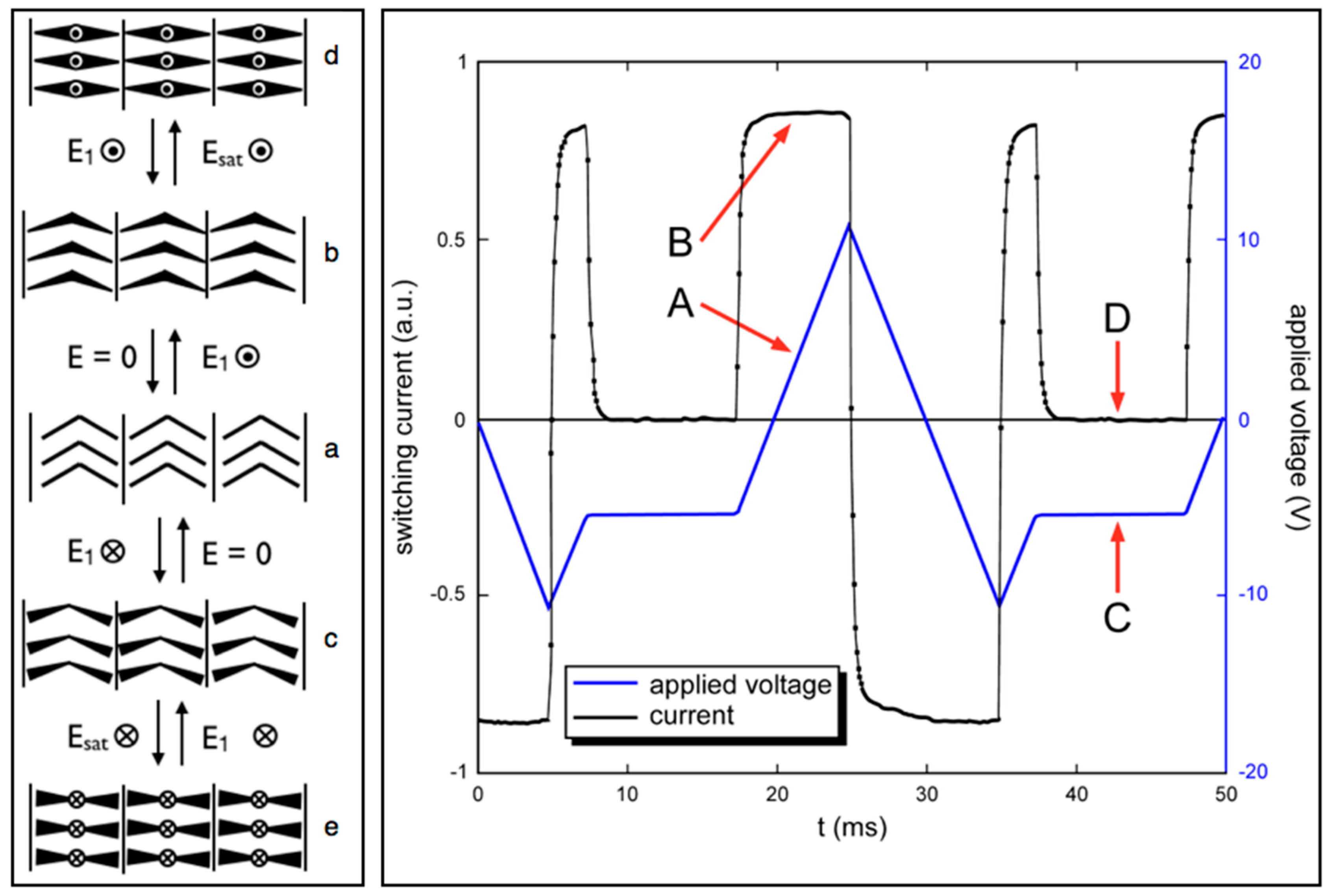
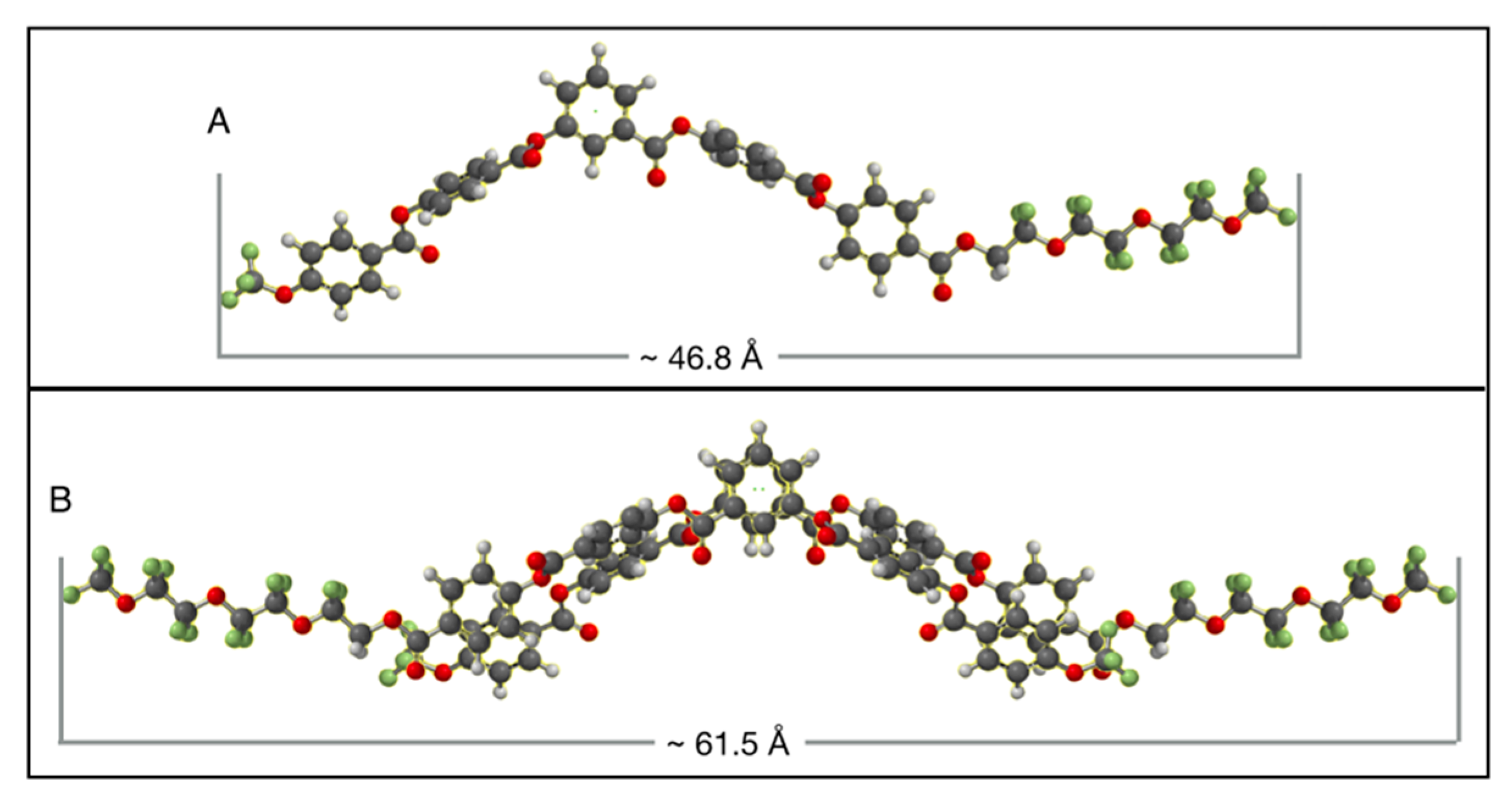
6. Speculation Regarding the Layer Structure of the SmAPF Phase of W789
7. Conclusions
8. Synthesis Experimental Workflow
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Jákli, A.; Chien, L.C.; Krüerke, D.; Rauch, S.; Sawadw, H.; Bault, P.; Heppke, G.; Fodor-Csorba, K.; Nair, G.G. Light shutters and electro-optical storage devices from antiferroelectric liquid crystals of bent-shape molecules. In Liquid Crystal Materials, Devices, and Applications IX; Chien, L.C., Ed.; SPIE: Bellingham, DC, USA, 2003; Volume 5003, pp. 73–80. [Google Scholar]
- Jakli, A.; Chien, L.C.; Kruerke, D.; Sawade, H.; Heppke, G. Light shutters from antiferroelectric liquid crystals of bent-shaped molecules. Liquid Cryst. 2002, 29, 377–381. [Google Scholar] [CrossRef]
- O’Callaghan, M.J.; Wand, M.D.; Walker, C.M.; Nakata, M. Charge controlled, fixed optic axis analog (“v-shaped”) switching of a bent-core ferroelectric liquid crystal. Appl. Phys. Lett. 2004, 85, 6344–6346. [Google Scholar] [CrossRef]
- Shimbo, Y.; Takanishi, Y.; Ishikawa, K.; Gorecka, E.; Pociecha, D.; Mieckowski, J.; Gomola, K.; Takezoe, H. Ideal liquid crystal display mode using achiral banana-shaped liquid crystals. Jpn. J. Appl. Phys. Part 2 Lett. Express Lett. 2006, 45, L282–L284. [Google Scholar] [CrossRef]
- Guo, L.F.; Gomola, K.; Gorecka, E.; Pociecha, D.; Dhara, S.; Araoka, F.; Ishikawa, K.; Takezoe, H. Transition between two orthogonal polar phases in symmetric bent-core liquid crystals. Soft Matter 2011, 7, 2895–2899. [Google Scholar] [CrossRef]
- Brand, H.R.; Cladis, P.E.; Pleiner, H. Symmetry and defects in the CM phase of polymeric liquid crystals. Macromolecules 1992, 25, 7223–7226. [Google Scholar] [CrossRef]
- Yablonskii, S.V.; Soto-Bustamante, E.A.; Vergara-Toloza, R.O.; Haase, W. Ferroelectricity in achiral liquid-crystal systems. Adv. Mater. 2004, 16, 1936–1940. [Google Scholar] [CrossRef]
- Reddy, R.A.; Zhu, C.; Shao, R.; Korblova, E.; Gong, T.; Shen, Y.; Garcia, E.; Glaser, M.A.; Maclennan, J.E.; Walba, D.M.; et al. Spontaneous Ferroelectric Order in a Bent-Core Smectic Liquid Crystal of Fluid Orthorhombic Layers. Science 2011, 332, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Korblova, E.; Walba, D.; Gong, T.; Reddy, A.; Zhu, C.; Shao, R.; Maclennan, J.; Glaser, M.; Clark, N. Design and synthesis of an achiral ferroelectric smectic liquid crystal. Proc. SPIE 2011, 8114, 81140X-1–81140X-9. [Google Scholar]
- Shen, Y.; Goodhew, L.; Shao, R.; Moran, M.; Korblova, E.; Walba, D.M.; Clark, N.A.; Maclennan, J.E.; Rudquist, P. Field alignment of bent-core smectic liquid crystals for analog optical phase modulation. Appl. Phys. Lett. 2015, 106, 191101-1–191101-4. [Google Scholar] [CrossRef]
- Sadashiva, B.K.; Reddy, R.A.; Pratibha, R.; Madhusudana, N.V. Biaxial smectic A phase in homologous series of compounds composed of highly polar unsymmetrically substituted bent-core molecules. J. Mater. Chem. 2002, 12, 943–950. [Google Scholar] [CrossRef]
- Wang, S.T.; Han, X.F.; Cady, A.; Liu, Z.Q.; Kamenev, A.; Glazman, L.; Sadashiva, B.K.; Reddy, R.A.; Huang, C.C. Optical investigations on the biaxial smectic-A phase of a bent-core compound. Phys. Rev. E 2004, 70, 061705. [Google Scholar] [CrossRef] [PubMed]
- Glaser, M.A.; Clark, N.A. Fluctuations and clinicity in tilted smectic liquid crystals. Phys. Rev. E 2002, 66, 021711-1–021711-4. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.; Reddy, R.A.; Hahn, H.; Lang, H.; Tschierske, C. The carbosilane unit as a stable building block for liquid crystal design: A new class of ferroelectric switching banana-shaped mesogens. Chem. Commun. (Camb.) 2004, 1898–1899. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.; Amaranatha Reddy, R.; Baumeister, U.; Tschierske, C. Banana-Shaped Liquid Crystals with Two Oligosiloxane End-Groups: Field-Induced Switching of Supramolecular Chirality. J. Am. Chem. Soc. 2004, 126, 14312–14313. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Shao, R.; Zhu, C.; Shen, Y.; Park, C.; Korblova, E.; Guerra, C.; Rego, J.A.; Hexemer, A.; Clark, N.A.; et al. Ferroelectric and antiferroelectric odd–even behavior in a tricarbosilane-terminated liquid crystal homologous series. Chem. Sci. 2014, 5, 1869–1874. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Gong, T.; Shao, R.F.; Korblova, E.; Maclennan, J.E.; Walba, D.M.; Clark, N.A. Effective conductivity due to continuous polarization reorientation in fluid ferroelectrics. Phys. Rev. E 2011, 84, 020701. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Iimuro, N.; Suzuki, T.; Iwane, H.; Miyachi, K.; Takanishi, Y.; Fukuda, A. Thresholdless antiferroelectricity in liquid crystals and its application to displays. J. Mater. Chem. 1996, 6, 671–973. [Google Scholar] [CrossRef]
- Rudquist, P.; Lagerwall, J.P.F.; Buivydas, M.; Gouda, F.; Lagerwall, S.T.; Clark, N.A.; Maclennan, J.E.; Shao, R.; Coleman, D.A.; Bardon, S.; et al. The case of thresholdless antiferroelectricity: Polarization-stabilized twisted SmC* liquid crystals give V-shaped electro-optic response. J. Mater. Chem. 1999, 9, 1257–1267. [Google Scholar] [CrossRef]
- Walba, D.M. Liquid Crystal Devices for Information Display and Photonics Applications. U.S. Patent Number 9,187,500 B2, 17 November 2015. [Google Scholar]
- Lim, C.F.; Tanski, J.M. Structural Analysis of Bisphenol-A and its Methylene, Sulfur, and Oxygen Bridged Bisphenol Analogs. J. Chem. Crystallogr. 2007, 37, 587–595. [Google Scholar] [CrossRef]
- Radcliffe, M.D.; Brostrom, M.L.; Epstein, K.A.; Rappaport, A.G.; Thomas, B.N.; Shao, R.F.; Clark, N.A. Smectic A and C materials with novel director tilt and layer thickness behaviour. Liq. Cryst. 1999, 26, 789–794. [Google Scholar] [CrossRef]
- Walba, D.M.; Korblova, E.; Eshdat, L.; Biewer, M.C.; Yang, H.; Jones, C.; Nakata, M.; Talarico, M.; Shao, R.; Clark, N.A. Chiral SmA* materials for display applications? J. Soc. Inf. Disp. 2007, 15, 585–588. [Google Scholar] [CrossRef]
- Gong, T. Design and Synthesis of Ferroelectric Liquid Crystals: 1) Studies on the de Vries Phase, and 2) Bent-core Achiral Ferroelectrics. Ph.D. Dissertation, University of Colorado Boulder, Boulder, CO, USA, 2011. [Google Scholar]
- Glaser, M.A.; Clark, N.A.; Walba, D.M.; Keyes, M.P.; Radcliffe, M.D.; Snustad, D.C. Mean field theory-based calculation of FLC polarization. Liq. Cryst. 2002, 29, 1073–1085. [Google Scholar] [CrossRef]
- Spartan ‘16 Parallel Suite (Wavefuction, Inc.) running on a 2014 Mac Pro workstation.
- Kramer, D.; Finkelmann, H. Breakdown of layering in frustrated smectic-A elastomers. Macromol. Rapid Commun. 2007, 28, 2318–2324. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korblova, E.D.; Guzman, E.; Maclennan, J.E.; Glaser, M.A.; Shao, R.; Garcia, E.; Shen, Y.; Visvanathan, R.; Clark, N.A.; Walba, D.M. New SmAPF Mesogens Designed for Analog Electrooptics Applications. Materials 2017, 10, 1284. https://doi.org/10.3390/ma10111284
Korblova ED, Guzman E, Maclennan JE, Glaser MA, Shao R, Garcia E, Shen Y, Visvanathan R, Clark NA, Walba DM. New SmAPF Mesogens Designed for Analog Electrooptics Applications. Materials. 2017; 10(11):1284. https://doi.org/10.3390/ma10111284
Chicago/Turabian StyleKorblova, Eva D., Edward Guzman, Joseph E. Maclennan, Matthew A. Glaser, Renfan Shao, Edgardo Garcia, Yongqiang Shen, Rayshan Visvanathan, Noel A. Clark, and David M. Walba. 2017. "New SmAPF Mesogens Designed for Analog Electrooptics Applications" Materials 10, no. 11: 1284. https://doi.org/10.3390/ma10111284




