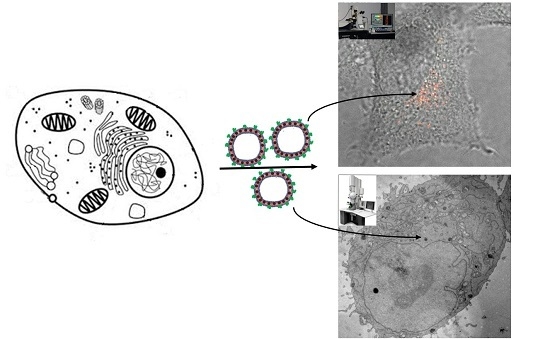
Fluorescent Magnetopolymersomes: A Theranostic Platform to Track Intracellular Delivery
Abstract
:1. Introduction
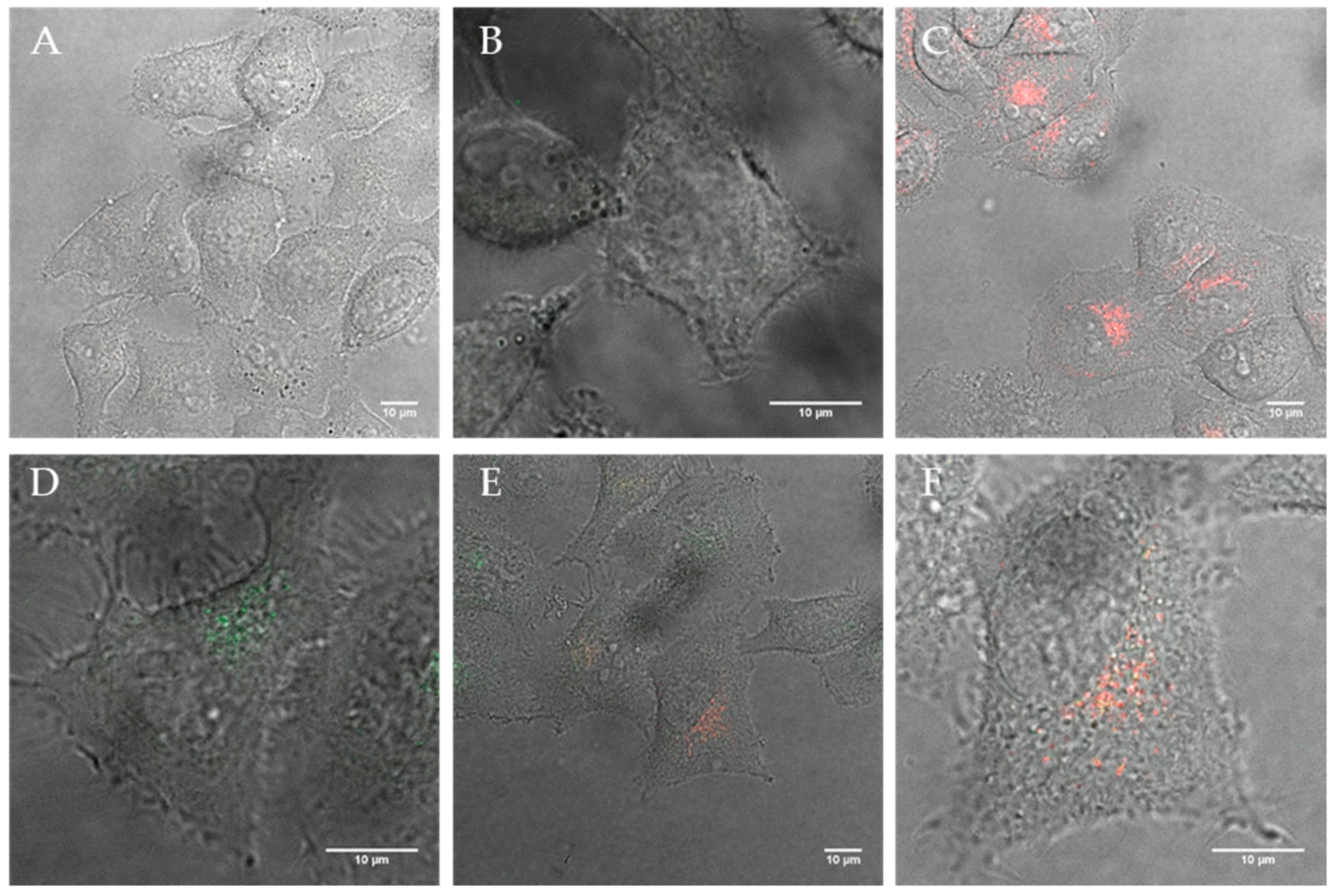
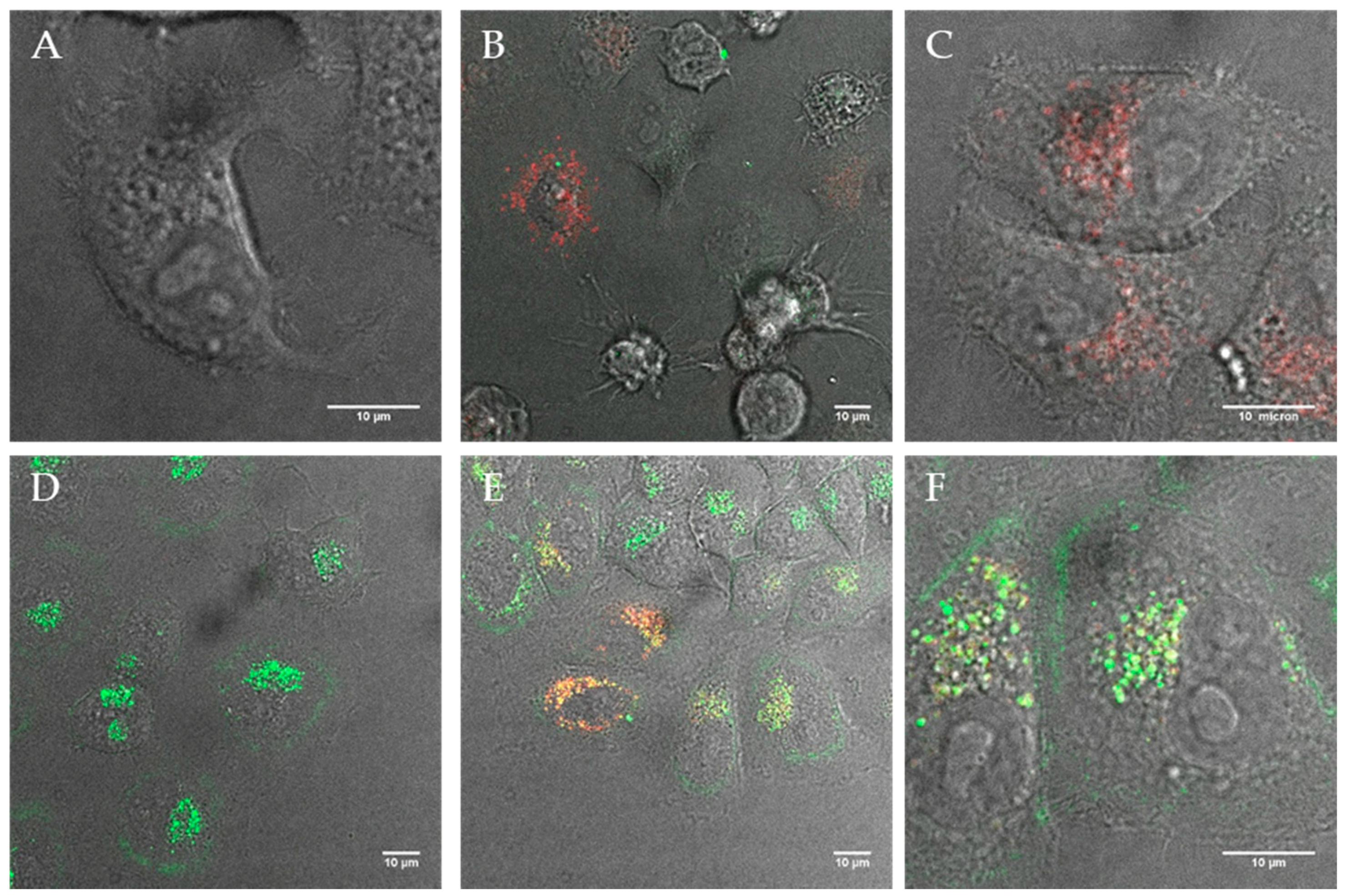
2. Results and Discussion
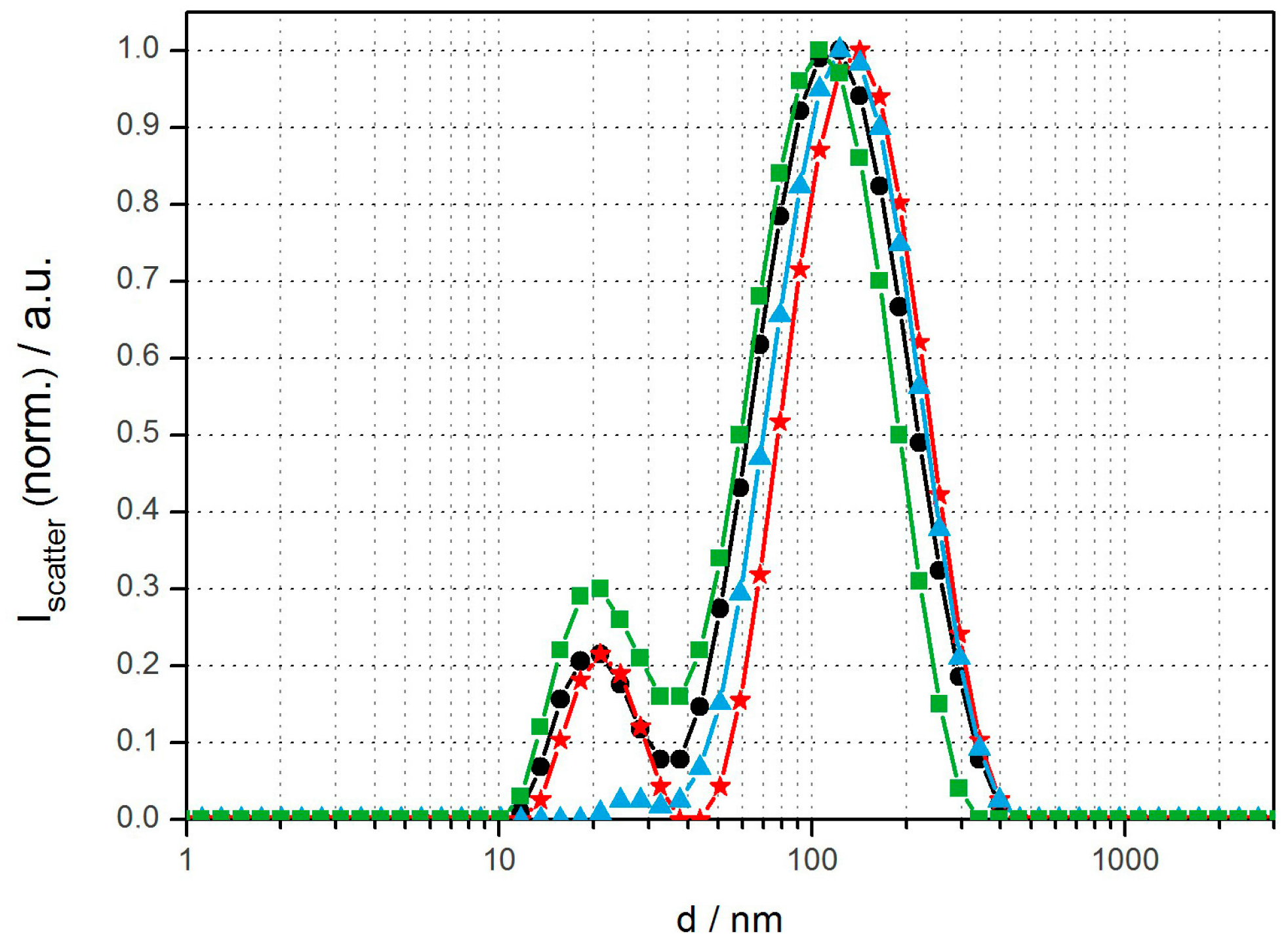
2.1. Preparation of Fluorescent Polymersomes
2.2. Surface Modification of Polymeric Vesicles
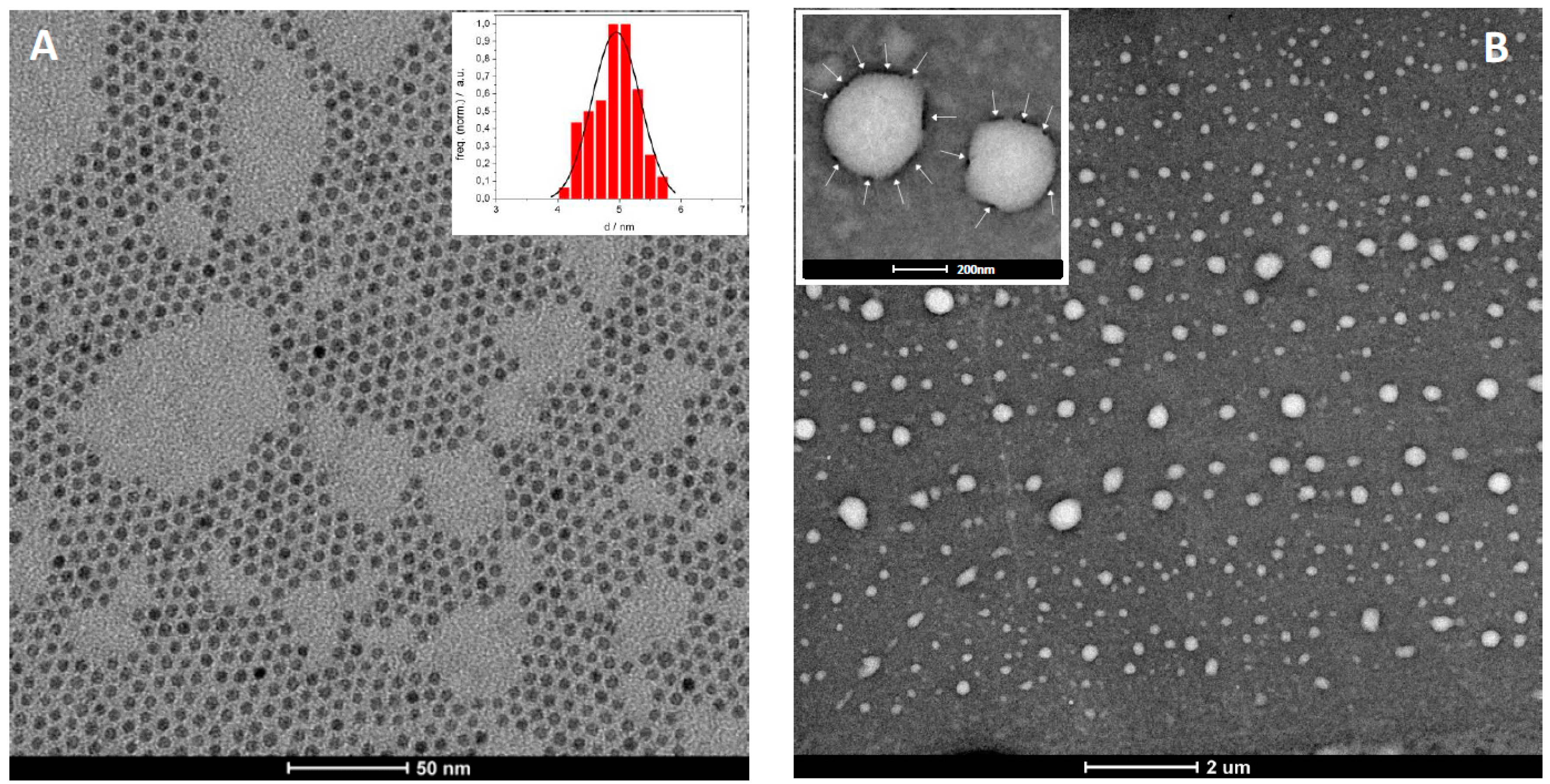
2.3. Magnetopolymersomes
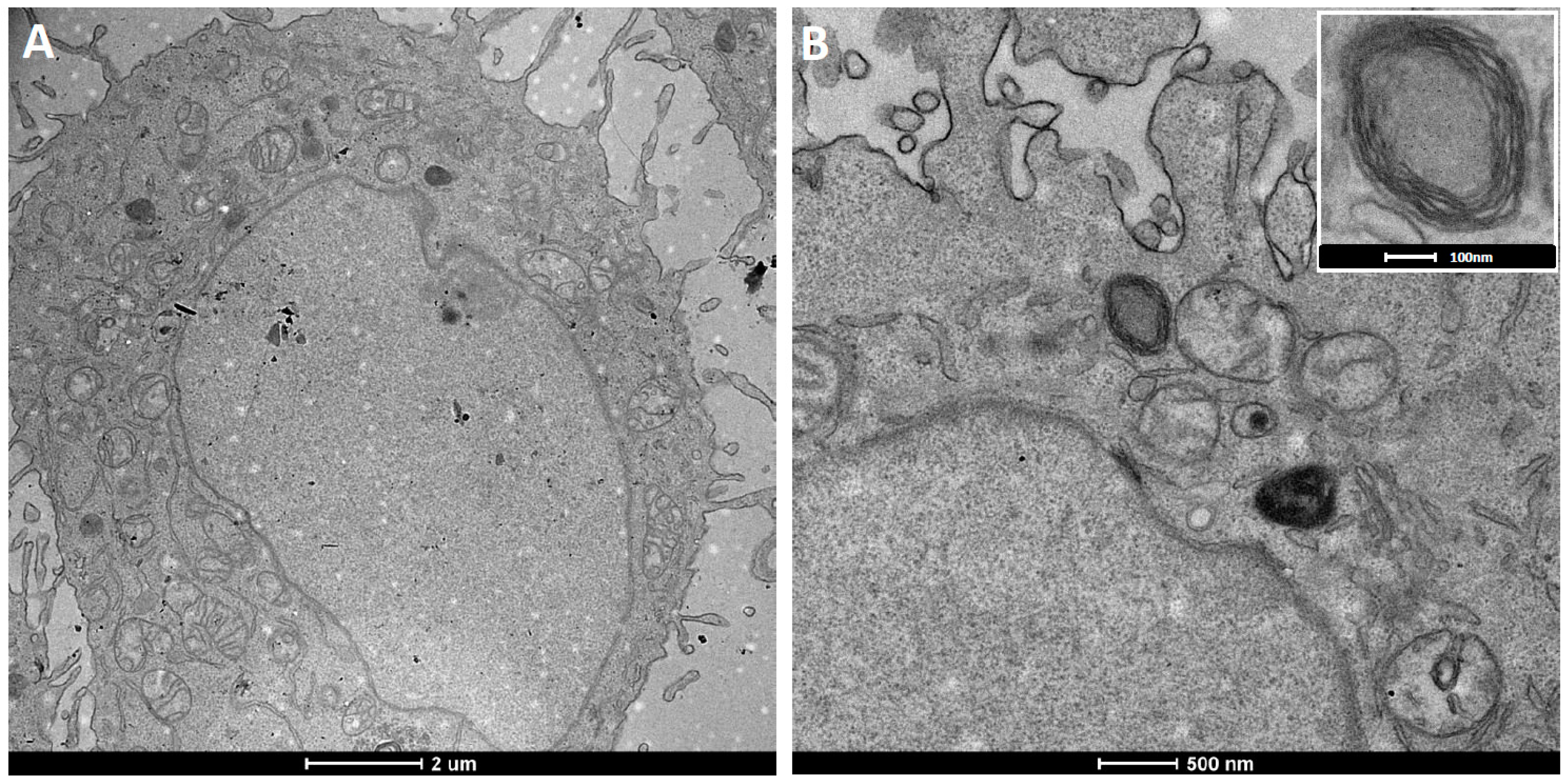
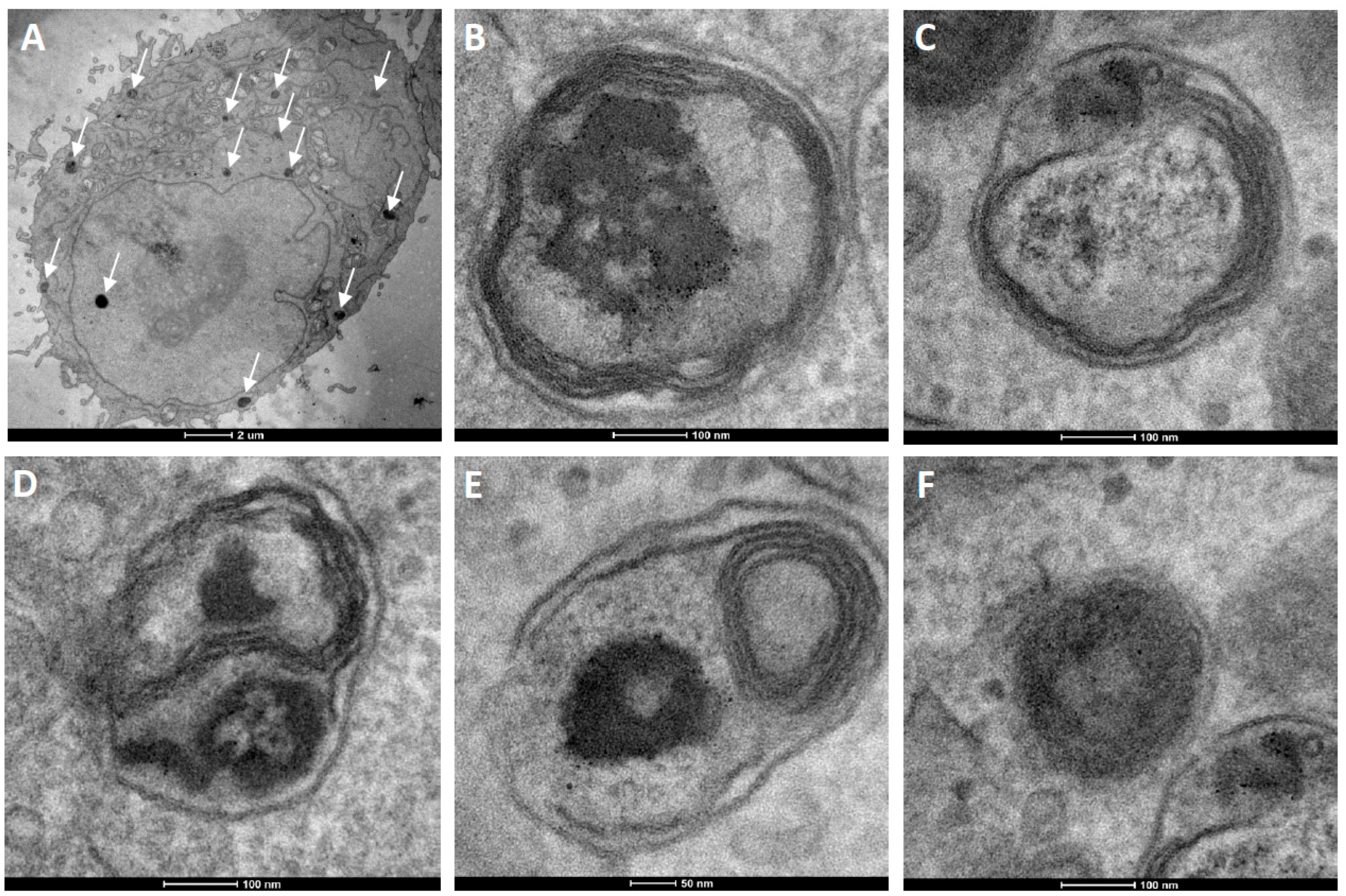
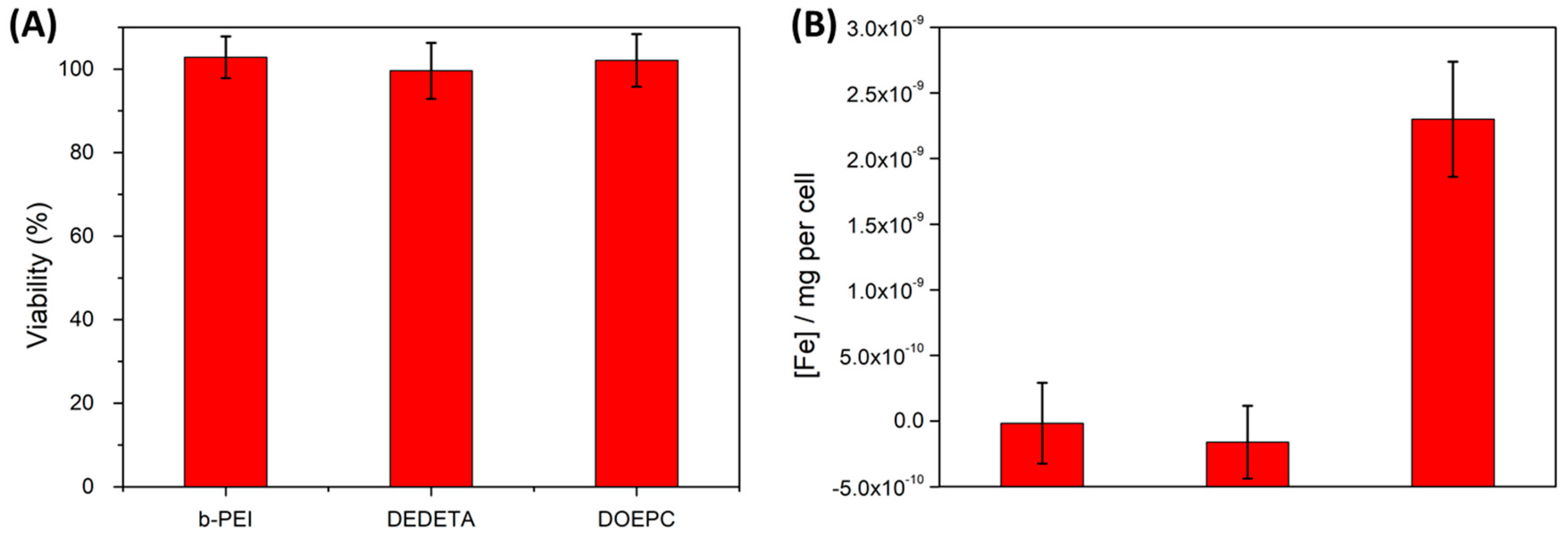
2.4. Cytotoxicity and Iron Content
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis
3.4. Sample Preparation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| b-PEI | branched-poly(ethylene imine) |
| DEAC | 7-(diethylamino)coumarin |
| DEDETA | N,N-diethyldiethylenetriamine |
| DOEPC | 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine chloride salt |
| HeLa | human cervical adenocarcinoma cells |
| PBD-b-PEO | polybutadiene-block-poly(ethylene oxide) |
| SPION | superparamagnetic iron oxide nanoparticle |
References
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, C.; Lomas, H.; Massignani, M.; Smart, T.; Battaglia, G. Polymersomes: Nature inspired nanometer sized compartments. J. Mater. Chem. 2009, 19, 3576–3590. [Google Scholar] [CrossRef]
- Le Meins, J.-F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Photos, P.J.; Bacakova, L.; Discher, B.; Bates, F.S.; Discher, D.E. Polymer vesicles in vivo: Correlations with PEG molecular weight. J. Control. Release 2003, 90, 323–334. [Google Scholar] [CrossRef]
- Schoettler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailaender, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lomas, H.; Massignani, M.; Abdullah, K.A.; Canton, I.; Lo Presti, C.; MacNeil, S.; Du, J.; Blanazs, A.; Madsen, J.; Armes, S.P.; et al. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss. 2008, 139, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, L. Recent Advances in Nonviral Vectors for Gene Delivery. Acc. Chem. Res. 2012, 45, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ren, T.; Gao, X. Cationic Transfection Lipids. Curr. Med. Chem. 2003, 10, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, H.; Barenholz, Y.; Domb, A.J. Polymers for DNA Delivery. Molecules 2005, 10, 34–64. [Google Scholar] [CrossRef] [PubMed]
- Yasayan, G.; Redhead, M.; Magnusson, J.P.; Spain, S.G.; Allen, S.; Davies, M.; Alexander, C.; Fernandez-Trillo, F. Well-defined polymeric vesicles with high stability and modulation of cell uptake by a simple coating protocol. Polym. Chem. 2012, 3, 2596–2604. [Google Scholar] [CrossRef]
- Su, X.; Mohamed Moinuddeen, S.K.; Mori, L.; Nallani, M. Hybrid polymersomes: Facile manipulation of vesicular surfaces for enhancing cellular interaction. J. Mater. Chem. B 2013, 1, 5751–5755. [Google Scholar] [CrossRef]
- Ye, F.; Barrefelt, Å.; Asem, H.; Abedi-Valugerdi, M.; El-Serafi, I.; Saghafian, M.; Abu-Salah, K.; Alrokayan, S.; Muhammed, M.; Hassan, M. Biodegradable polymeric vesicles containing magnetic nanoparticles, quantum dots and anticancer drugs for drug delivery and imaging. Biomaterials 2014, 35, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Levy, M.; Wilhelm, C.; Luciani, N.; Deveaux, V.; Gendron, F.; Luciani, A.; Devaud, M.; Gazeau, F. Nanomagnetism reveals the intracellular clustering of iron oxide nanoparticles in the organism. Nanoscale 2011, 3, 4402–4410. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sanson, C.; Diou, O.; Thévenot, J.; Ibarboure, E.; Soum, A.; Brûlet, A.; Miraux, S.; Thiaudière, E.; Tan, S.; Brisson, A.; et al. Doxorubicin Loaded Magnetic Polymersomes: Theranostic Nanocarriers for MR Imaging and Magneto-Chemotherapy. ACS Nano 2011, 5, 1122–1140. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Pérez-Andrés, E.; Thevenot, J.; Sandre, O.; Berra, E.; Lecommandoux, S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. Second Symp. Innov. Polym. Control. Deliv. SIPCD 2012 2013, 169, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-H.; Huang, W.-C.; Chang, C.-W.; Shen, M.-Y.; Shih, Z.-F.; Huang, Y.-F.; Lin, S.-C.; Chiu, H.-C. Functionalized polymersomes with outlayered polyelectrolyte gels for potential tumor-targeted delivery of multimodal therapies and MR imaging. J. Control. Release 2013, 168, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Kurzhals, S.; Virk, M.; Reimhult, E. Triggered Release from Thermoresponsive Polymersomes with Superparamagnetic Membranes. Materials 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Bello, G.; Virk, M.; Kurzhals, S.; Scheberl, A.; Gal, N.; Matysik, A.; Kraut, R.; Reimhult, E. Magneto-Thermal Release from Nanoscale Unilamellar Hybrid Vesicles. ChemNanoMat 2016, 2, 1111–1120. [Google Scholar] [CrossRef]
- Shaghasemi, B.S.; Virk, M.M.; Reimhult, E. Optimization of Magneto-thermally Controlled Release Kinetics by Tuning of Magnetoliposome Composition and Structure. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kim, D.-H.; Vitol, E.A.; Liu, J.; Balasubramanian, S.; Gosztola, D.J.; Cohen, E.E.; Novosad, V.; Rozhkova, E.A. Stimuli-Responsive Magnetic Nanomicelles as Multifunctional Heat and Cargo Delivery Vehicles. Langmuir 2013, 29, 7425–7432. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Reimhult, E. Controlled magnetosomes: Embedding of magnetic nanoparticles into membranes of monodisperse lipid vesicles. J. Colloid Interface Sci. 2016, 466, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bleul, R.; Thiermann, R.; Marten, G.U.; House, M.J.; Pierre, T.G.S.; Hafeli, U.O.; Maskos, M. Continuously manufactured magnetic polymersomes—A versatile tool (not only) for targeted cancer therapy. Nanoscale 2013, 5, 11385–11393. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Polymer vesicles in various media. Curr. Opin. Colloid Interface Sci. 2000, 5, 125–131. [Google Scholar] [CrossRef]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8. [Google Scholar] [CrossRef]
- MacDonald, R.I. Characteristics of self-quenching of the fluorescence of lipid-conjugated rhodamine in membranes. J. Biol. Chem. 1990, 265, 13533–13539. [Google Scholar] [PubMed]
- Chang, Y.-W.; Silas, J.A.; Ugaz, V.M. A Direct Probe of the Interplay between Bilayer Morphology and Surface Reactivity in Polymersomes. Langmuir 2010, 26, 12132–12139. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.D.; Rawle, R.J.; Boxer, S.G. Choose Your Label Wisely: Water-Soluble Fluorophores Often Interact with Lipid Bilayers. PLoS ONE 2014, 9, e87649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, T.; Zhao, Y.; Fan, D.; Ding, L.; Zhang, S. Cytotoxic Activity of Some Novel Dicoumarin Derivatives in vitro. Chem. Res. Chin. Univ. 2009, 25, 644–647. [Google Scholar]
- Bangar Raju, B.; Varadarajan, T.S. Substituent and Solvent Effects on the Twisted Intramolecular Charge Transfer of Three New 7-(Diethylamino)coumarin-3-aldehyde Derivatives. J. Phys. Chem. 1994, 98, 8903–8905. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG–PLA and PEG–PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Appelhans, D.; Wiedemuth, R.; Formanek, P.; Boye, S.; Lederer, A.; Temme, A.; Voit, B. Overcoming Concealment Effects of Targeting Moieties in the PEG Corona: Controlled Permeable Polymersomes Decorated with Folate-Antennae for Selective Targeting of Tumor Cells. Small 2015, 11, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Elias, D.R.; Kamat, N.P.; Johnston, E.D.; Poloukhtine, A.; Popik, V.; Hammer, D.A.; Tsourkas, A. Improved Tumor Targeting of Polymer-Based Nanovesicles Using Polymer–Lipid Blends. Bioconjug. Chem. 2011, 22, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Vanderlick, T.K.; Beales, P.A. Formation and dissolution of phospholipid domains with varying textures in hybrid lipo-polymersomes. Soft Matter 2012, 8, 7982–7988. [Google Scholar] [CrossRef]
- Bixner, O.; Lassenberger, A.; Baurecht, D.; Reimhult, E. Complete Exchange of the Hydrophobic Dispersant Shell on Monodisperse Superparamagnetic Iron Oxide Nanoparticles. Langmuir 2015, 31, 9198–9204. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Haynes, A.S.; Kikkawa, J.M.; Park, S.-J. Controlling the Self-Assembly Structure of Magnetic Nanoparticles and Amphiphilic Block-Copolymers: From Micelles to Vesicles. J. Am. Chem. Soc. 2011, 133, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Koski, J.; Meng, X.; Riggleman, R.A.; Zhang, P.; Park, S.-J. Size-Controlled Self-Assembly of Superparamagnetic Polymersomes. ACS Nano 2014, 8, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Scheberl, A.; Stadlbauer, A.; Stiglbauer, A.; Helbich, T.; Reimhult, E. Individually Stabilized, Superparamagnetic Nanoparticles with Controlled Shell and Size Leading to Exceptional Stealth Properties and High Relaxivities. ACS Appl. Mater. Interfaces 2017, 9, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Rijcken, C.J.F.; Lammers, T.; Seevinck, P.R.; Storm, G.; van Nostrum, C.F.; Hennink, W.E. Superparamagnetic Iron Oxide Nanoparticles Encapsulated in Biodegradable Thermosensitive Polymeric Micelles: Toward a Targeted Nanomedicine Suitable for Image-Guided Drug Delivery. Langmuir 2009, 25, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Lecommandoux, S.; Sandre, O.; Chécot, F.; Rodriguez-Hernandez, J.; Perzynski, R. Magnetic Nanocomposite Micelles and Vesicles. Adv. Mater. 2005, 17, 712–718. [Google Scholar] [CrossRef]
- Sari, N.; Kahraman, E.; Sari, B.; Özgün, A. Synthesis of Some Polymer-Metal Complexes and Elucidation of their Structures. J. Macromol. Sci. Part A 2006, 43, 1227–1235. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Gilkes, R.J.; Cornell, R.M.; Posner, A.M.; Quirk, J.P. Dissolution of iron oxides and oxyhydroxides in hydrochloric and perchloric acids. Clays Clay Miner. 1981, 29, 269–276. [Google Scholar] [CrossRef]
- Zirbs, R.; Lassenberger, A.; Vonderhaid, I.; Kurzhals, S.; Reimhult, E. Melt-grafting for the synthesis of core-shell nanoparticles with ultra-high dispersant density. Nanoscale 2015, 7, 11216–11225. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of Iron Oxide Nanocubes: High-Resolution In Situ Monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Javed, Y.; Lartigue, L.; Volatron, J.; Elgrabli, D.; Marangon, I.; Pugliese, G.; Caron, B.; Figuerola, A.; Luciani, N.; et al. The One Year Fate of Iron Oxide Coated Gold Nanoparticles in Mice. ACS Nano 2015, 9, 7925–7939. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Ling, D.; Hyeon, T. Iron Oxide Nanoparticles: Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications (Small 9–10/2013). Small 2013, 9, 1449. [Google Scholar] [CrossRef]
- Liao, N.; Wu, M.; Pan, F.; Lin, J.; Li, Z.; Zhang, D.; Wang, Y.; Zheng, Y.; Peng, J.; Liu, X.; et al. Poly (dopamine) coated superparamagnetic iron oxide nanocluster for noninvasive labeling, tracking, and targeted delivery of adipose tissue-derived stem cells. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Glauert, A.M.; Lewis, P.R. Biological Specimen Preparation for Transmission Electron Microscopy; Practical Methods in Electron Microscopy; Princeton University Press: Princeton, NJ, USA, 1998; ISBN 978-1-85578-061-3. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bixner, O.; Gal, N.; Zaba, C.; Scheberl, A.; Reimhult, E. Fluorescent Magnetopolymersomes: A Theranostic Platform to Track Intracellular Delivery. Materials 2017, 10, 1303. https://doi.org/10.3390/ma10111303
Bixner O, Gal N, Zaba C, Scheberl A, Reimhult E. Fluorescent Magnetopolymersomes: A Theranostic Platform to Track Intracellular Delivery. Materials. 2017; 10(11):1303. https://doi.org/10.3390/ma10111303
Chicago/Turabian StyleBixner, Oliver, Noga Gal, Christoph Zaba, Andrea Scheberl, and Erik Reimhult. 2017. "Fluorescent Magnetopolymersomes: A Theranostic Platform to Track Intracellular Delivery" Materials 10, no. 11: 1303. https://doi.org/10.3390/ma10111303