A Statistical Study on the Effect of Hydrostatic Pressure on Metastable Pitting Corrosion of X70 Pipeline Steel
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Sample Preparation
2.2. Electrochemical Measurements during Hydrostatic Pressure Loading
2.3. Stable Pit Propagation Measurements
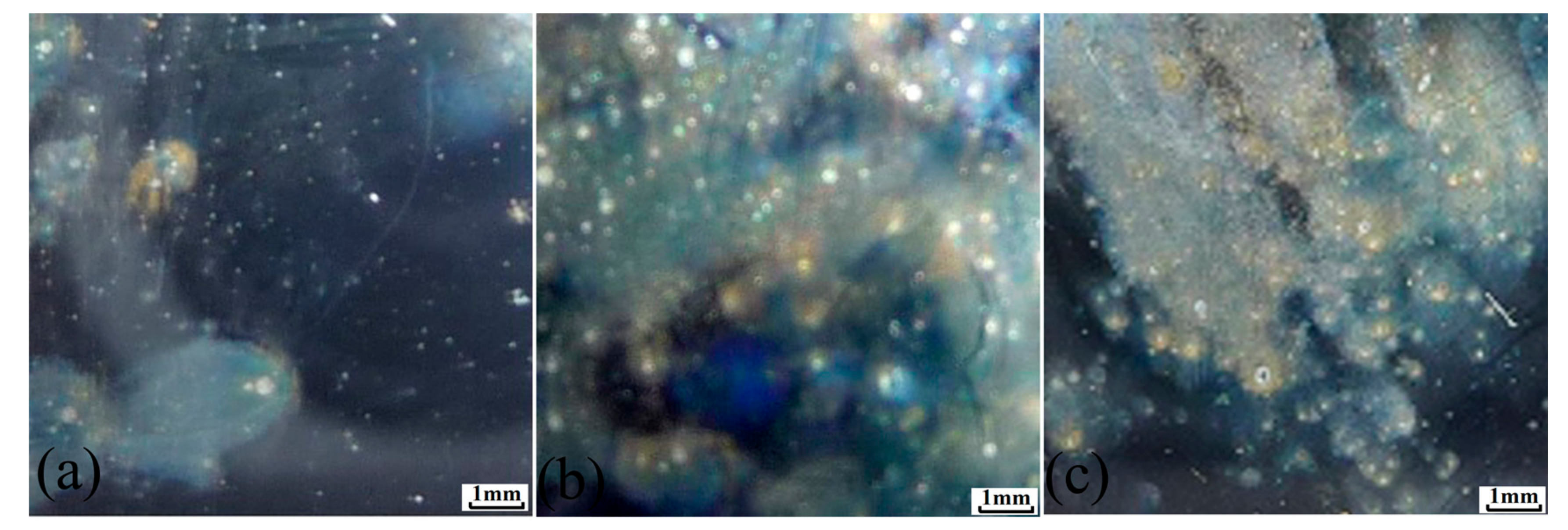
2.4. Microstructure Characterization
3. Results
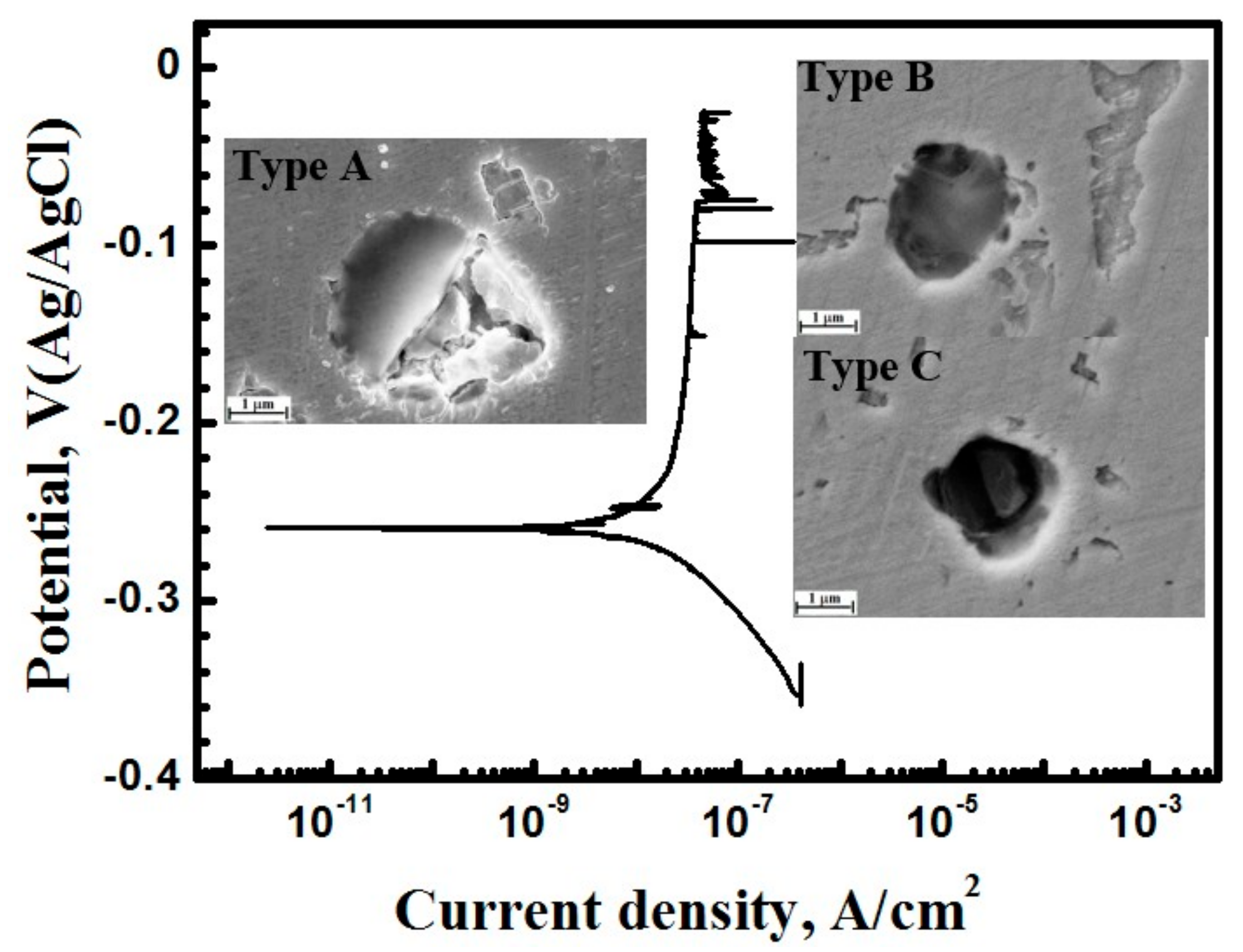
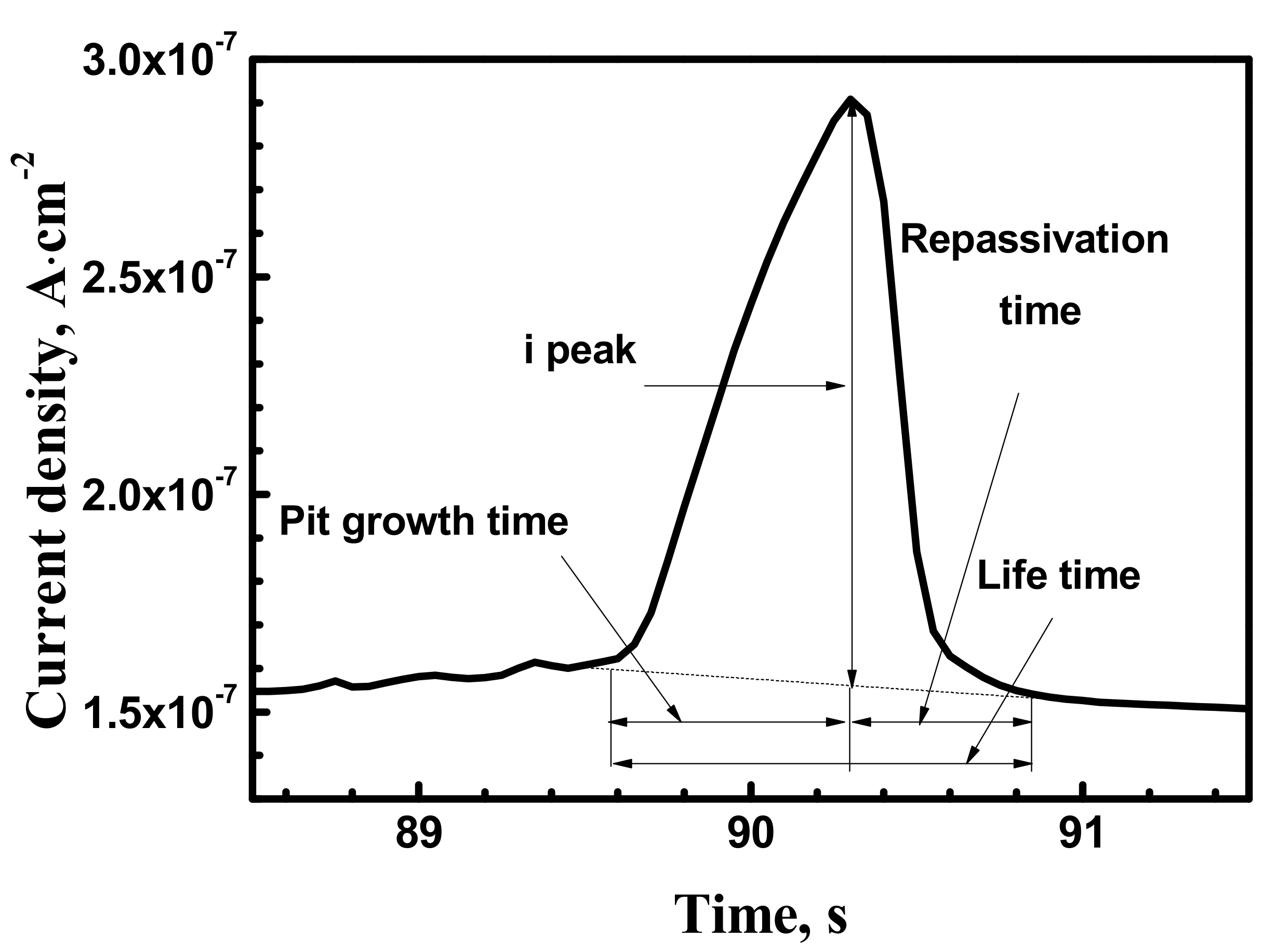
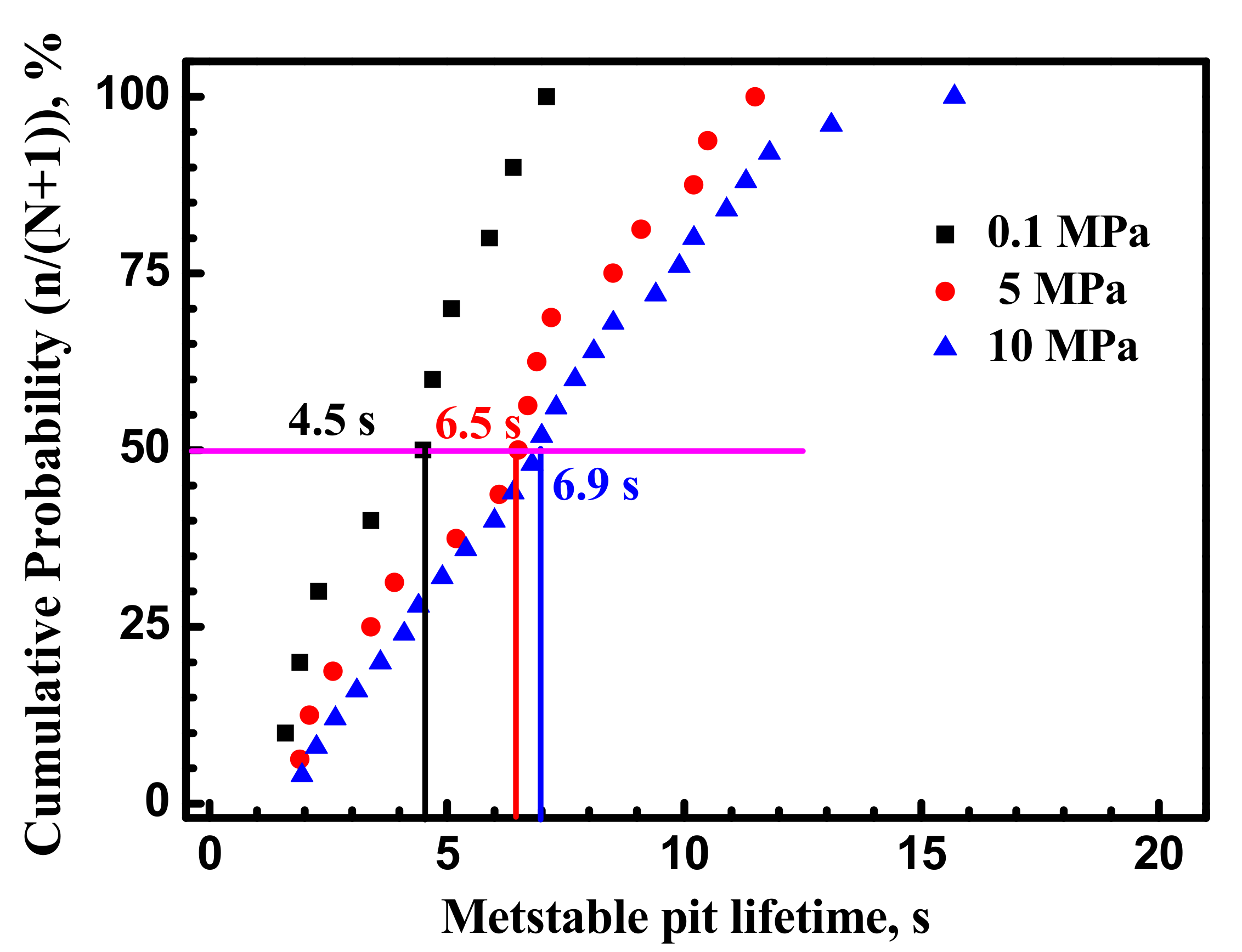
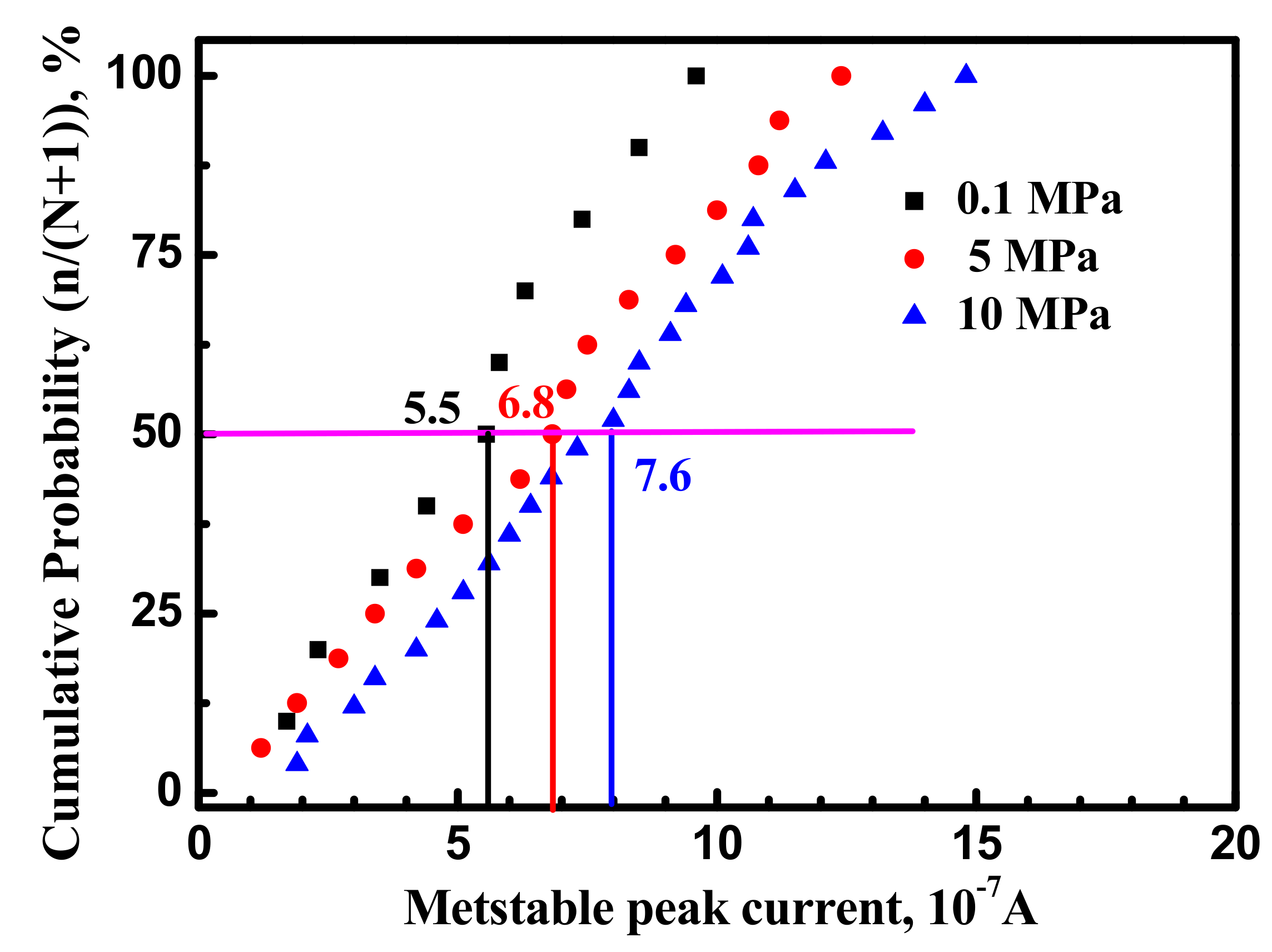
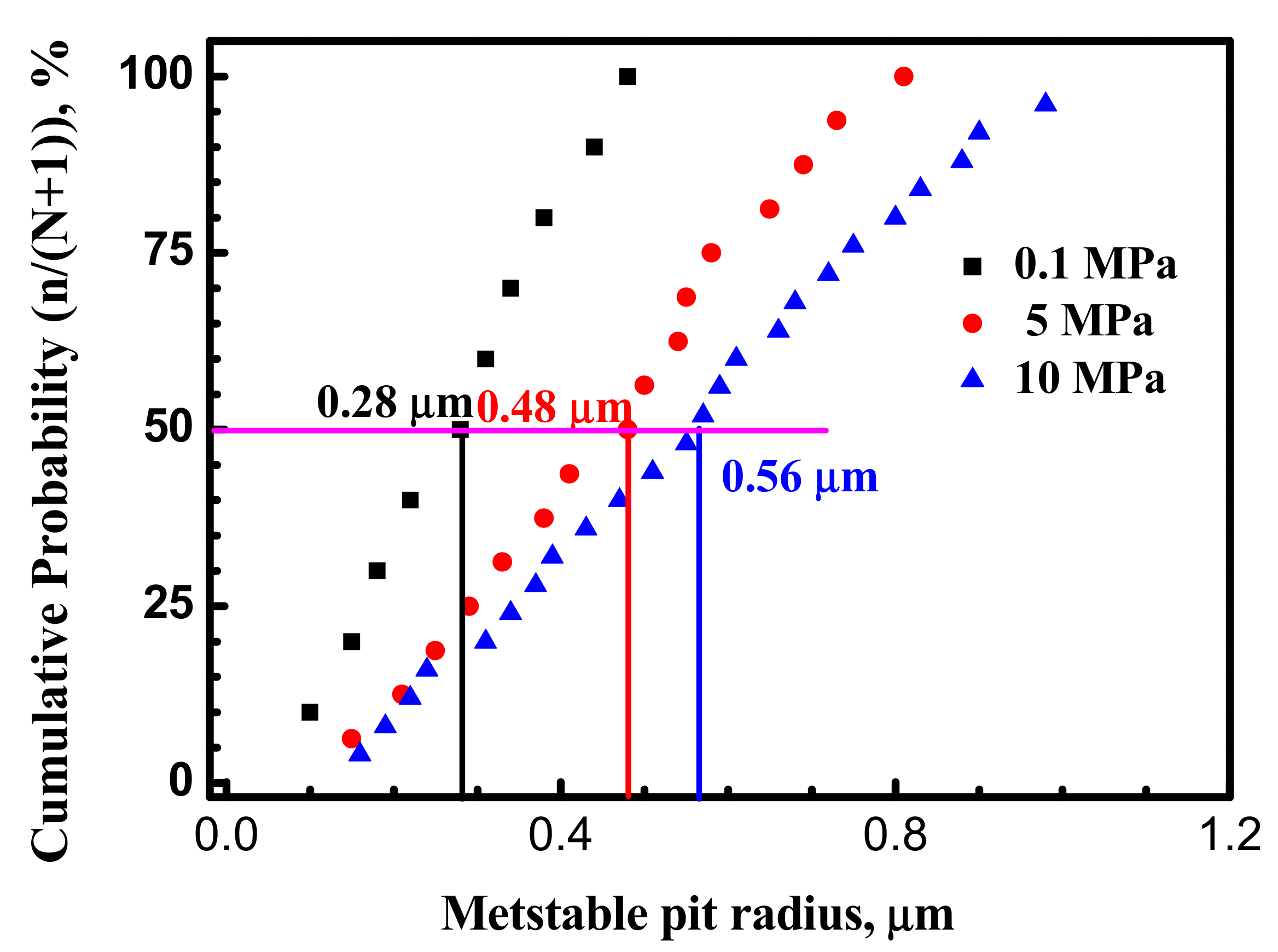
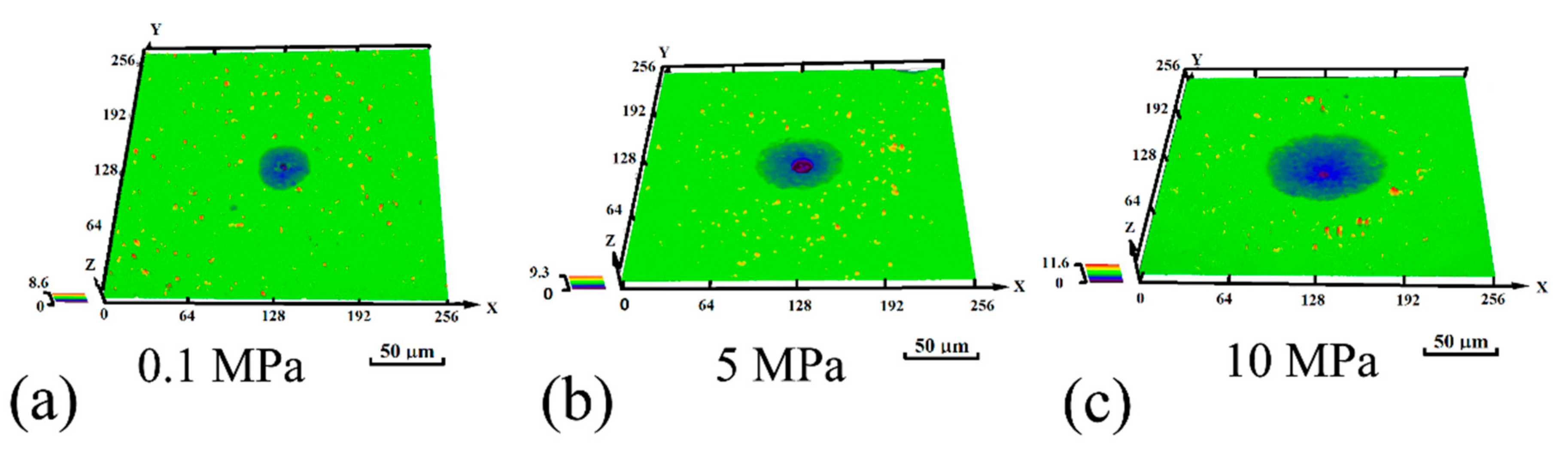
3.1. Metastable Pitting Analysis
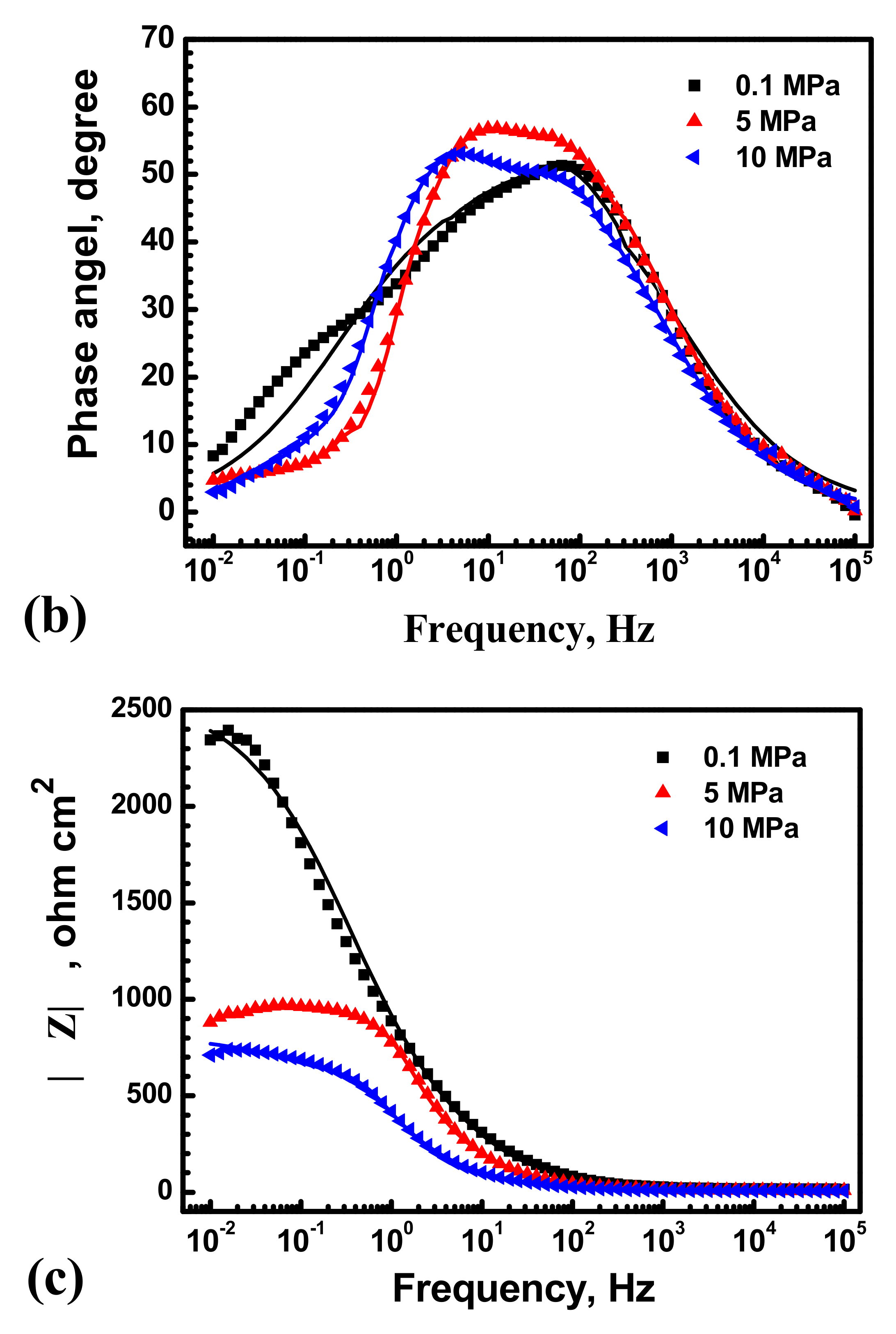
3.2. Electrochemical Results
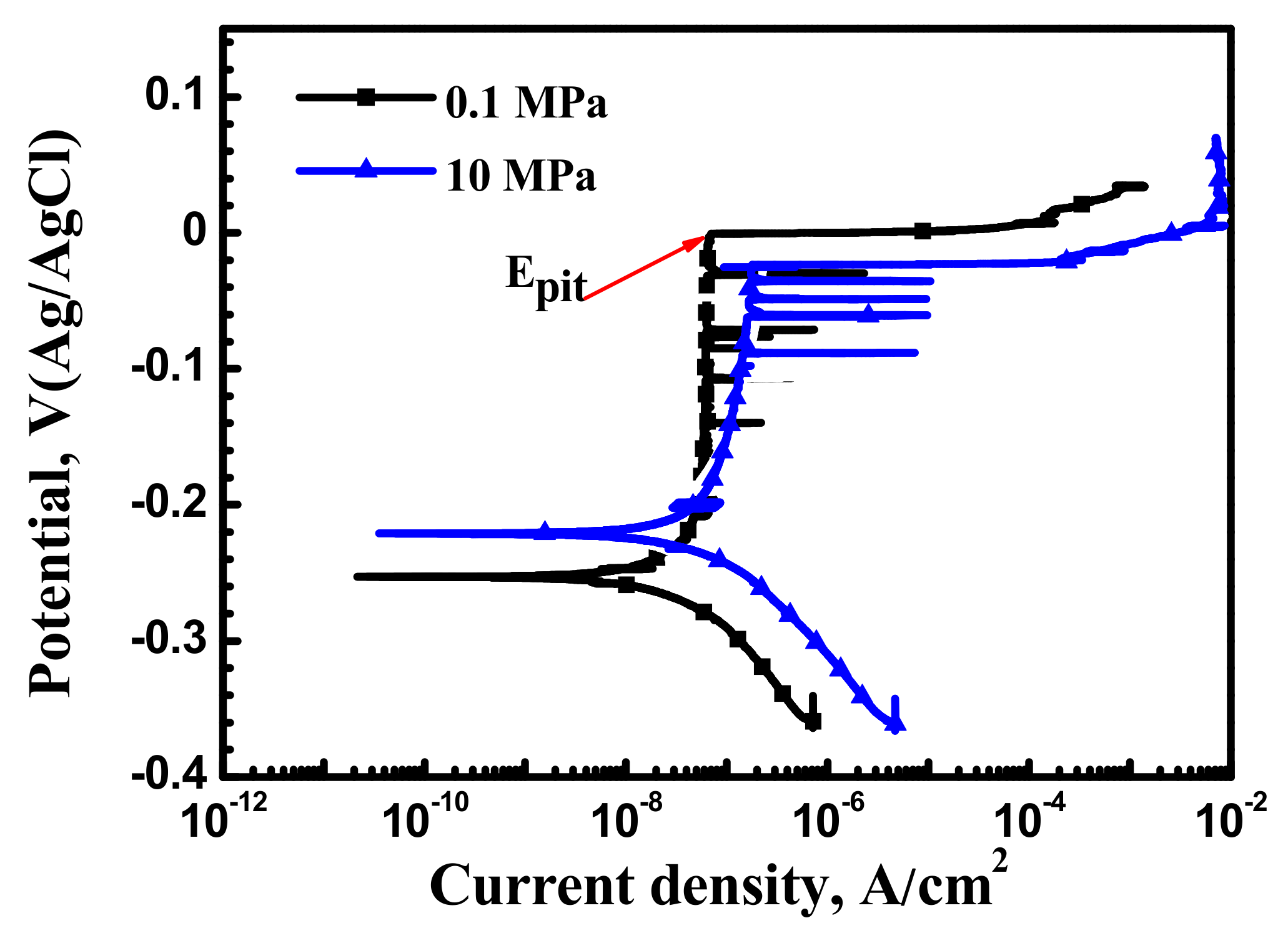
3.2.1. Potentiodynamic Polarization Curves
3.2.2. Metastable Pitting Electrochemistry
4. Discussion
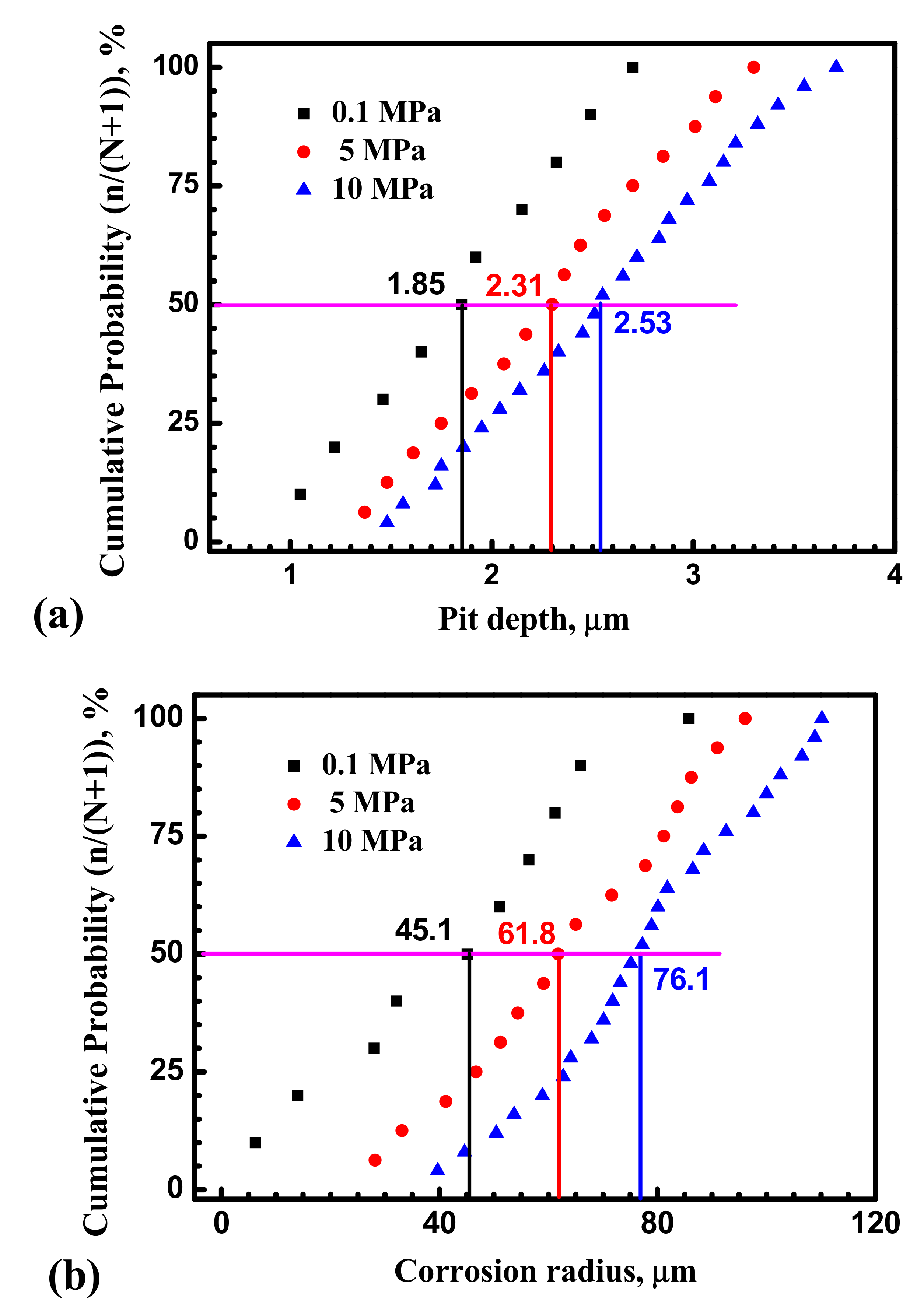
4.1. Hydrostatic Pressure Effect on Metastable Pit Initiation
4.2. Hydrostatic Pressure Effect on Metastable Pit Growth
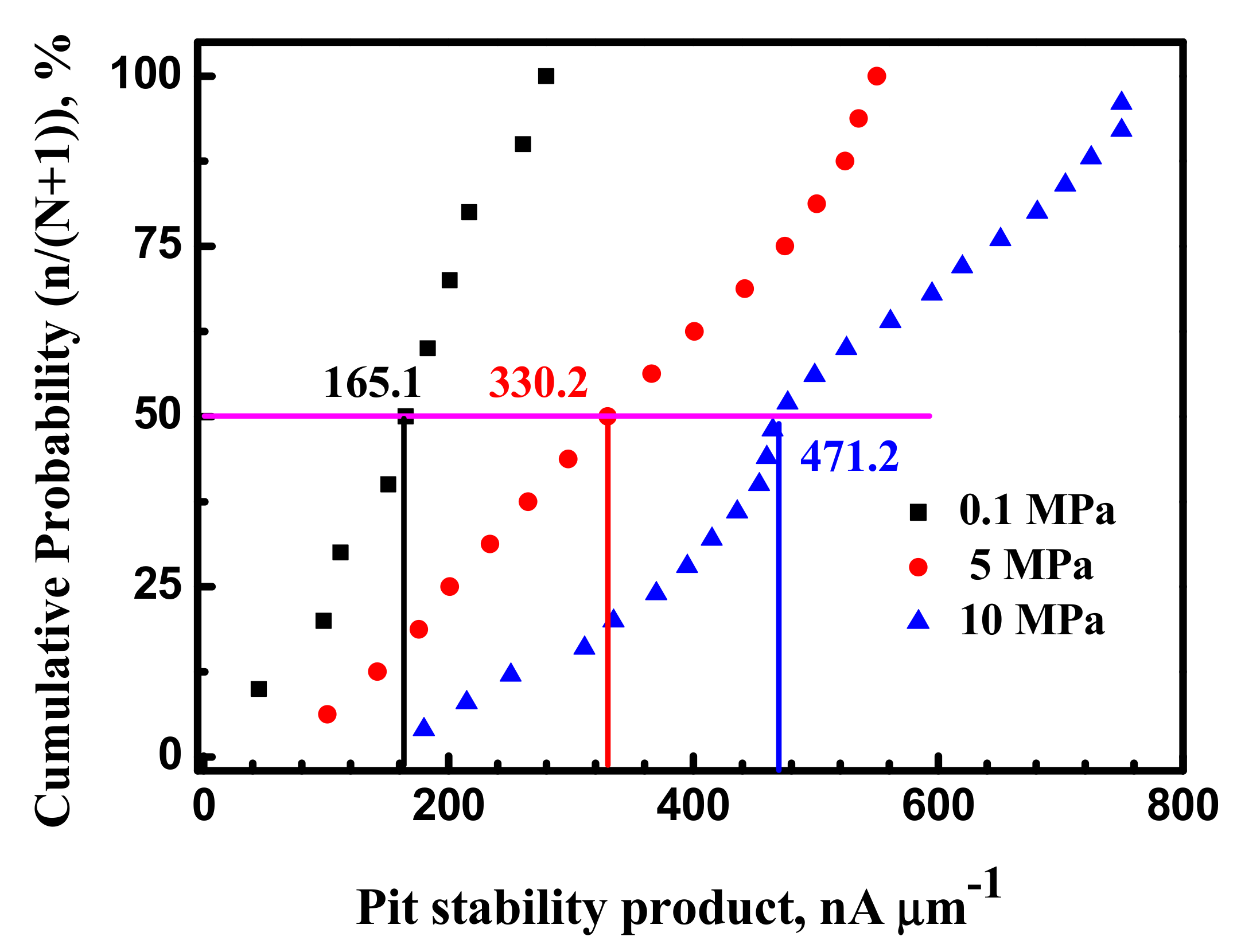
4.3. Hydrostatic Pressure Effect on Metastable Pitting Stabilization
5. Conclusions
- (1)
- Potentiodynamic measurements indicated that increasing hydrostatic pressure decreases the breakdown potential and leads to reduced transpassivity region.
- (2)
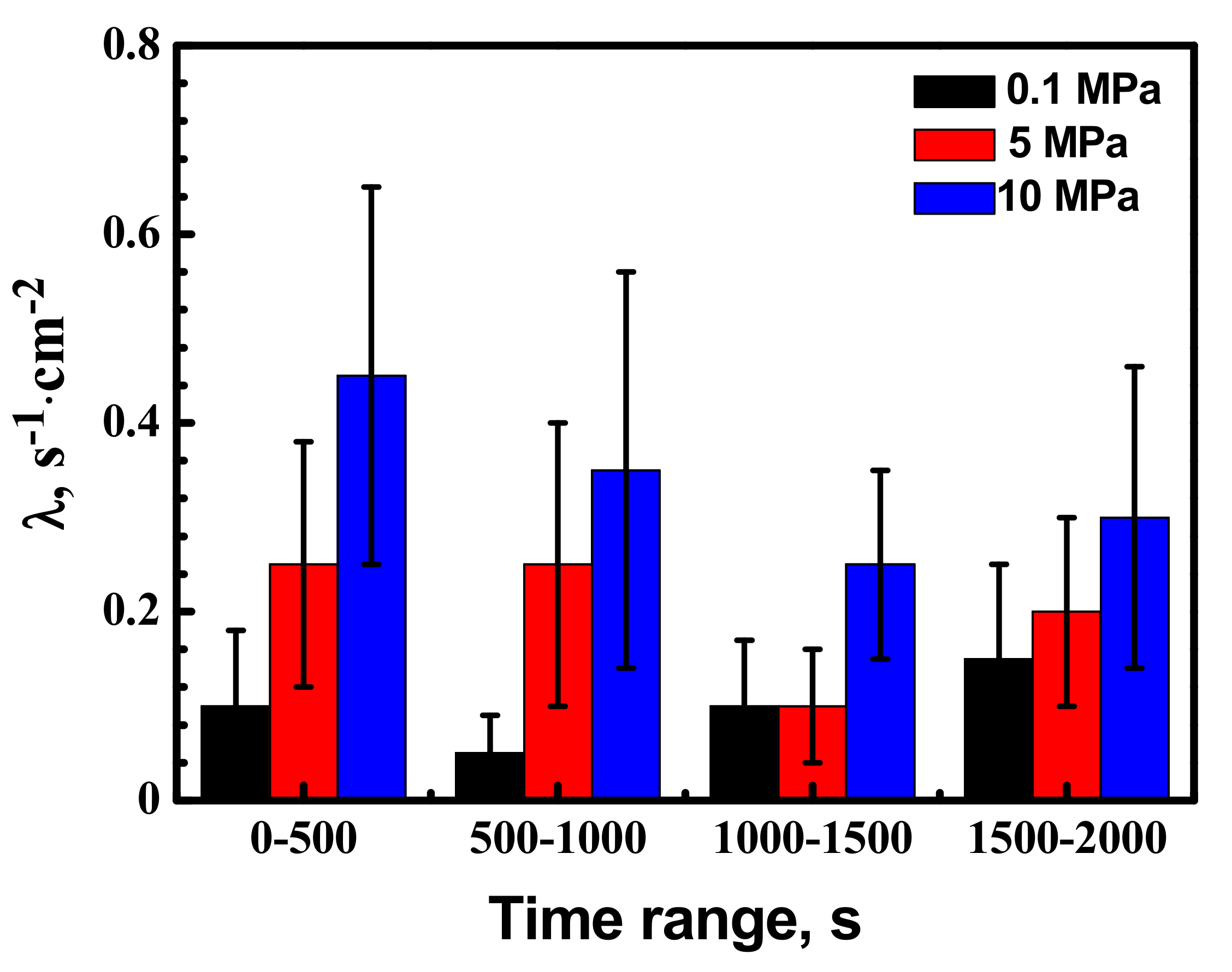
- Hydrostatic pressure can promote the adsorption of Cl− on metal surface. Potentiostatic measurement indicated that the rate of metastable pit formation in the X70 steel increased with hydrostatic pressure, indicating that the pitting generation rate was increased. EIS results indicate Hydrostatic pressure decreases the charge transfer resistance and increases the dissolution rate within the cavities. The results also revealed that increasing hydrostatic pressure leads to an increase in the average values of metastable pitting peak current, pit radius and pit lifetime. Hydrostatic pressure improves the probability of metastable pits transition to stability. This means that hydrostatic pressure promotes metastable pitting initiation and propagation.
- (3)
- Metastable pit measurement and corrosion tests both indicated that pitting initiation and propagation are accelerated by hydrostatic pressure. Result validity is verified by evaluating metastable pitting to predict pitting corrosion resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Traverso, P.; Canepa, E. A review of studies on corrosion of metals and alloys in deep-sea environment. Ocean. Eng. 2014, 87, 10–15. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sukiman, N.L.; Cavanaugh, M.K.; Hutchinson, C.R.; Birbilis, N. Metastable pitting characteristics of aluminium alloys measured using current transients during potentiostatic polarization. Electrochim. Acta 2012, 66, 245–254. [Google Scholar] [CrossRef]
- Tian, W.M.; Du, N.; Li, S.M.; Chen, S.B.; Wu, Q.Y. Metastable pitting corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2014, 85, 372–379. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G. Influence of hydrostatic pressure on pitting of aluminium in sea water. Br. Corros. J. 1985, 20, 183–186. [Google Scholar] [CrossRef]
- Beccaria, A.M. The effect of hydrostatic pressure on the corrosion of nickel in slightly alkaline solutions containing Cl− ions. Corros. Sci. 1989, 29, 408–416. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G. The effect of salt concentration on nickel corrosion behaviour in slightly alkaline solutions at different hydrostatic pressures. Corros. Sci. 1993, 34, 989–1005. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G. Influence of passive film composition and sea water pressure on resistance to localized corrosion of some stainless steels in sea water. Br. Corros. J. 1995, 30, 283–286. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Beccaria, A.M. Corrosion protection of AA6061 T6 by fluoropolymer coatings in NaCl solution. Surf. Coat. Technol. 2002, 155, 176–183. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.G.; Shao, Y.W. A stochastic analysis of the effect of hydrostatic pressure on the pit corrosion of Fe-20Cr alloy. Electrochim. Acta 2009, 54, 3915–3922. [Google Scholar] [CrossRef]
- Yang, Y.G.; Zhang, T.; Shao, Y.W.; Meng, G.Z.; Wang, F.H. Effect of hydrostatic pressure on the corrosion behaviour of Ni–Cr–Mo–V high strength steel. Corros. Sci. 2010, 52, 2697–2706. [Google Scholar] [CrossRef]
- Yang, Y.G.; Zhang, T.; Shao, Y.W. New understanding of the effect of hydrostatic pressure on the corrosion of Ni–Cr–Mo–V high strength steel. Corros. Sci. 2013, 73, 250–261. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Metastable pitting corrosion of stainless steel and the transition to stability. Philos. Trans. R. Soc. Lond. A 1992, 341, 531–559. [Google Scholar] [CrossRef]
- Abbasi Aghuy, A.; Zakeri, M.; Moayed, M.H.; Mazinani, M. Effect of grain size on pitting corrosion of 304L austenitic stainless steel. Corros. Sci. 2015, 94, 368–376. [Google Scholar] [CrossRef]
- Mahdi, E.; Rauf, A.; Eltai, E.O. Effect of temperature and erosion on pitting corrosion of X100 steel in aqueous silica slurries containing bicarbonate and chloride content. Corros. Sci. 2014, 83, 48–58. [Google Scholar] [CrossRef]
- Guan, L.; Zhang, B.; Yong, X.P. Effects of cyclic stress on the metastable pitting characteristic for 304 stainless steel under potentiostatic polarization. Corros. Sci. 2015, 93, 80–89. [Google Scholar] [CrossRef]
- Gholami, M.; Hoseinpoor, M.; Moayed, M.H. A statistical study on the effect of annealing temperature on pitting corrosion resistance of 2205 duplex stainless steel. Corros. Sci. 2015, 94, 156–164. [Google Scholar] [CrossRef]
- Amin, M.A. Metastable and stable pitting events on Al induced by chlorate and perchlorate anions-Polarization, XPS and SEM studies. Electrochim. Acta 2009, 54, 1857–1863. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Choi, M.S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of pitting corrosion in hyperduplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Lee, I.S. Effects of sulfur addition on pitting corrosion and machinability behavior of super duplex stainless steel containing rare earth metals: Part 2. Corros. Sci. 2010, 52, 3537–3547. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Lee, I.S. Effects of copper addition on the formation of inclusions and the resistance to pitting corrosion of high performance duplex stainless steels. Corros. Sci. 2011, 53, 1408–1416. [Google Scholar] [CrossRef]
- Sun, H.J.; Liu, L.; Li, Y.; Wang, F.H. Effect of Hydrostatic Pressure on the Corrosion Behavior of a Low Alloy Steel. J. Electrochem. Soc. 2013, 160, C89–C96. [Google Scholar] [CrossRef]
- Sun, H.J.; Liu, L.; Li, Y.; Wang, F.H. The performance of Al–Zn–In–Mg–Ti sacrificial anode in simulated deep water environment. Corros. Sci. 2013, 77, 77–87. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Li, C.Y.; Qi, Y.M. Mechanism of (Mg, Al, Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Daufin, G.; Pagetti, J.; Labbe, J.P. Pitting initiation on stainless steel: Electrochemical and micrographic aspects. Corrosion 1985, 41, 533–539. [Google Scholar] [CrossRef]
- Yang, Z.; Kan, B.; Li, J.; Su, Y.; Qiao, L.; Volinsky, A.A. Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments. Materials 2017, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.M.; Zuo, Y.; Wang, J.N. The metastable pitting potential and its relation to the pitting potential for four materials in chloride solutions. Corros. Sci. 2014, 80, 111–119. [Google Scholar] [CrossRef]
- Tang, Y.M.; Zuo, Y. The metastable pitting of mild steel in bicarbonate solutions. Mater. Chem. Phys. 2004, 88, 221–226. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Meng, G.Z. Effect of pitting nucleation on critical pitting temperature of 316L stainless steel by nitric acid passivation. Corros. Sci. 2015, 91, 232–244. [Google Scholar] [CrossRef]
- Zhang, F.; Pan, J.S.; Lin, C.J. Localized corrosion behaviour of reinforcement steel in simulated concrete pore solution. Corros. Sci. 2009, 51, 2130–2138. [Google Scholar] [CrossRef]
- Williams, D.E.; Stewart, J.; Balkwill, P.H. The nucleation, growth and stability of micropits in stainless steel. Corros. Sci. 1994, 36, 1213–1215. [Google Scholar] [CrossRef]
- Williams, D.E.; Westcott, C.; Fleischmann, M. Stochastic Models of Pitting Corrosion of Stainless Steels. J. Electrochem. Soc. 1985, 132, 1796. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Solid Surfaces; World Scientific: Singapore, 1994. [Google Scholar]
- Yan, M.C.; Xu, J.; Yu, L.B. EIS analysis on stress corrosion initiation of pipeline steel under disbonded coating in near-neutral pH simulated soil electrolyte. Corros. Sci. 2016, 110, 23–34. [Google Scholar]
- Naghizadeh, M.; Nakhaie, D.; Zakeri, M. The effect of dichromate ion on the pitting corrosion of AISI 316 stainless steel Part II: Pit initiation and transition to stability. Corros. Sci. 2015, 94, 420–427. [Google Scholar] [CrossRef]
- Frankel, G.S.; Stockert, L.; Hunkeler, F.; Boehni, H. Metastable pitting of stainless steel. Corrosion 1987, 43, 429–436. [Google Scholar] [CrossRef]


| C | Si | Mn | Cr | Ni | Ti | V | Nb | Others | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.066 | 0.29 | 1.39 | 0.032 | 0.20 | 0.015 | 0.037 | 0.056 | 0.300 | Bal. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Kan, B.; Li, J.; Qiao, L.; Volinsky, A.A.; Su, Y. A Statistical Study on the Effect of Hydrostatic Pressure on Metastable Pitting Corrosion of X70 Pipeline Steel. Materials 2017, 10, 1307. https://doi.org/10.3390/ma10111307
Yang Z, Kan B, Li J, Qiao L, Volinsky AA, Su Y. A Statistical Study on the Effect of Hydrostatic Pressure on Metastable Pitting Corrosion of X70 Pipeline Steel. Materials. 2017; 10(11):1307. https://doi.org/10.3390/ma10111307
Chicago/Turabian StyleYang, Zixuan, Bo Kan, Jinxu Li, Lijie Qiao, Alex A. Volinsky, and Yanjing Su. 2017. "A Statistical Study on the Effect of Hydrostatic Pressure on Metastable Pitting Corrosion of X70 Pipeline Steel" Materials 10, no. 11: 1307. https://doi.org/10.3390/ma10111307





