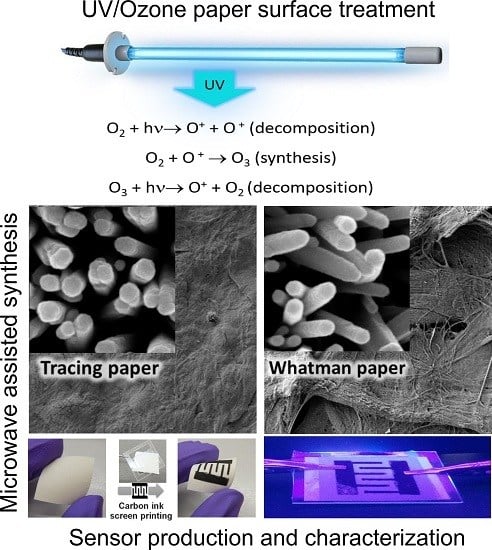
Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications
Abstract
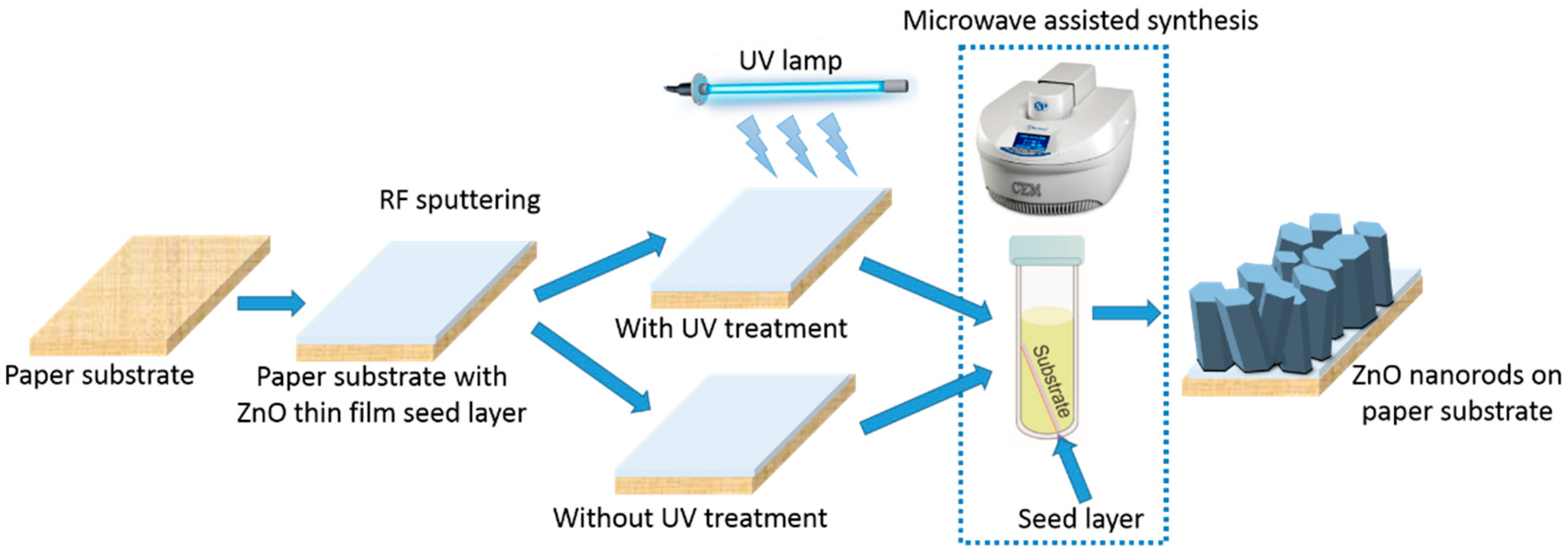
:1. Introduction
2. Results and Discussion
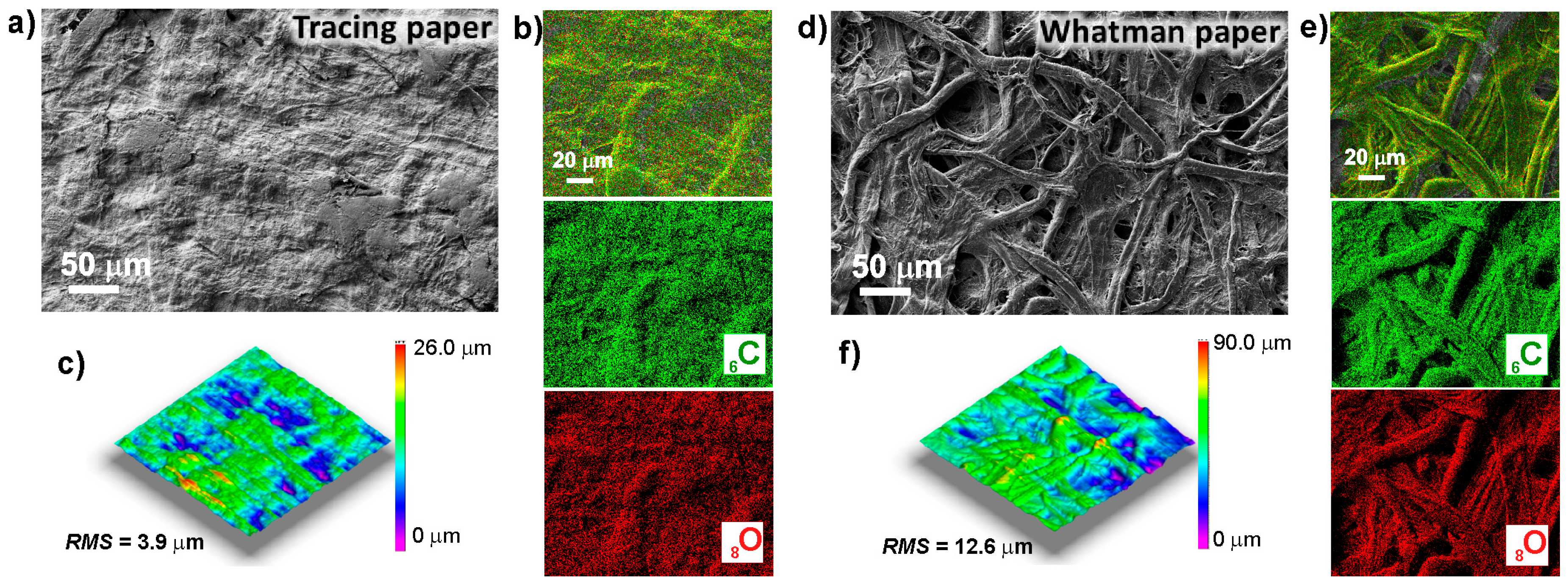
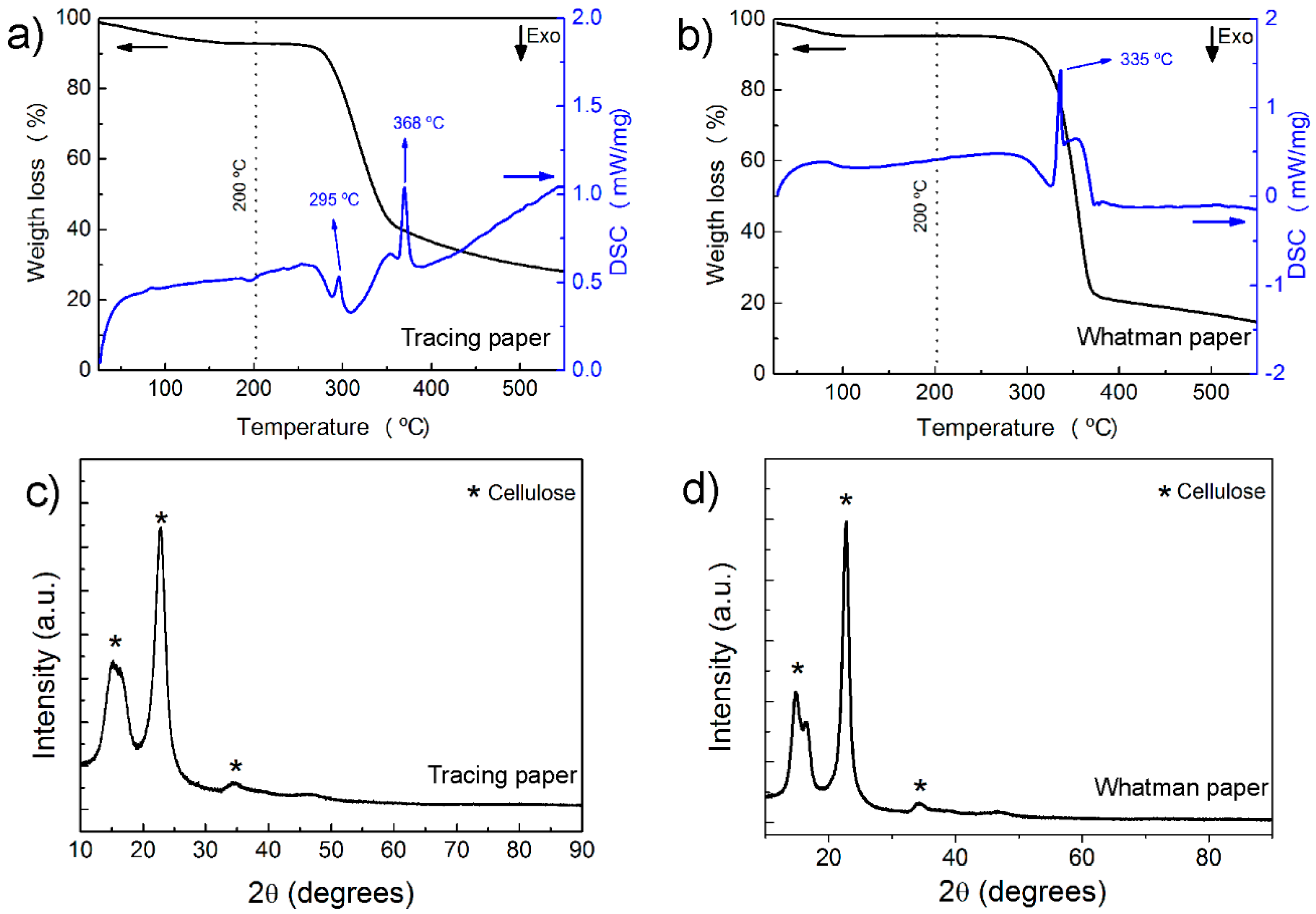
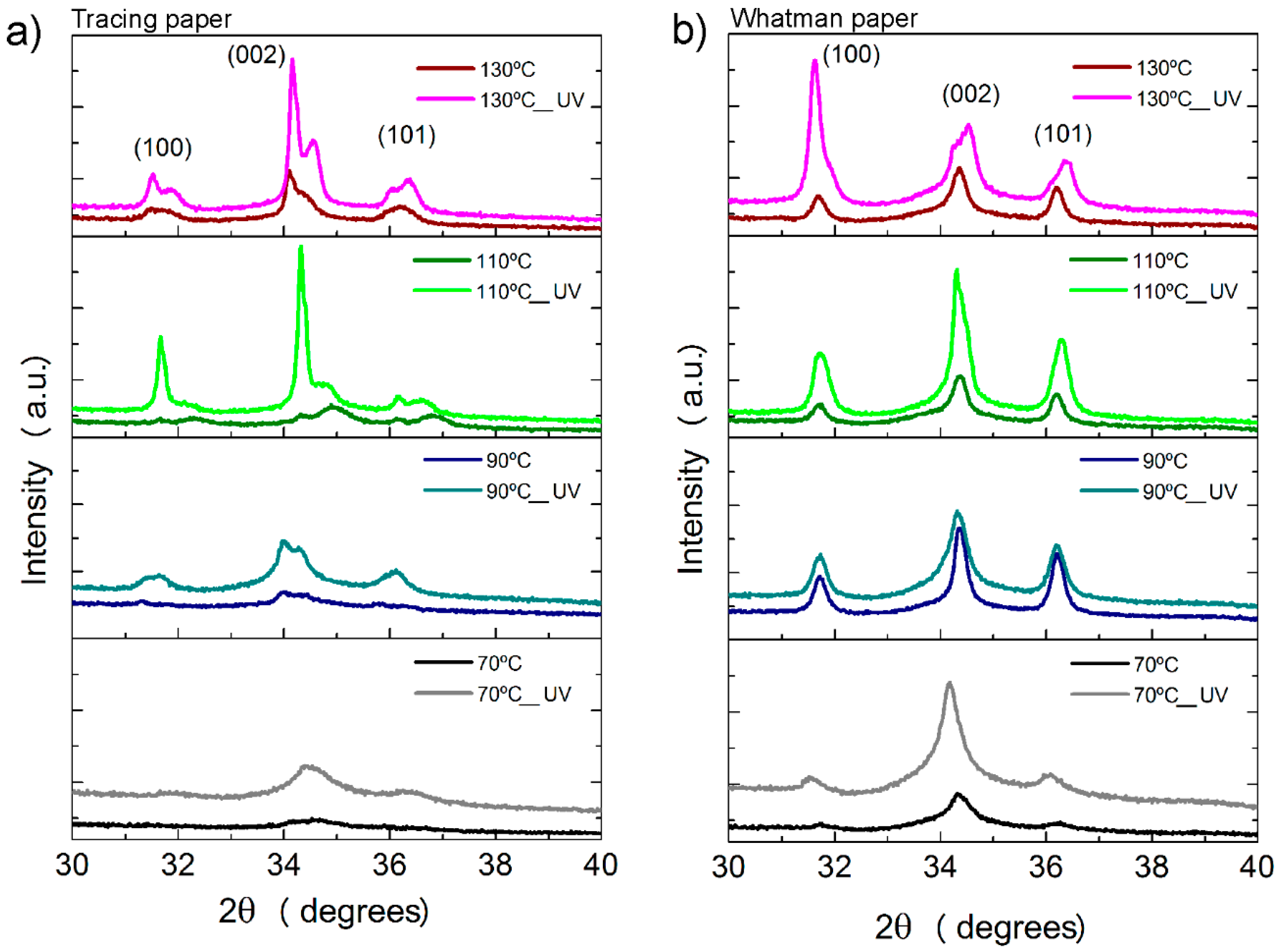
2.1. Characterization of Paper Substrates: SEM, Thermal Analysis, XRD
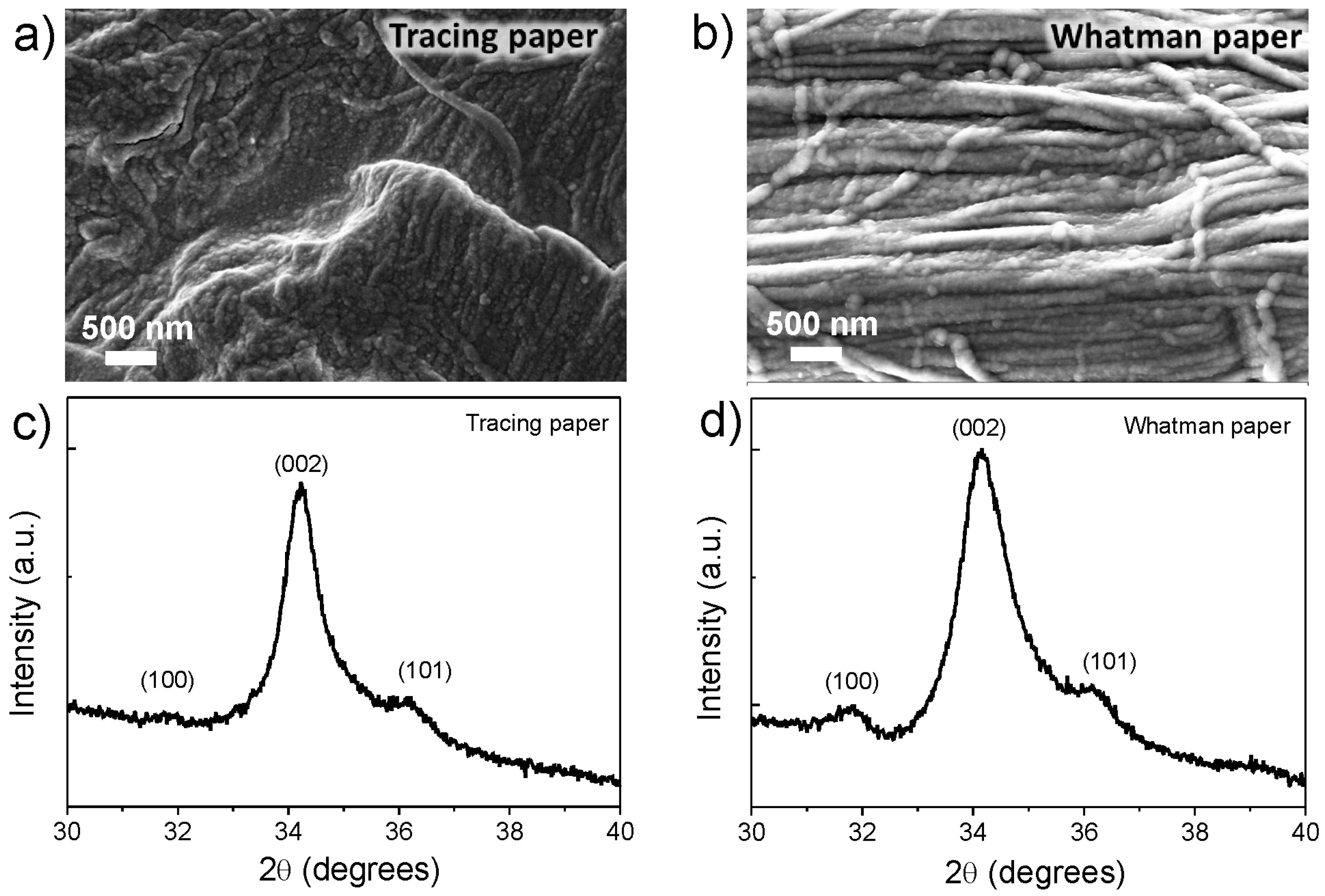
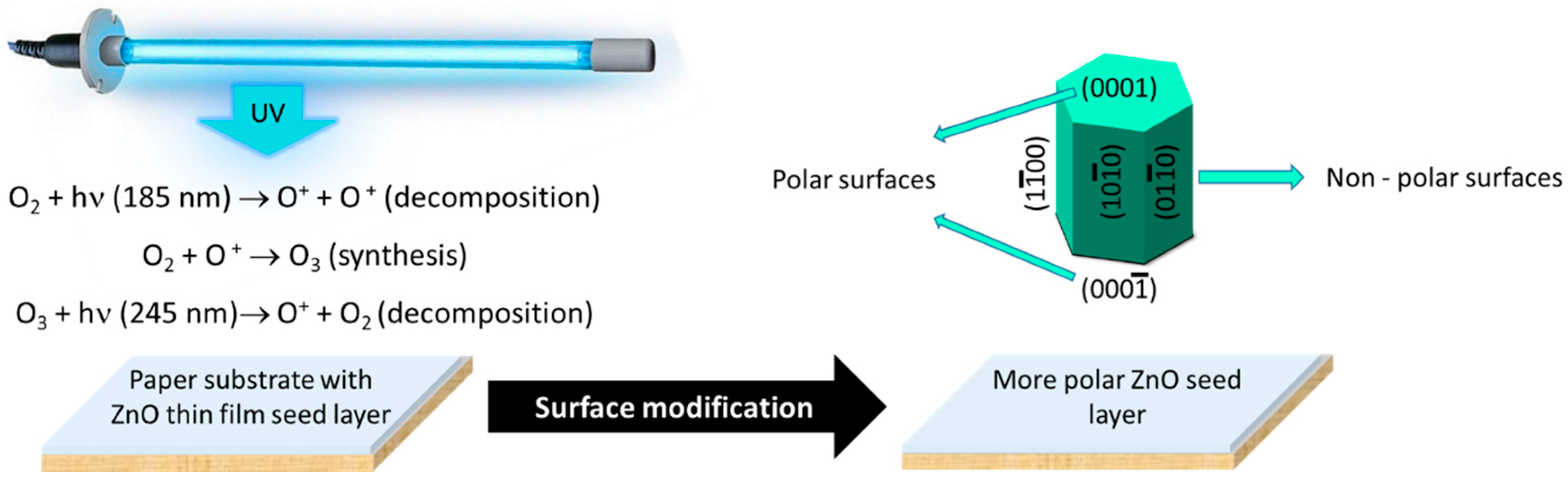
2.2. UV/Ozone ZnO Seed Layer Treatment
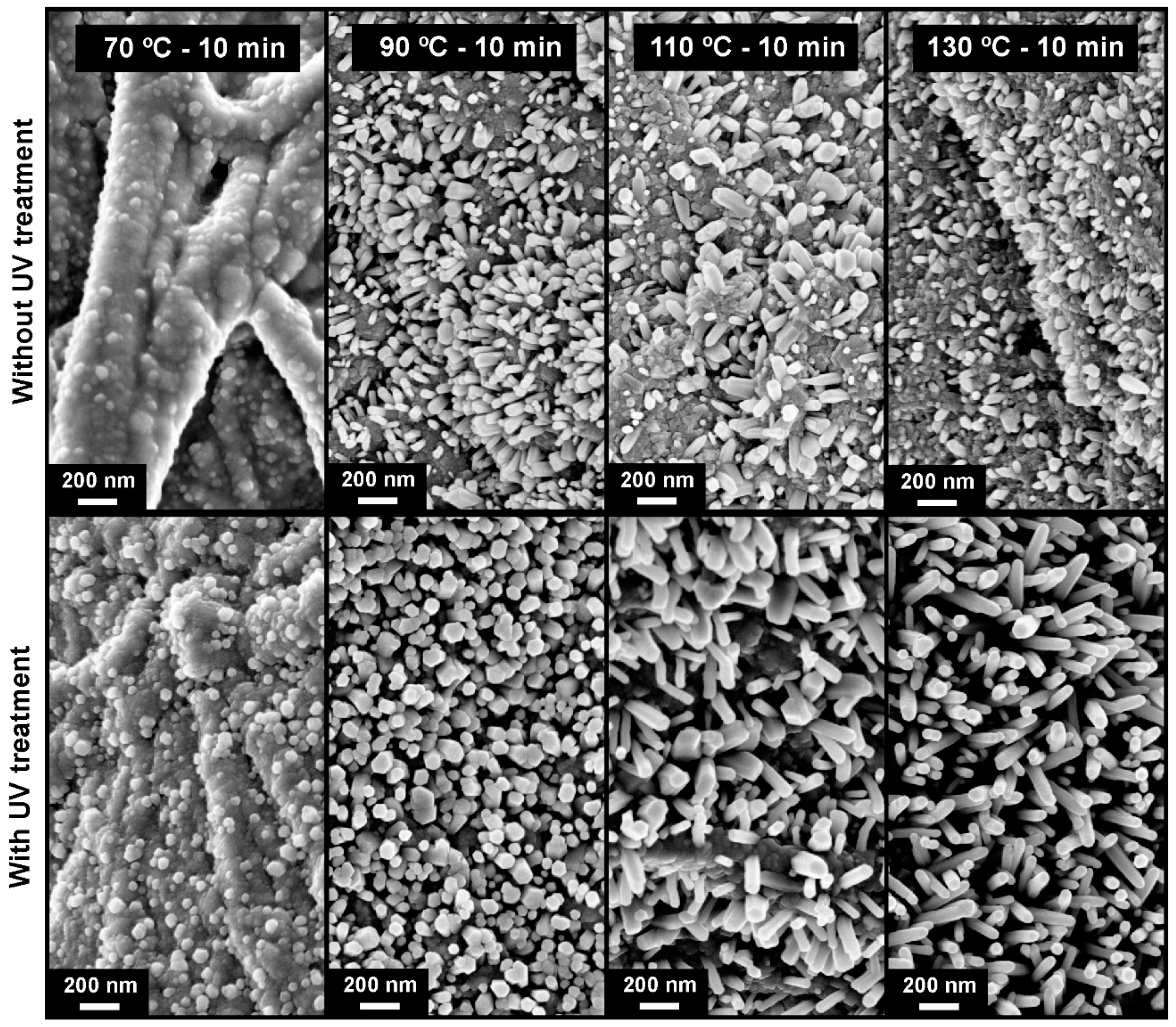
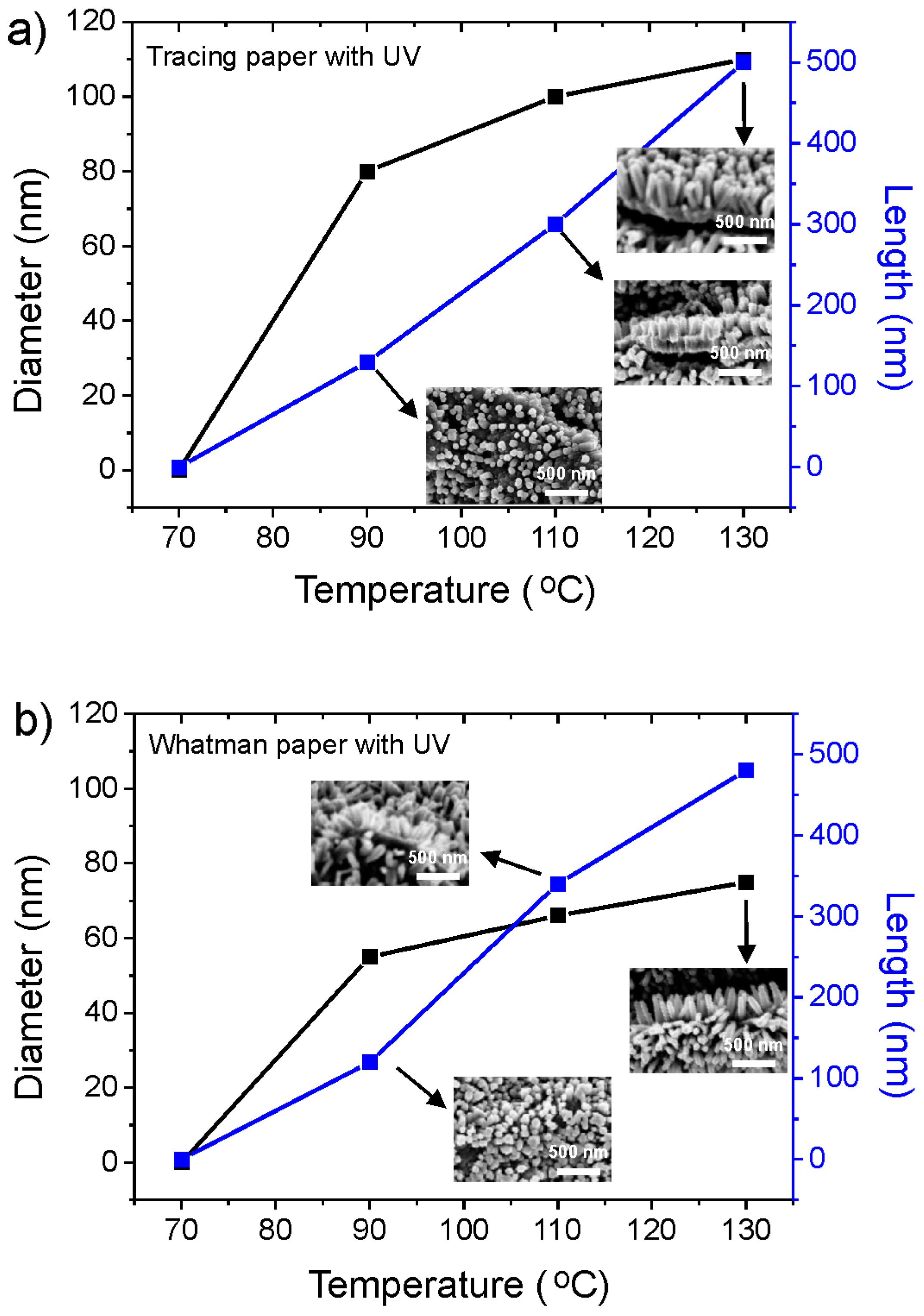
2.3. Crystallographic Structure and Morphology Analysis of ZnO Nanorods
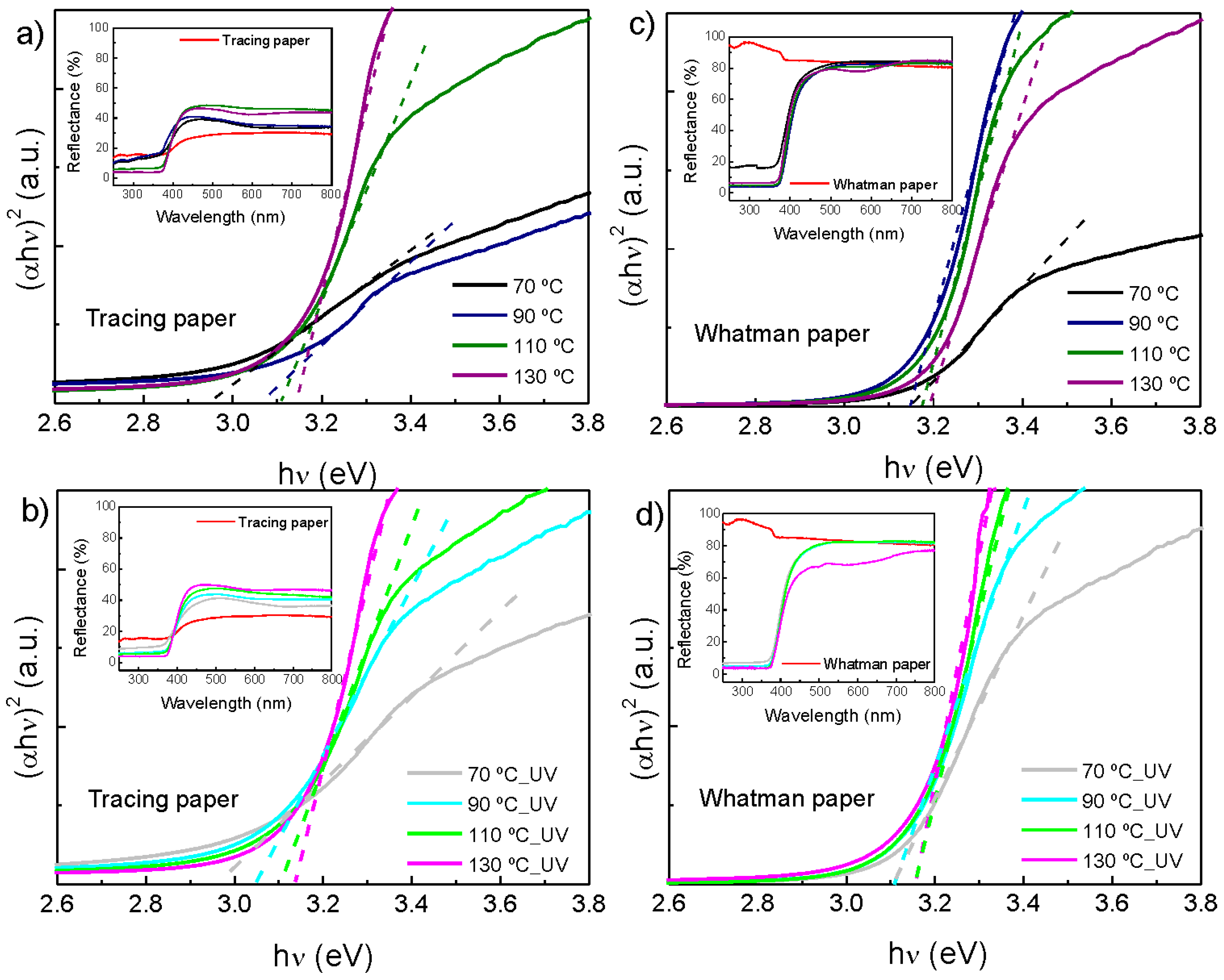
2.4. Optical Properties
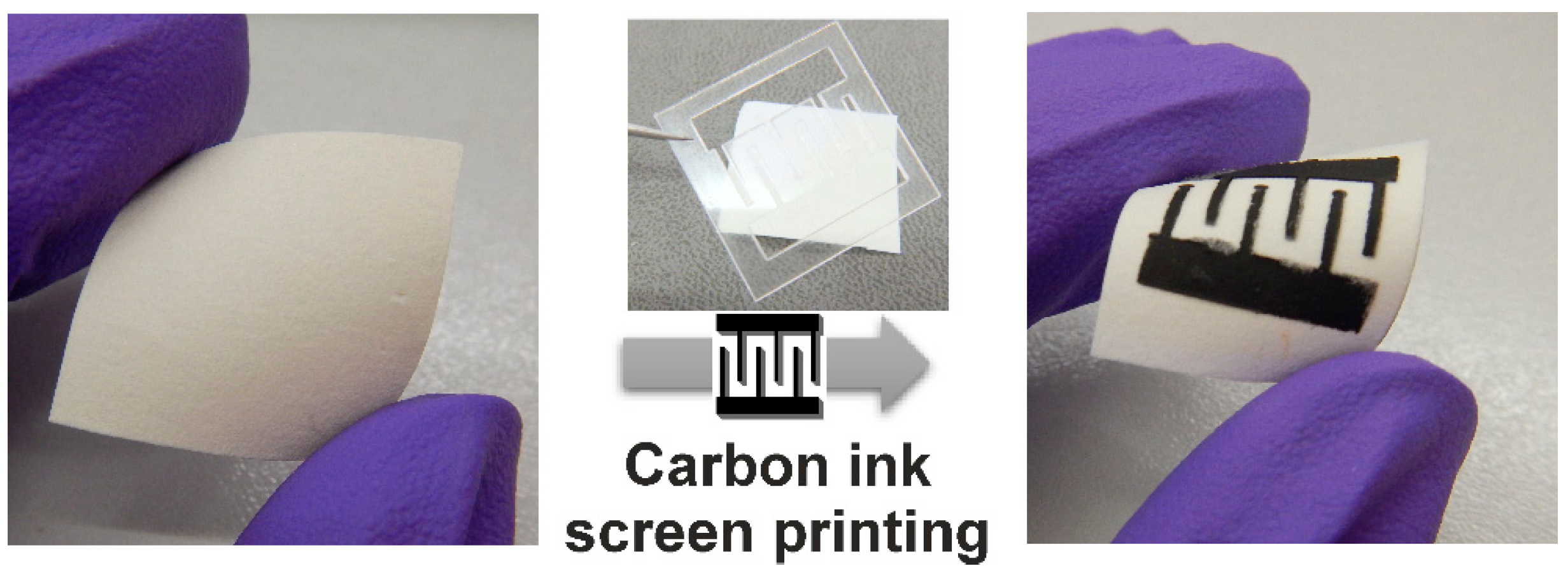
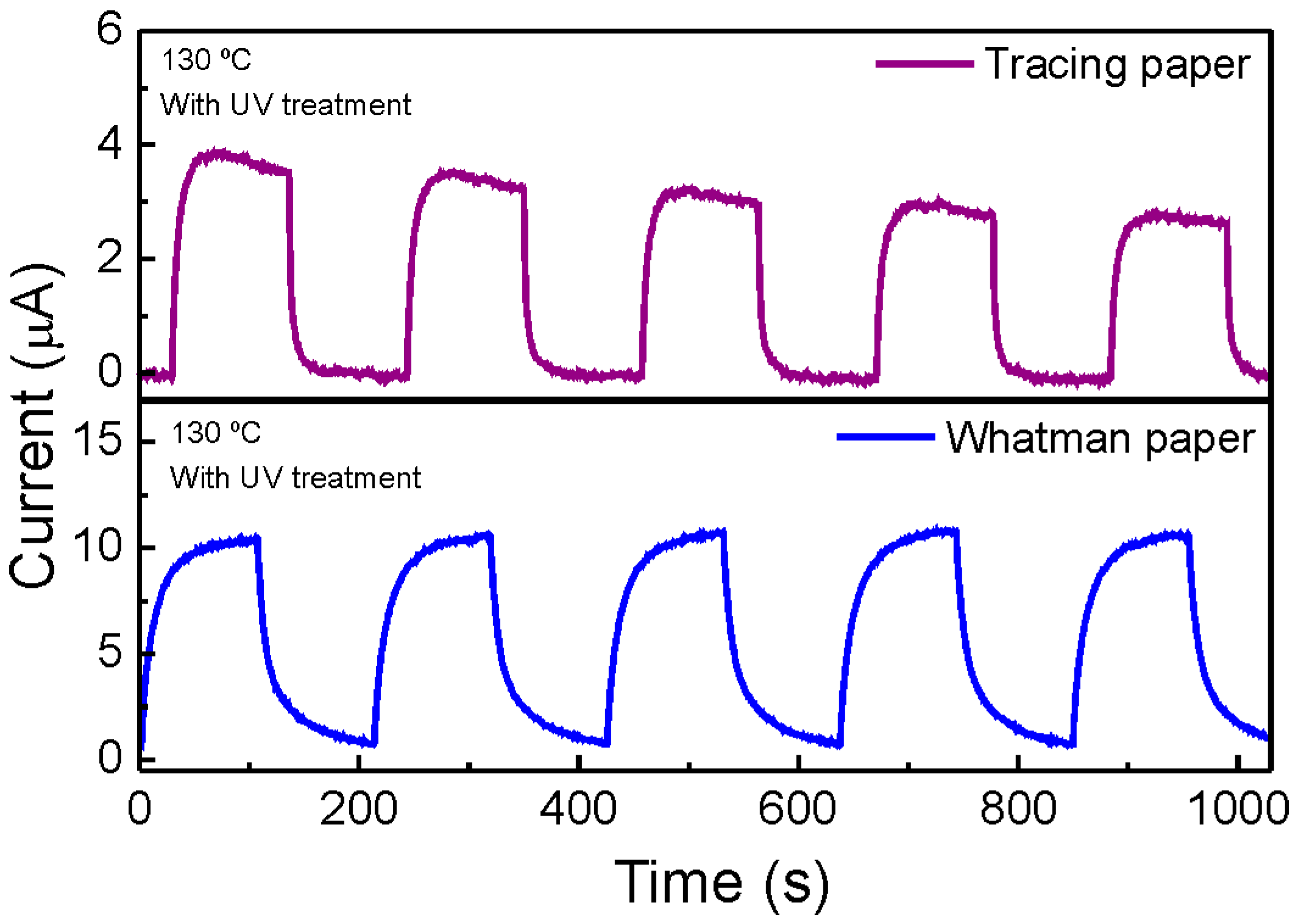
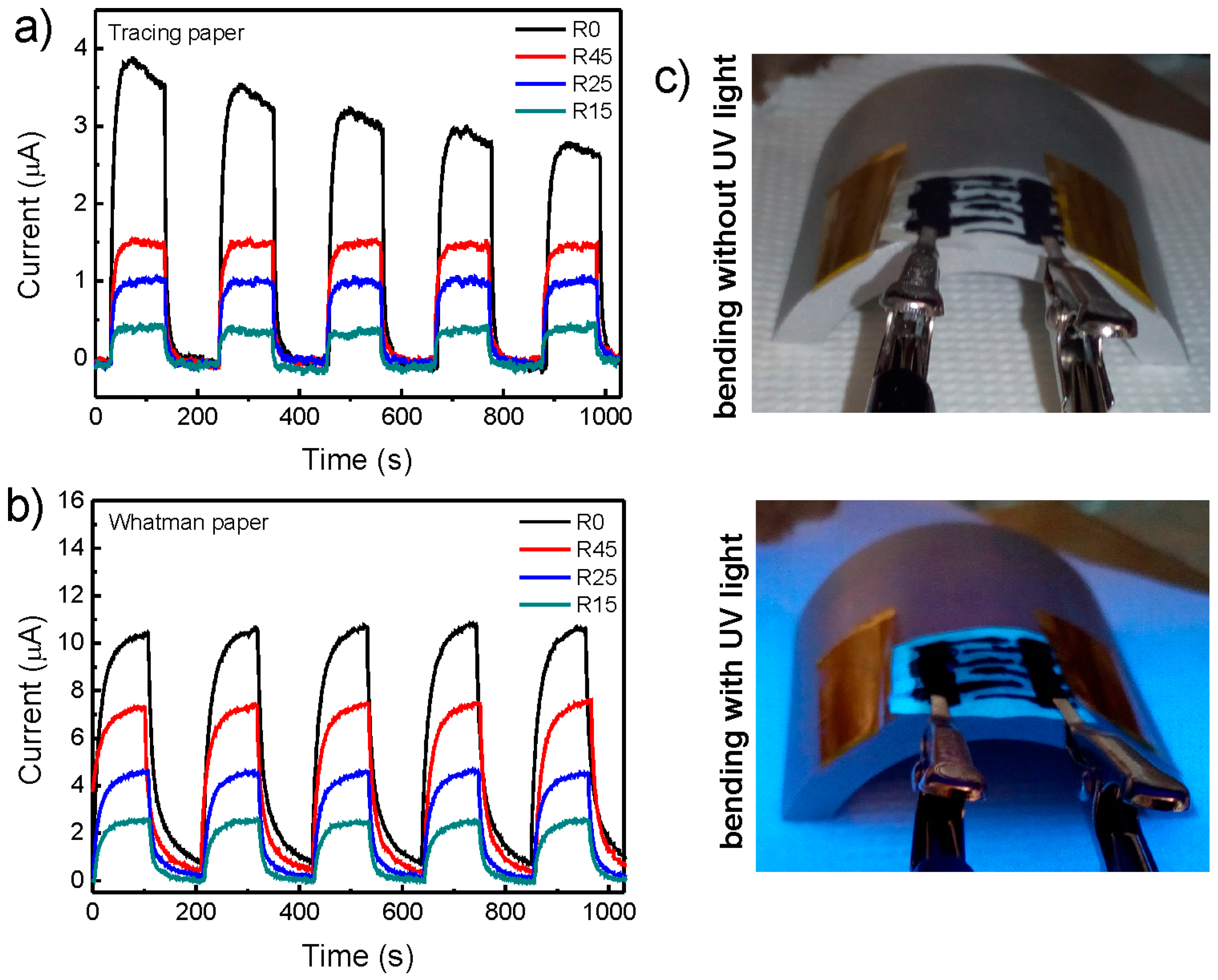
2.5. Application of ZnO Nanorods in Paper-Based UV Sensors
3. Experimental Details
3.1. Synthesis of ZnO Nanostructures
3.2. Characterization Techniques
3.3. Characterization of ZnO Nanorods on Tracing and Whatman Substrate as a UV Sensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fortunato, E.; Correia, N.; Barquinha, P.; Pereira, L.; Goncalves, G.; Martins, R. High-Performance Flexible Hybrid Field-Effect Transistors Based on Cellulose Fiber Paper. IEEE Electron Device Lett. 2008, 29, 988–990. [Google Scholar] [CrossRef]
- Shah, J.; Brown, R.M. Towards electronic paper displays made from microbial cellulose. Appl. Microbiol. Biotechnol. 2005, 66, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Tobjörk, D.; Österbacka, R. Paper Electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1536. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kerr, L.L. Dye sensitized solar cells on paper substrates. Sol. Energy Mater. Sol. Cells 2011, 95, 2531–2535. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Santos, L.; Costa, M.N.; Dantas, J.M.; Duarte, P.; Gonçalves, A.; Martins, R.; Salgueiro, C.A.; Fortunato, E. Office paper platform for bioelectrochromic detection of electrochemically active bacteria using tungsten trioxide nanoprobes. Sci. Rep. 2015, 5, 9910. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Martins, R.; Inácio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 94006. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.; Quaresma, P.; de Almeida, M.P.; Araújo, A.; Pereira, E.; Fortunato, E.; Martins, R.; Franco, R.; Águas, H. Office paper decorated with silver nanostars—An alternative cost effective platform for trace analyte detection by SERS. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Morkoc, H.; Ozgur, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology. Available online: http://eu.wiley.com/WileyCDA/WileyTitle/productCd-3527408134.html (accessed on 14 March 2016).
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Hayes, B.L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: Matthews, NC, USA, 2002. [Google Scholar]
- Nunes, D.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Cu2O polyhedral nanowires produced by microwave irradiation. J. Mater. Chem. C 2014, 2, 6097. [Google Scholar] [CrossRef]
- Gonçalves, A.; Resende, J.; Marques, A.C.; Pinto, J.V.; Nunes, D.; Marie, A.; Goncalves, R.; Pereira, L.; Martins, R.; Fortunato, E. Smart optically active VO2 nanostructured layers applied in roof-type ceramic tiles for energy efficiency. Sol. Energy Mater. Sol. Cells 2016, 150, 1–9. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2015, 278, 262–270. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Pereira, S.; Martins, R.; Fortunato, E. Photocatalytic Activity of TiO2 Nanostructured Arrays Prepared by Microwave-Assisted Solvothermal Method. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; Cao, W.B., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.; Pimentel, A.; Oliveira, M.J.; Mendes, M.J.; Franco, R.; Fortunato, E.; Águas, H.; Martins, R. Direct growth of plasmonic nanorod forests on paper substrates for low-cost flexible 3D SERS platforms. Flex. Print. Electron. 2017, 2, 14001. [Google Scholar] [CrossRef]
- Major, S.; Kumar, S.; Bhatnagar, M.; Chopra, K.L. Effect of hydrogen plasma treatment on transparent conducting oxides. Appl. Phys. Lett. 1986, 49, 394. [Google Scholar] [CrossRef]
- Park, J.-S.; Jeong, J.K.; Mo, Y.-G.; Kim, H.D.; Kim, S.-I. Improvements in the device characteristics of amorphous indium gallium zinc oxide thin-film transistors by Ar plasma treatment. Appl. Phys. Lett. 2007, 90, 262106. [Google Scholar] [CrossRef]
- Angermann, H.; Korte, L.; Rappich, J.; Conrad, E.; Sieber, I.; Schmidt, M.; Hübener, K.; Hauschild, J. Optimisation of electronic interface properties of a-Si:H/c-Si hetero-junction solar cells by wet-chemical surface pre-treatment. Thin Solid Films 2008, 516, 6775–6781. [Google Scholar] [CrossRef]
- Ip, K.; Gila, B.; Onstine, A.; Lambers, E.; Heo, Y.; Baik, K.; Norton, D.; Pearton, S.; Kim, S.; LaRoche, J.; et al. Effect of ozone cleaning on Pt/Au and W/Pt/Au Schottky contacts to n-type ZnO. Appl. Surf. Sci. 2004, 236, 387–393. [Google Scholar] [CrossRef]
- Cho, J.M.; Kwak, S.-W.; Aqoma, H.; Kim, J.W.; Shin, W.S.; Moon, S.-J.; Jang, S.-Y.; Jo, J. Effects of ultraviolet–ozone treatment on organic-stabilized ZnO nanoparticle-based electron transporting layers in inverted polymer solar cells. Org. Electron. 2014, 15, 1942–1950. [Google Scholar] [CrossRef]
- Manekkathodi, A.; Lu, M.-Y.; Wang, C.W.; Chen, L.-J. Direct growth of aligned zinc oxide nanorods on paper substrates for low-cost flexible electronics. Adv. Mater. 2010, 22, 4059–4063. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Pereira, L.M.N.; Martins, R.F.P. Fully Transparent ZnO Thin-Film Transistor Produced at Room Temperature. Adv. Mater. 2005, 17, 590–594. [Google Scholar] [CrossRef]
- Pimentel, A.C.; Gonçalves, A.; Marques, A.; Martins, R.; Fortunato, E. Zinc oxide thin films used as an ozone sensor at room temperature. MRS Proc. 2011, 915, 915-R07-4. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, Z.; Yan, X.; Liao, Q. ZnO nanostructures in enzyme biosensors. Sci. China Mater. 2015, 58, 60–76. [Google Scholar] [CrossRef]
- Pimentel, A.; Fortunato, E.; Gonçalves, A.; Marques, A.; Águas, H.; Pereira, L.; Ferreira, I.; Martins, R. Polycrystalline intrinsic zinc oxide to be used in transparent electronic devices. Thin Solid Films 2005, 487, 212–215. [Google Scholar] [CrossRef]
- Panda, S.K.; Jacob, C. Preparation of transparent ZnO thin films and their application in UV sensor devices. Solid State Electron. 2012, 73, 44–50. [Google Scholar] [CrossRef]
- Chang, H.; Sun, Z.; Ho, K.Y.-F.; Tao, X.; Yan, F.; Kwok, W.-M.; Zheng, Z. A highly sensitive ultraviolet sensor based on a facile in situ solution-grown ZnO nanorod/graphene heterostructure. Nanoscale 2011, 3, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, H.; Zhao, D.; Li, B.; Zhang, Z.; Jiang, M.; Shen, D. High responsivity ZnO nanowires based UV detector fabricated by the dielectrophoresis method. Sens. Actuators B Chem. 2012, 166–167, 12–16. [Google Scholar] [CrossRef]
- Chai, G.; Lupan, O.; Chow, L.; Heinrich, H. Crossed zinc oxide nanorods for ultraviolet radiation detection. Sens. Actuators A Phys. 2009, 150, 184–187. [Google Scholar] [CrossRef]
- Yao, I.-C.; Tseng, T.-Y.; Lin, P. ZnO nanorods grown on polymer substrates as UV photodetectors. Sens. Actuators A Phys. 2012, 178, 26–31. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Razak, K.A.; Lockman, Z.; Abdul Aziz, A. Structural and morphology of ZnO nanorods synthesized using ZnO seeded growth hydrothermal method and its properties as UV sensing. PLoS ONE 2012, 7, e50405. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.H.; Chia, Z.X.; Kevin, M.; Wong, A.S.W.; Ho, G.W. A facile approach towards ZnO nanorods conductive textile for room temperature multifunctional sensors. Sens. Actuators B Chem. 2010, 151, 121–126. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, K.; Yang, F.; Feng, G.; Sun, Y.; Dai, Y. Thermal degradation kinetics and heat properties of cellulosic cigarette paper: Influence of potassium carboxylate as combustion improver. Cellulose 2013, 20, 3205–3217. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Poletto, M.; Zattera, A.J.; Amico, S.C. Correlation of the thermal stability and the decomposition kinetics of six different vegetal fibers. Cellulose 2014, 21, 177–188. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Lujun, Y.; Maojun, Z.; Changli, L.; Li, M.; Wenzhong, S. Facile synthesis of superhydrophobic surface of ZnO nanoflakes: Chemical coating and UV-induced wettability conversion. Nanoscale Res. Lett. 2012, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, R.M.; McLachlan, M.A. Surface Structure Modification of ZnO and the Impact on Electronic Properties. Adv. Mater. 2016, 28, 3893–3921. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.N.; Fukushima, Y.; Hirano, Y.; Tokuoka, Y.; Takahashi, M.; Kawashima, N. Surface modification of polystyrene and poly(methyl methacrylate) by active oxygen treatment. Colloids Surfaces B Biointerfaces 2003, 29, 171–179. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y.; Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Vig, J.R. UV/ozone cleaning of surfaces. J. Vac. Sci. Technol. A Vac. Surf. Films 1985, 3, 1027–1034. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 13001. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Jiang, F.; Gao, R.; Peng, Z. Microstructure and nonohmic properties of SnO2-Ta2O5-ZnO system doped with ZrO2. Sci. World J. 2014, 2014, 754890. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Diao, P.; Wang, X.; Cai, S. The effect of hydrothermal growth temperature on preparation and photoelectrochemical performance of ZnO nanorod array films. J. Solid State Chem. 2005, 178, 3210–3215. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors; Dover Publications, Inc.: Mineola, NY, USA, 1971. [Google Scholar]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Tai, W.-P.; Shu, S.-J. Effect of preheating temperature on structural and optical properties of ZnO thin films by sol–gel process. Thin Solid Films 2005, 491, 153–160. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloys Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Chou, C.-S.; Wu, Y.-C.; Lin, C.-H. Oxygen sensor utilizing ultraviolet irradiation assisted ZnO nanorods under low operation temperature. RSC Adv. 2014, 4, 52903–52910. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Zhai, T.; Fang, X.; Liao, M.; Xu, X.; Zeng, H.; Yoshio, B.; Golberg, D. A Comprehensive Review of One-Dimensional Metal-Oxide Nanostructure Photodetectors. Sensors 2009, 9, 6504–6529. [Google Scholar] [CrossRef] [PubMed]
- Mamat, M.H.; Khusaimi, Z.; Zahidi, M.M.; Mahmood, M.R. Nanorods; Yaln, O., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Kalantar-zadeh, K.; Fry, B. Sensor Characteristics and Physical Effects. In Nanotechnology-Enabled Sensors; Springer: Boston, MA, USA, 2008; pp. 13–62. [Google Scholar]
- Fryxell, G.E.; Cao, G. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors; Imperial College Press: London, UK, 2007. [Google Scholar]
- Cullity, B.D. Elements of X Ray Diffraction; Addison-Wesley Publisher Companym Inc.: Boston, MA, USA, 1956. [Google Scholar]
- Chen, T.-P.; Young, S.-J.; Chang, S.-J.; Hsiao, C.-H.; Hsu, Y.-J. Bending effects of ZnO nanorod metal–semiconductor–metal photodetectors on flexible polyimide substrate. Nanoscale Res. Lett. 2012, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

| Synthesis Temperature | 70 °C | 90 °C | 110 °C | 130 °C | |
|---|---|---|---|---|---|
| Tracing paper | Without UV treatment | 2.95 eV | 3.07 eV | 3.10 eV | 3.14 eV |
| With UV treatment | 2.96 eV | 3.05 eV | 3.11 eV | 3.14 eV | |
| Whatman paper | Without UV treatment | 3.15 eV | 3.15 eV | 3.18 eV | 3.19 eV |
| With UV treatment | 3.10 eV | 3.10 eV | 3.16 eV | 3.16 eV | |
| Paper | Radius | Response Time (s) | Recovery Time (s) | Responsivity (µA/W) |
|---|---|---|---|---|
| Tracing | R0 | 30 | 27 | 0.39 |
| R45 | 27 | 25 | 0.19 | |
| R25 | 24 | 26 | 0.12 | |
| R15 | 21 | 30 | 0.044 | |
| Whatman | R0 | 57 | 65 | 1.20 |
| R45 | 57 | 62 | 0.84 | |
| R25 | 62 | 57 | 0.57 | |
| R15 | 61 | 48 | 0.32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications. Materials 2017, 10, 1308. https://doi.org/10.3390/ma10111308
Pimentel A, Samouco A, Nunes D, Araújo A, Martins R, Fortunato E. Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications. Materials. 2017; 10(11):1308. https://doi.org/10.3390/ma10111308
Chicago/Turabian StylePimentel, Ana, Ana Samouco, Daniela Nunes, Andreia Araújo, Rodrigo Martins, and Elvira Fortunato. 2017. "Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications" Materials 10, no. 11: 1308. https://doi.org/10.3390/ma10111308