Co-Precipitation Synthesis and Optical Properties of Mn4+-Doped Hexafluoroaluminate w-LED Phosphors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of K2MnF6
2.3. Synthesis of M3AlF6:Mn4+
2.4. Characterization
3. Results and Discussion
3.1. Structural Properties
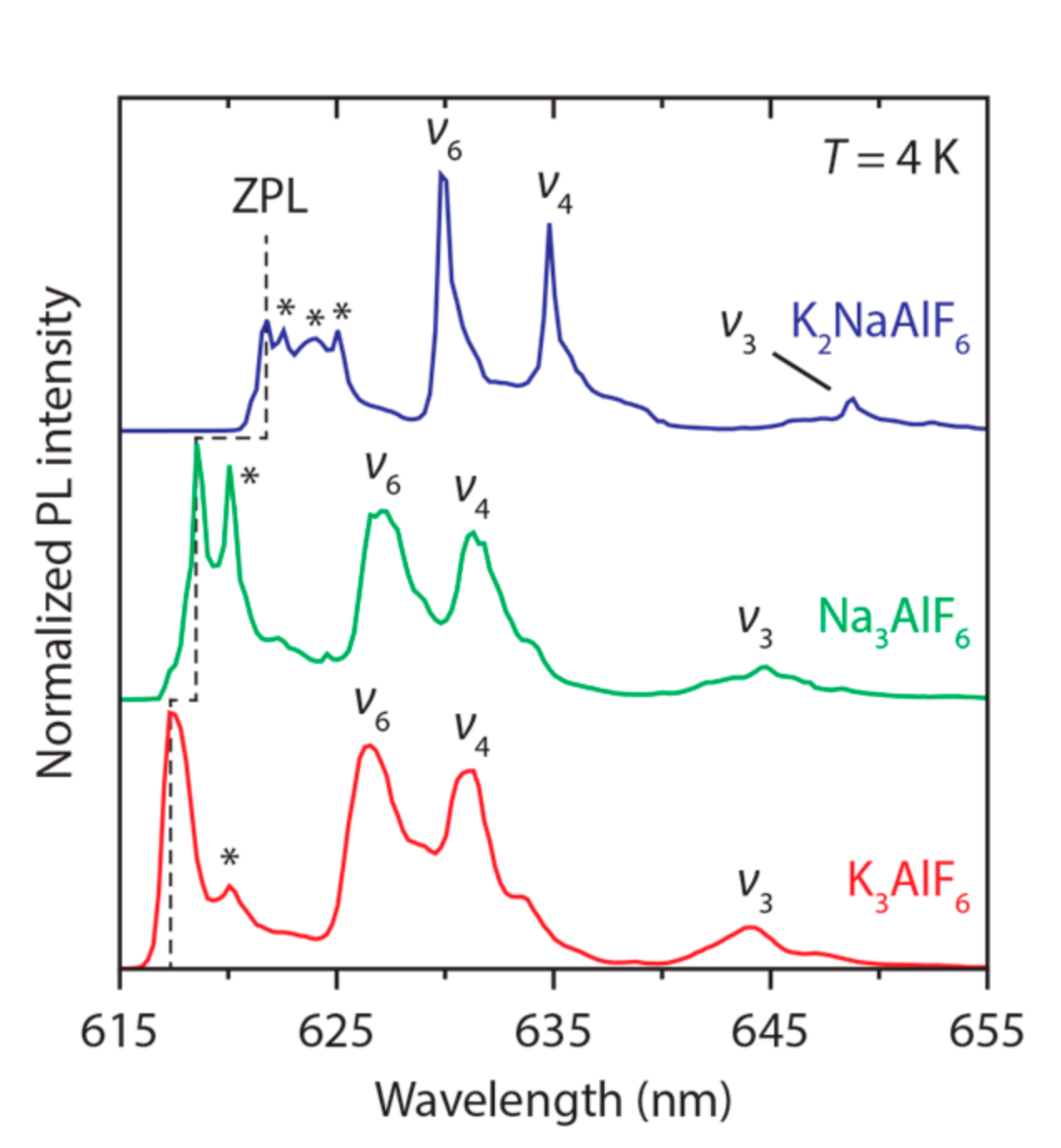
3.2. Mn4+Luminescence
3.3. Thermal Quenching in M3AlF6:Mn4+
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Charge of the LED brigade: A global switch to LEDs will change the lighting business. Economist. 2011. http://www.economist.com/node/21526373.
- Krames, M.R.; Shchekin, O.B.; Mueller-Mach, R.; Mueller, G.O.; Zhou, L.; Harbers, G.; Craford, M.G. Status and future of high-power light-emitting diodes for solid-state lighting. IEEE/OSA J. Disp. Technol. 2007, 3, 160–175. [Google Scholar] [CrossRef]
- Harbers, G.; Bierhuizen, S.J.; Krames, M.R. Performance of high power light emitting diodes in display illumination applications. IEEE/OSA J. Disp. Technol. 2007, 3, 98–109. [Google Scholar] [CrossRef]
- Nakamura, S. Current status of GaN-based solid-state lighting. MRS Bull. 2009, 34, 101–107. [Google Scholar] [CrossRef]
- Smet, P.F.; Parmentier, A.B.; Poelman, D. Selecting conversion phosphors for white light-emitting diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar] [CrossRef] [Green Version]
- Setlur, A.A. Phosphors for LED-based solid-state lighting. Electrochem. Soc. Interface 2009, 18, 32–36. [Google Scholar]
- Xie, R.J.; Hirosaki, N. Silicon-based oxynitride and nitride phosphors for white LEDs—A review. Sci. Technol. Adv. Mater. 2007, 8, 588–600. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, C.C.; Luo, W.; Shu, S.; Liu, Z.; Liu, Y.; Kong, J.; Ma, E.; Cao, Y.; Liu, R.-S.; et al. Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 2014, 5, 4312. [Google Scholar] [CrossRef] [PubMed]
- Setlur, A.A.; Radkov, E.V.; Henderson, C.S.; Her, J.-H.; Srivastava, A.M.; Karkada, N.; Kishore, M.S.; Kumar, N.P.; Aesram, D.; Deshpande, A.; et al. Energy-efficient, high-color-rendering LED lamps using oxyfluoride and fluoride phosphors. Chem. Mater. 2010, 22, 4076–4082. [Google Scholar] [CrossRef]
- Sijbom, H.F.; Joos, J.J.; Martin, L.I.D.J.; Van den Eeckhout, K.; Poelman, D.; Smet, P.F. Luminescent behavior of the K2SiF6:Mn4+ red phosphor at high fluxes and at the microscopic level. ECS J. Solid State Sci. Technol. 2016, 5, R3040–R3048. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Meijerink, A.; Liu, R.-S. Critical red components for next-generation white LEDs. J. Phys. Chem. Lett. 2016, 7, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-D.; Liu, R.-S. Narrow-band red-emitting Mn4+-doped hexafluoride phosphors: Synthesis, optoelectronic properties, and applications in white light-emitting diodes. J. Mater. Chem. C 2016, 4, 10759–10775. [Google Scholar] [CrossRef]
- Hoshino, R.; Adachi, S. Photo-induced degradation and thermal decomposition in ZnSnF6·6H2O:Mn4+ red-emitting phosphor. Opt. Mater. 2015, 48, 36–43. [Google Scholar] [CrossRef]
- Sijbom, H.F.; Verstraete, R.; Joos, J.J.; Poelman, D.; Smet, P.F. K2SiF6:Mn4+ as a red phosphor for displays and warm-white LEDs: A review of properties and perspectives. Opt. Mater. Express 2017, 7, 3332–3365. [Google Scholar] [CrossRef]
- Murphy, J.E.; Garcia-Santamaria, F.; Setlur, A.A.; Sista, S. PFS, K2SiF6:Mn4+: The red-line emitting LED phosphor behind GE’s TriGain TechnologyTM platform. SID Symp. Dig. Tech. Pap. 2015, 46, 927–930. [Google Scholar] [CrossRef]
- Nguyen, H.-D.; Lin, C.C.; Liu, R.-S. Waterproof alkyl phosphate coated fluoride phosphors for optoelectronic materials. Angew. Chem. Int. Ed. 2015, 54, 10862–10866. [Google Scholar] [CrossRef] [PubMed]
- Song, E.H.; Wang, J.Q.; Ye, S.; Jiang, X.F.; Peng, M.Y.; Zhang, Q.Y. Room-temperature synthesis and warm-white LED applications of Mn4+ ion doped fluoroaluminate red phosphor Na3AlF6:Mn4+. J. Mater. Chem. C 2016, 4, 2480–2487. [Google Scholar] [CrossRef]
- Song, E.; Wang, J.; Shi, J.; Deng, T.; Ye, S.; Peng, M.; Wang, J.; Wondraczek, L.; Zhang, Q. Highly efficient and thermally stable K3AlF6:Mn4+ as a red phosphor for ultra-high-performance warm white light-emitting diodes. ACS Appl. Mater. Interfaces 2017, 9, 8805–8812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, L.; Brik, M.G.; Zhang, X.; Huang, L.; Xuan, T.; Wang, J. Facile synthesis, morphology and photoluminescence of a novel red fluoride nanophosphor K2NaAlF6:Mn4+. J. Mater. Chem. C 2017, 5, 6420–6426. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, L.; Zou, R.; Zhang, J.; Yu, J.; Wu, M.; Wang, J.; Su, Q. Hydrothermal synthesis, morphology and photoluminescent properties of an Mn4+-doped novel red fluoride phosphor elpasolite K2LiAlF6. J. Mater. Chem. C 2016, 4, 5690–5695. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Thompson, W.T.; Goad, D.G.W. Some thermodynamic properties of K3AlF6–KAlF4 melts. Can. J. Chem. 1976, 54, 3342–3349. [Google Scholar] [CrossRef]
- Rumble, J.R. Handbook of Chemistry and Physics, 98th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lv, L.; Jiang, X.; Huang, S.; Chen, X.; Pan, Y. The formation mechanism, improved photoluminescence and LED applications of red phosphor K2SiF6:Mn4+. J. Mater. Chem. C 2014, 2, 3879–3884. [Google Scholar] [CrossRef]
- Setlur, A.A.; Lyons, R.J.; Nammalwar, P.K.; Hanumanlha, R.; Murphy, J.E.; Garcia, F. Moisture-Resistant Phosphor Compositions and Associate Methods. World Patent WO 2,015,102,876, 9 July 2015. [Google Scholar]
- Shriver, D.F.; Atkins, P.W. Inorganic Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Bode, H.; Jenssen, H.; Bandte, F. Über eine neue darstellung des kalium-hexafluoromanganats (IV). Angew. Chem. 1953, 65, 304. [Google Scholar] [CrossRef]
- Roesky, H.W. Efficient Preparations of Fluorine Compounds; Roesky, H.W., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; ISBN 9781118409466. [Google Scholar]
- Morss, L.R. Crystal structure of dipotassium sodium fluoroaluminate (elpasolite). J. Inorg. Nucl. Chem. 1974, 36, 3876–3878. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Ferguson, R.B. Refinement of the crystal structure of cryolite. Can. Mineral. 1975, 13, 377–382. [Google Scholar]
- Abakumov, A.M.; King, G.; Laurinavichute, V.K.; Rozova, M.G.; Woodward, P.M.; Antipov, E.V. The crystal structure of α-K3AlF6: Elpasolites and double perovskites with broken corner-sharing connectivity of the octahedral framework. Inorg. Chem. 2009, 48, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Takahashi, T. Photoluminescent properties of K2GeF6:Mn4+ red phosphor synthesized from aqueous HF/KMnO4 solution. J. Appl. Phys. 2009, 106, 013516. [Google Scholar] [CrossRef]
- Xu, Y.K.; Adachi, S. Properties of Na2SiF6:Mn4+ and Na2GeF6:Mn4+ red phosphors synthesized by wet chemical etching. J. Appl. Phys. 2009, 105, 013525. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, Y.; Huang, S.; Chen, X.; Wang, J.; Liu, G. Hydrothermal synthesis and photoluminescence properties of red phosphor BaSiF6:Mn4+ for LED applications. J. Mater. Chem. C 2014, 2, 2301–2306. [Google Scholar] [CrossRef]
- Fang, M.-H.; Nguyen, H.-D.; Lin, C.C.; Liu, R. Preparation of a novel red Rb2SiF6:Mn4+ phosphor with high thermal stability through a simple one-step approach. J. Mater. Chem. C 2015, 3, 7277–7280. [Google Scholar] [CrossRef]
- Zhou, Q.; Tan, H.; Zhou, Y.; Zhang, Q.; Wang, Z.; Yan, J.; Wu, M. Optical performance of Mn4+ in a new hexa-coordinative fluorozirconate complex of Cs2ZrF6. J. Mater. Chem. C 2016, 4, 7443–7448. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Yang, Z.; Liu, Y.; Yang, H.; Tan, H.; Zhang, Q.; Zhou, Q. Synthesis of K2XF6:Mn4+ (X = Ti, Si and Ge) red phosphors for white LED applications with low-concentration of HF. Opt. Mater. 2015, 49, 235–240. [Google Scholar] [CrossRef]
- De Mello Donegá, C. Synthesis and properties of colloidal heteronanocrystals. Chem. Soc. Rev. 2011, 40, 1512–1546. [Google Scholar] [CrossRef] [PubMed]
- Paulusz, A.G. Efficient Mn(IV) emission in fluorine coordination. J. Electrochem. Soc. 1973, 120, 942–947. [Google Scholar] [CrossRef]
- Takahashi, T.; Adachi, S. Mn4+-activated red photoluminescence in K2SiF6 phosphor. J. Electrochem. Soc. 2008, 155, E183–E188. [Google Scholar] [CrossRef]
- Senden, T.; Broers, F.T.H.; Meijerink, A. Comparative study of the Mn4+ 2E → 4A2 luminescence in isostructural RE2Sn2O7:Mn4+ pyrochlores (RE3+ = Y3+, Lu3+ or Gd3+). Opt. Mater. 2016, 60, 431–437. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin, Germany, 1994. [Google Scholar]
- Garcia-Santamaria, F.; Murphy, J.E.; Setlur, A.A.; Sista, S.P. Concentration quenching in K2SiF6:Mn4+ phosphors. ECS J. Solid State Sci. Technol. 2018, 7, R3030–R3033. [Google Scholar] [CrossRef]
- Chodos, S.L.; Black, A.M.; Flint, C.D. Vibronic spectra and lattice dynamics of Cs2MnF6 and A12MIVF6:MnF62−. J. Chem. Phys. 1976, 65, 4816–4824. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M. On the optical properties of the Mn4+ ion in solids. J. Lumin. 2013, 133, 69–72. [Google Scholar] [CrossRef]
- Brik, M.G.; Camardello, S.J.; Srivastava, A.M. Influence of covalency on the Mn4+ 2Eg → 4A2g emission energy in crystals. ECS J. Solid State Sci. Technol. 2014, 4, R39–R43. [Google Scholar] [CrossRef]
- Sakurai, S.; Nakamura, T.; Adachi, S. Rb2SiF6:Mn4+ and Rb2TiF6:Mn4+ red-emitting phosphors. ECS J. Solid State Sci. Technol. 2016, 5, R206–R210. [Google Scholar] [CrossRef]
- Bachmann, V.; Ronda, C.; Meijerink, A. Temperature quenching of yellow Ce3+ luminescence in YAG:Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- Senden, T.; van Dijk-Moes, R.J.A.; Meijerink, A. Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors. ECS J. Solid State Sci. Technol. 2017. Unpublished work. [Google Scholar]






| Phosphor | Na2CO3 | K2CO3 | KF | 40% HF |
|---|---|---|---|---|
| Na3AlF6:Mn4+ | 15 mmol | - | - | 15 mL |
| K2NaAlF6:Mn4+ | 5 mmol | 10 mmol | - | 15 mL |
| K3AlF6:Mn4+ | - | - | 40 mmol 1 | 3 mL |
| Lattice | Space Group | Al3+ Symmetry | Al–F Distance (Å) | ZPL Energy (cm−1) |
|---|---|---|---|---|
| Na3AlF6 | P21/n | Ci | 1.808 | 16,167 |
| K2NaAlF6 | Fmm | Oh | 1.778 | 16,082 |
| K3AlF6 | I41/a | C1 | 1.810 | 16,200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senden, T.; Geitenbeek, R.G.; Meijerink, A. Co-Precipitation Synthesis and Optical Properties of Mn4+-Doped Hexafluoroaluminate w-LED Phosphors. Materials 2017, 10, 1322. https://doi.org/10.3390/ma10111322
Senden T, Geitenbeek RG, Meijerink A. Co-Precipitation Synthesis and Optical Properties of Mn4+-Doped Hexafluoroaluminate w-LED Phosphors. Materials. 2017; 10(11):1322. https://doi.org/10.3390/ma10111322
Chicago/Turabian StyleSenden, Tim, Robin G. Geitenbeek, and Andries Meijerink. 2017. "Co-Precipitation Synthesis and Optical Properties of Mn4+-Doped Hexafluoroaluminate w-LED Phosphors" Materials 10, no. 11: 1322. https://doi.org/10.3390/ma10111322





