1. Introduction
The application of glasses as “biomaterials” has revolutionized the field of human biomedicine and has brought the concept of “surface active” materials which have the ability to elicit a special response on their surface when in contact with biological fluids [
1,
2]. It has been well accepted and agreed that the key feature which leads to the bone-bonding ability of bioactive glasses (and of bioactive materials in general), is the formation of hydroxycarbonate apatite (HСA) on their surface. This layer considerably enhances the interfacial adhesion of the implant to bone tissue and therefore a stable interface is maintained long enough to favor further cellular interaction such as the incorporation of collagen and interaction with other biomolecules and tissue growth factors, which then favor the development of a biological bond with the tissues [
3]. Consequently, this interface requires tailor-made biomaterials with specific and adjustable chemical reactivity, considering the need to match the rates of implant dissolution and tissue growth. Typically, silicate-based bioactive glasses are able in the very early stage of mineralization to form a silica gel layer onto their surface, which is of crucial importance for their bone-bonding behavior. There has been also an alternative opinion which considers the mineralization of HСA layer on the glass surface not critical for the bioactivity of the material [
4], as the ionic dissolution products from the bioactive material appear to stimulate the growth and differentiation of cells at the genetic level, an effect which has been considered to be dose dependent. Despite the controversy related to the importance of HСA layer formation on the surface of bioactive glasses/ceramics, it is still considered to be the marker of bioactivity during the initial screening of biomaterials [
4].
In the last few years, efforts concerning the design and processing of bioactive materials in the system CaO-MgO-SiO
2-Na
2O-P
2O
5-CaF
2 resulted in new series of glasses, which were shown to exhibit high bioactivity in vitro, that is, excellent biomineralization upon immersion in simulated body fluid (SBF), stimulation of osteoblast proliferation in cell culture medium, and no evidence of any toxicity or other detrimental effects in the functionality of cells [
5,
6,
7]. Subsequent clinical trials were successfully undertaken with glass particulates that formed a cohesive mass with patient’s blood, demonstrating its homeostatic effect [
8]. Furthermore, aiming at better quality of a grafting procedure, injectable pastes were produced using a melt-quenched CaO-MgO-SiO
2-Na
2O-P
2O
5-CaF
2 bioactive glass and two organic carriers, namely polyethylene glycol (PEG) and glycerol (gly) [
9]. The prepared homogeneous mixtures appeared in the form of moldable pastes and demonstrated cohesive injectability [
9]. The excellent bioactivity of those pastes in vitro was expressed by high mineralization rates of crystalline hydroxyapatite (HA), which was identified by X-ray diffraction analysis (XRD) already after 12 h of immersion in simulated body fluid (SBF) [
9]. More recent experimental data clearly indicated the fact that the processability and bioactivity of the pastes can be adjusted by the organic carrier. In a recent case study, it was shown the occurrence of chemical bonding between the surface of bioactive glass particulates and glycerol (gly), whereas only physical interactions were detected in the case of organic carrier based on PEG [
10].
The aim of present study was to elucidate the effect of alumina incorporation on the surface mineralization and degradation of bioglass (BG) and a bioglass-glycerol (BG-gly) paste. Within this context, the effect of the synthesis of BG in an alumina crucible (unlike in other reported studies, which used a Pt crucible [
5,
6,
7,
9,
10]), as well as the effect of glycerol interaction with the BG surface on the bioactivity was carefully assessed via SBF tests and extensive structural analysis of the studied materials. It is shown that the synthesis condition of BG affects its network connectivity and consequently alters its bioactivity; moreover, the BG-gly sample exhibits strong chemical interactions between gly and the BG surface, which boost the bioactivity of the paste in comparison to that of the neat BG.
3. Results
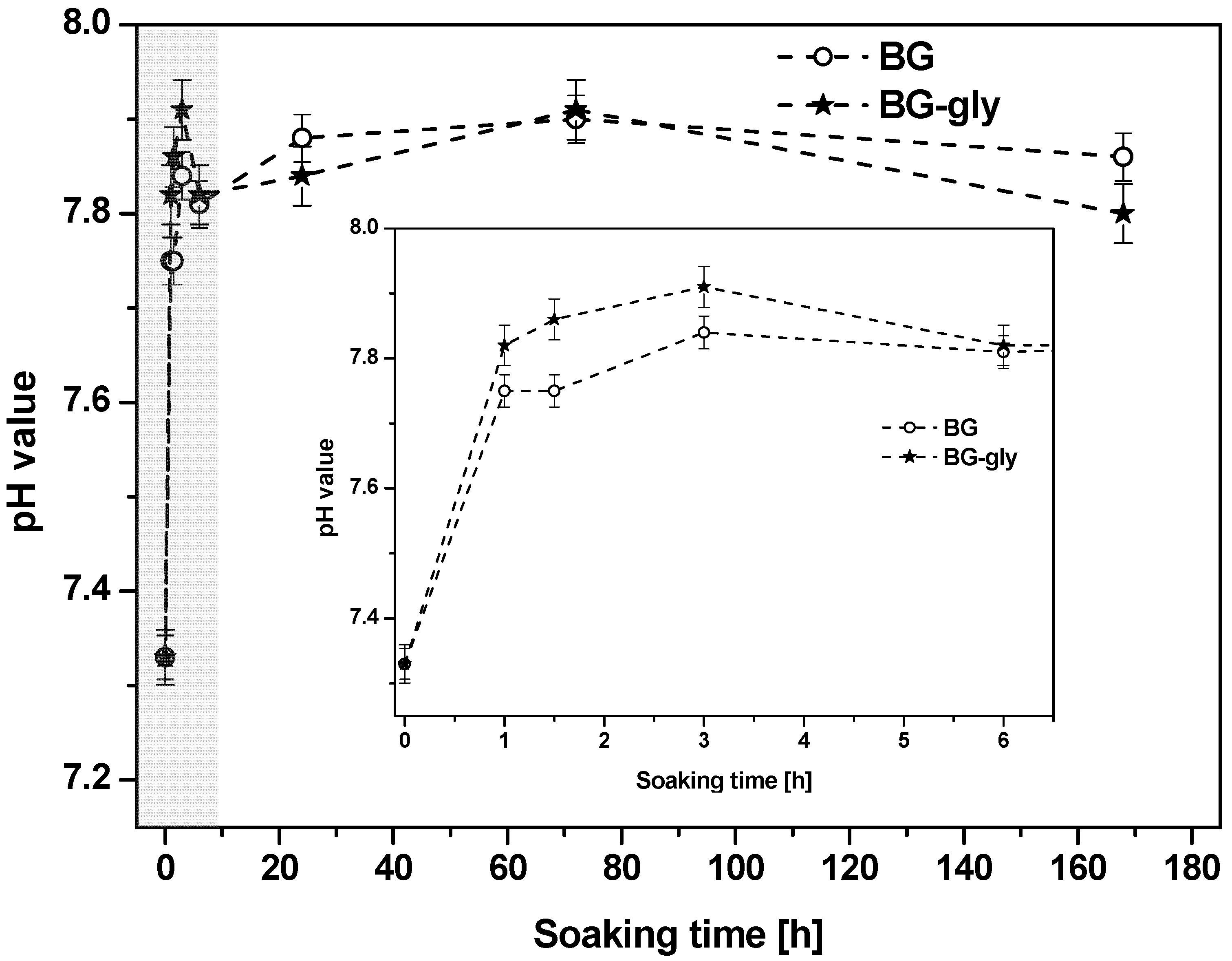
Figure 1 shows the evolution of the pH value of the SBF after testing BG and BG-gly for 1, 1.5, 3, 6, 24, 72 and 168 h. The change in pH occurs immediately after soaking the materials in SBF and is particularly pronounced within the first hour of incubation time. Thus, it seems that the dissolution takes place immediately after immersion in SBF and in all cases alkaline reaction occurred, that is, abrupt increase of pH value (
Figure 1), attributed to the rapid ion exchange between M
+/M
2+ cations from the glass (network modifiers, i.e., M
+ = Na
+ and M
2+ = Ca
2+, Mg
2) with hydronium ions (H
3O
+) from the solution. Subsequently, a steady increase of the pH value is observed over 3 days of soaking in SBF. In this period, namely, between 1 and 3 h of SBF exposure, the pH of the solution of BG-gly appears to be slightly higher than that of BG, whereas no significant difference between BG and BG-gly is observed at longer exposure times. The pH change correlates to the evolution of the ionic concentrations of Ca
2+, Mg
2+, P
5+, Si
4+, Na
+ and Al
3+ cations in the liquid.
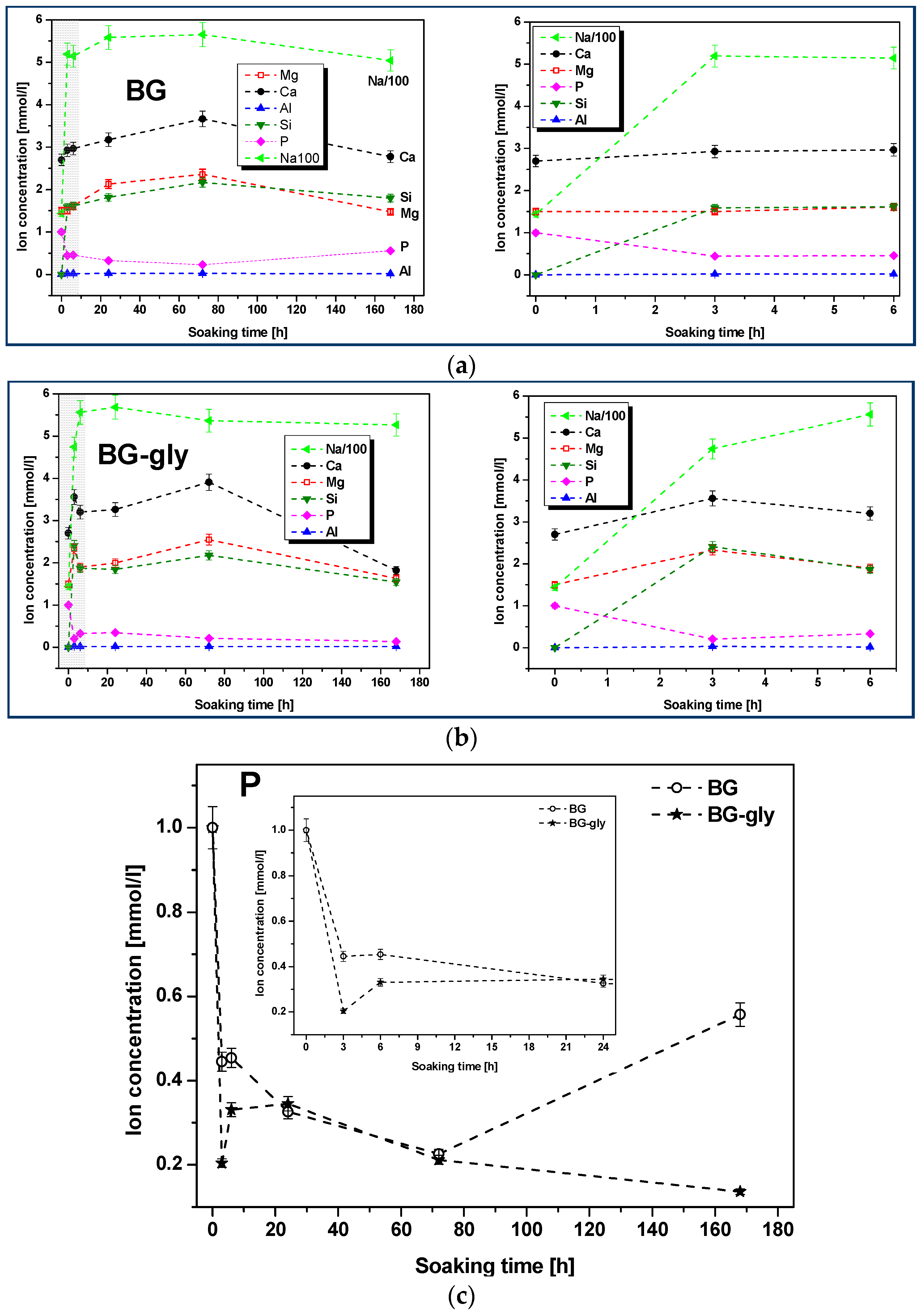
Figure 2a,b shows the plots of the molar concentrations of the mentioned species as functions of the SBF immersion time for BG and BG-gly. It can be observed that the increase of the concentration of the Na
+, Ca
2+ and Mg
2+ cations is steep in the early stage of the immersion. Subsequently, the concentration of Na
+, Ca
2+ and Mg
2+ continuously increased until 72 h immersion in SBF. It is worth mentioning that, although the concentrations of the alkaline/alkaline-earth ions leached from BG and BG-gly into the SBF solution are comparable with each other, the concentrations of Ca
2+ and Mg
2 are quite different. Thus, at the initial stages (e.g., after 3 h) of exposure to SBF, the BG-gly paste featured considerably higher Ca
2+ and Mg
+2 concentrations as compared to the neat BG sample, that is, ca. 25 and 50% higher concentrations, respectively (
Figure 2d,e). The phosphate concentration rapidly decreased in the early stage of the immersion and again, the concentration drop was more pronounced in the case of BG-gly than for the neat BG, indicating the active role of phosphate on the transformation of its surface (
Figure 2c). The concentration of Al
3+ was very low in the SBF solution for both BG and BG-gly, being at the limit of the sensitivity of the ICP-OES apparatus.
The difference in leaching behavior of BG and BG-gly, which has a crucial importance for the mineralization of HA, is likely governed by the surface chemistry, which was assessed by means of XRD and SEM.
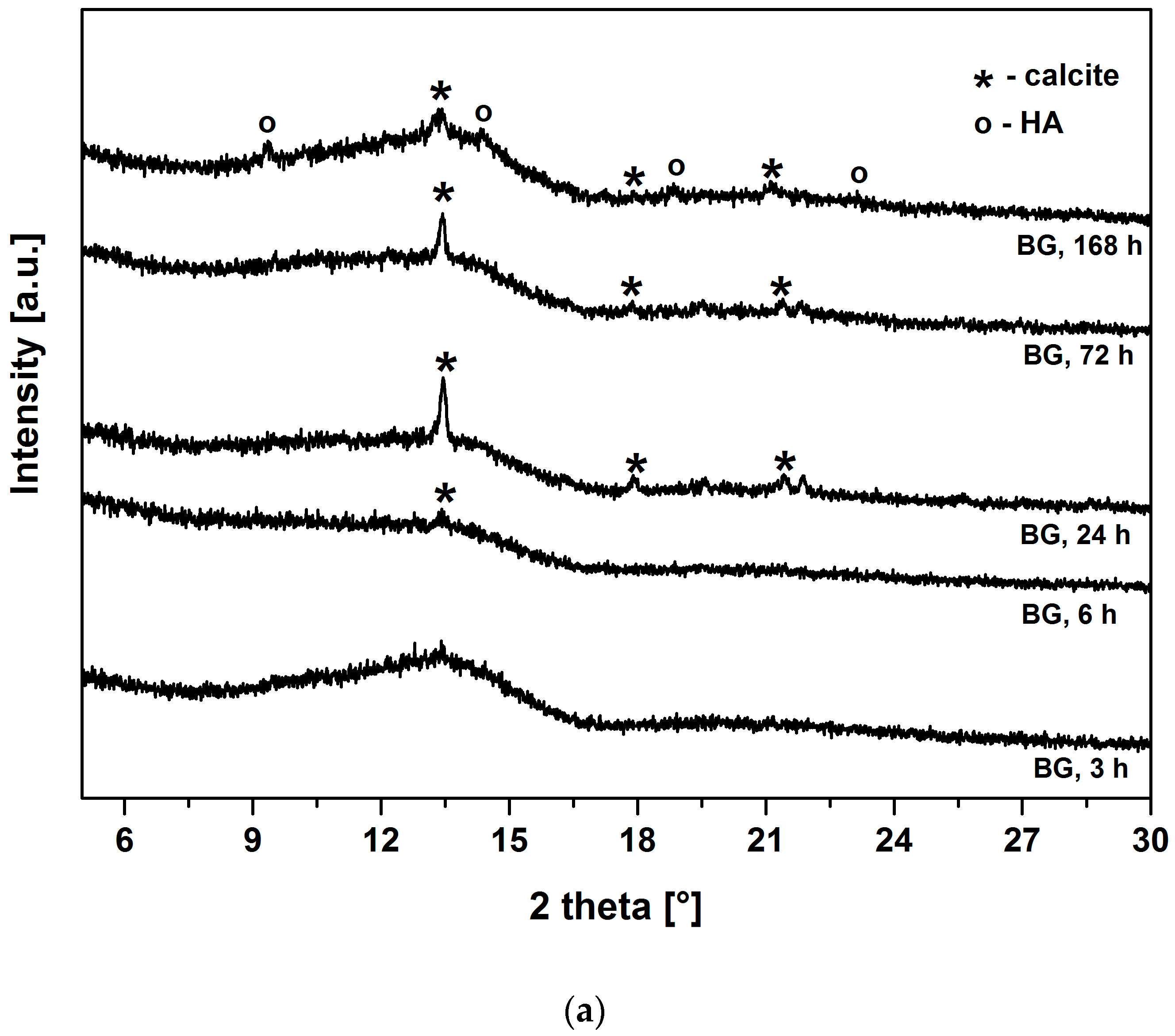
Figure 3a,b show the XRD patterns of BG and BG-gly, respectively, after immersion in SBF for various periods of time. Both BG and BG-gly are nearly featureless after 3 h of immersion; whereas calcite with its main reflection at 2θ = 13.42° was revealed as a primary crystalline phase in BG-gly after 6 h of SBF exposure (
Figure 3b). In the case of BG, the reflections of calcite are clearly distinguishable already after 24 h of immersion in SBF (
Figure 3a). Calcite with its characteristic reflections remains the only crystalline phase at the surface of BG after 72 h of exposure to SBF, although the intensity of the reflections decreases in time. A new reflection at 2θ = 14.57°, was revealed in BG after 168 h of soaking in SBF was assigned to HA and, thus, the surface of BG consisted of calcite and hydroxyapatite (
Figure 3a). In the case of the BG-gly paste, the reflections of the calcite phase completely vanished after 168 h of soaking in SBF and HA was the only crystalline phase at the surface of the BG-gly sample (
Figure 3b).
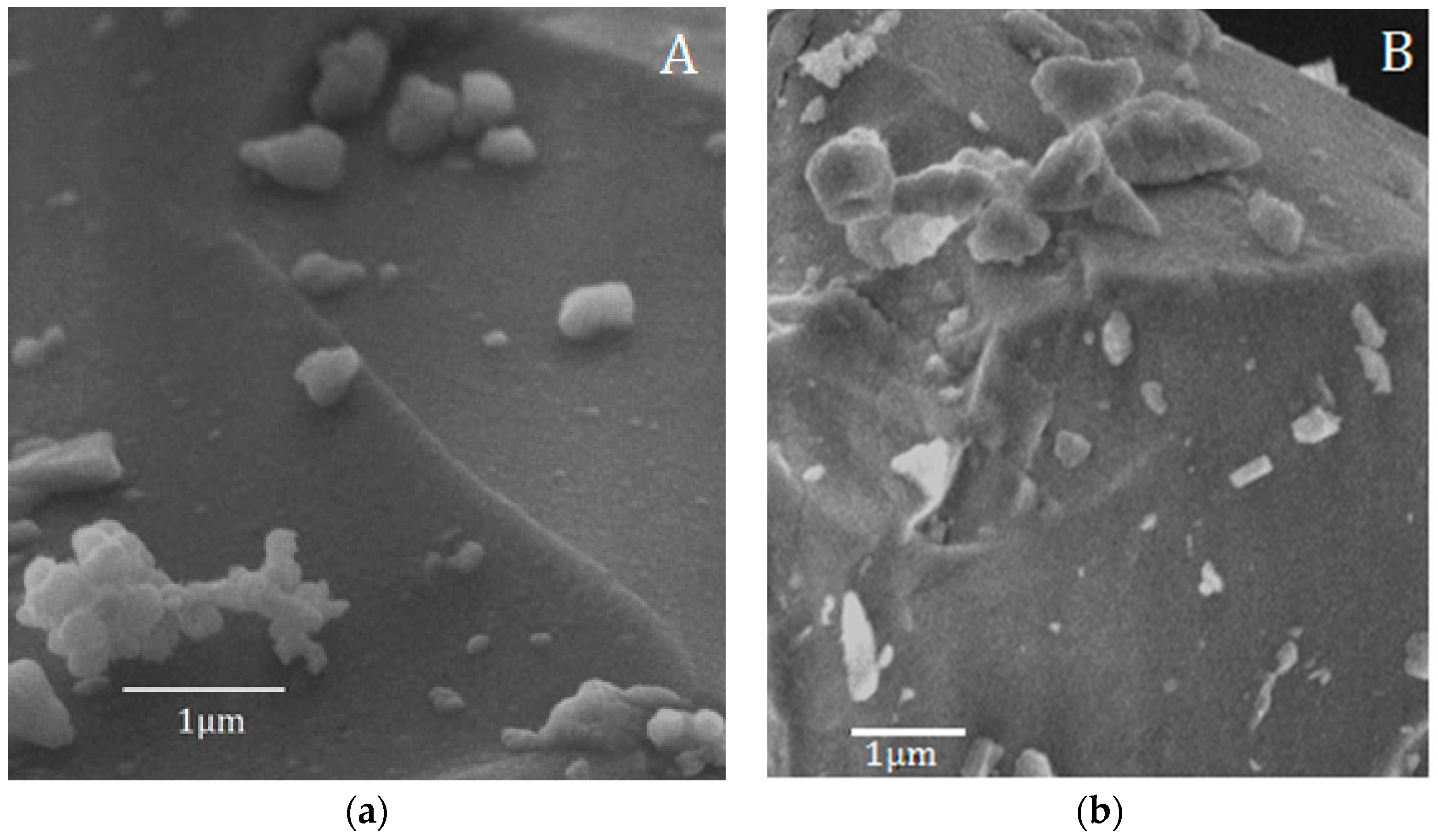
These results are in accordance with the SEM observation of the samples after 168 h of soaking in SBF (
Figure 4), which demonstrated that the surface of the BG-gly sample features submicron precipitates enriched in Ca, P, and Si according to EDS (not shown). EDS from larger areas of the surfaces revealed the presence of Al in both BG (1.33 wt %) and BG-gly (1.37 wt %) samples, clearly evidencing that this element becomes incorporated into the glass structure during its preparation stage in an alumina crucible. This is in very good agreement with observations from Goel et al. [
14], who showed that the aluminium uptake from the alumina crucibles during the glass preparation process may be as high as 1.5–2.0 wt % Al
2O
3 (i.e., 0.8–1.1 wt % Al). It should be noted that the ICP-OES measurements revealed negligible leaching of Al from the samples into the SBF solution. The slightly higher content of Al on the surface of BG and BG-gly (as compared to results of the study from [
14]) is probably related to the leaching of the other ions into SBF solution [
6].
It is well known that alumina may act as a network former or modifier, depending on the ratio between the aluminum cations and the other network modifiers. When fulfilling the function of a network former, aluminum is incorporated within the glassy network in the form of AlO
4 tetrahedra, provided that the total concentration of alkali and/or alkaline earth oxides equals or exceeds that of alumina [
15]. Consequently, each added aluminum ion is able to remove one non-bridging oxygen (NBO) from the glass structure due to (i) the lack of the oxygen provided by alumina and (ii) the necessity to maintain local charge neutrality of the AlO
4 units. As a result, the network connectivity of the glass structure is expected to increase. Moreover, it has been accepted that the presence of Al
3+ increases the chemical durability of glasses by forming Al–OH groups on their surface when in contact with body fluids [
16]. According to Shelby et al. [
15], the addition of alumina to the glass typically improves its durability in neutral solutions, but results in very rapid dissolution in highly acidic solutions due to attack at the Al–O bonds. Hench and Anderson concluded that alumina inhibits HA formation, thereby retarding the mineralization of osteoids into bone tissue [
17]. The formation of Al–OH bonds on the glass surface hinders the apatite formation possibly by the following two mechanisms: (i) it decreases the concentration of silanol groups on the glass surface (which act as nucleating centers for apatite formation); (ii) the Al
2O
3 gel is positively charged at pH = 7.4, while the SiO
2 gel-like layer is negatively charged [
18]. Nevertheless, minor amounts of Al
2O
3 (0.5–2 mol %) may, for instance, improve the sintering ability of bioactive glasses and reduce their crystallization tendency. Thus, Al
2O
3-containing apatite-mullite glass-ceramics have been shown to be biocompatible in vivo [
19], but featured the absence of HA formation on their surface due to the lack of silanol (Si–OH) groups.
Considering the behavior of BG upon SBF exposure in the present study and comparing this with the behavior of the BG prepared in a Pt crucible, we can confirm the literature known effect of Al which improves the network connectivity and consequently pushes down the HA crystallization. We consider that the small amount of Al in the BG glass strengthens the glass network and induces an enrichment of Al–OH groups at the surface of BG upon exposure to SBF, thus suppressing the HA mineralization.
The chemical degradation tests performed for BG and BG-gly in Tris-HCl revealed a considerably mass loss of 2.81 wt % for the BG sample and of 28.46 wt % for BG-gly. Considering that the BG-gly sample consists of 27 wt % glycerol (which gets dissolved upon SBF immersion), we can estimate the mass loss of BG in the BG-gly paste to ca. 1.46 wt %. This can be explained by the fact that upon immersion of BG-gly into SBF, first glycerol should get dissolved; as some glycerol is chemically bonded to the surface of BG [
10], one may speculate that the chemically bonded BG might be responsible for the lower mass loss observed for the BG-gly samples. In the case that the chemically bonded glycerol also gets leached into SBF (in time), the lower mass loss of BG-gly as compared to that of BG may be considered as a consequence of the lower dissolution rate of BG in BG-gly due to the presence of the surface-bonded glycerol. However, the chemically bonded glycerol may not get released upon SBF exposure and thus the mass loss of the BG-gly sample might be even lower than 1.46 wt %. Interestingly, the pH value of the solution after immersion for 120 h in Tris-HCl increased for both investigated samples from the initial value of 7.4 to the value of 8.7.
4. Discussion
The mechanism of apatite formation is the subject of much scientific interest and can be described as occurring through a series of various chemical reactions. As the result of ion exchange, the glass forms Si-OH groups on its surface. Simultaneously with the ion exchange, water directly attacks the glass network (so-called congruent dissolution [
15]). The release of alkali and alkaline earth ions from the glass causes/affects the crystallization kinetics of HA, while the presence of the silanol groups on the surface of the glass is of crucial importance to induce (i) heterogeneous nucleation of apatite and (ii) the release of Ca ions from the materials. As a consequence, amorphous calcium phosphates are formed on the surface of the glass and convert subsequently to crystalline HA [
1,
2,
20].
In our previous study [
9], BG melt glass was prepared in a Pt crucible and its bioactivity was studied upon SBF exposure. Both BG and the corresponding BG-gly paste featured high bioactivity in vitro and showed the formation of crystalline hydroxyapatite (HA) already after 12 h immersion in SBF. In the present study, the same BG glass prepared in alumina crucible and the corresponding BG-gly paste have been shown to contain small amounts of aluminum in their network. As a consequence, the mineralization of HA on the surface of the BG samples has been suppressed. Likewise, ZnO was reported to suppress in a similar manner the HA mineralization upon SBF exposure [
21]. The reason for this behavior was considered to rely on the significant decrease of the release of silicon from the glass-ceramic as well as a lack of silanol groups that caused suppression of apatite formation on its surface. In the absence of ZnO or at low contents, the glass-ceramic was able to show HA mineralization upon SBF exposure [
21].
The release of different species to solution has been known to depend on the strength of cation-oxygen bonds that must be broken to generate a detached unit that can be solvated and released into the solution. The experimental observation revealed that, for instance, sodium bonded to silicate groups is released more easily than sodium bonded to aluminate groups in aluminosilicate glasses [
22]. This may be a reason of the alteration in the HA mineralization behavior of BG and BG-gly prepared in this study as compared to those based on BG prepared in Pt crucible.
The surface of both materials from the present study (BG and BG-gly) apparently is able to promote HA precipitation. The XRD results (
Figure 3) indicate that calcite has been formed at the expenses of or concurrently to HA at the early stage of immersion in SBF. Calcite precipitation might occur due to depletion in PO
43− ions followed by increase in Ca/P ratio and the saturation of the solution with respect to CaCO
3 [
23]. However, the calcite formation might be also attributed to the effect of the particle size of BG [
24] (as the mean particle size of the BG glass synthesized in alumina crucible was about twice smaller than of the BG glass prepared in Pt crucible [
9]).
One important and intriguing result of the present study is the bioactivity of the BG-gly paste, which was higher than that of the neat BG. Despite the BG-gly sample also showing early calcite precipitation, at SBF exposure time longer than three days HA mineralization is observed, and after SBF exposure of seven days HA is the only crystalline phase present on the surface of BG.
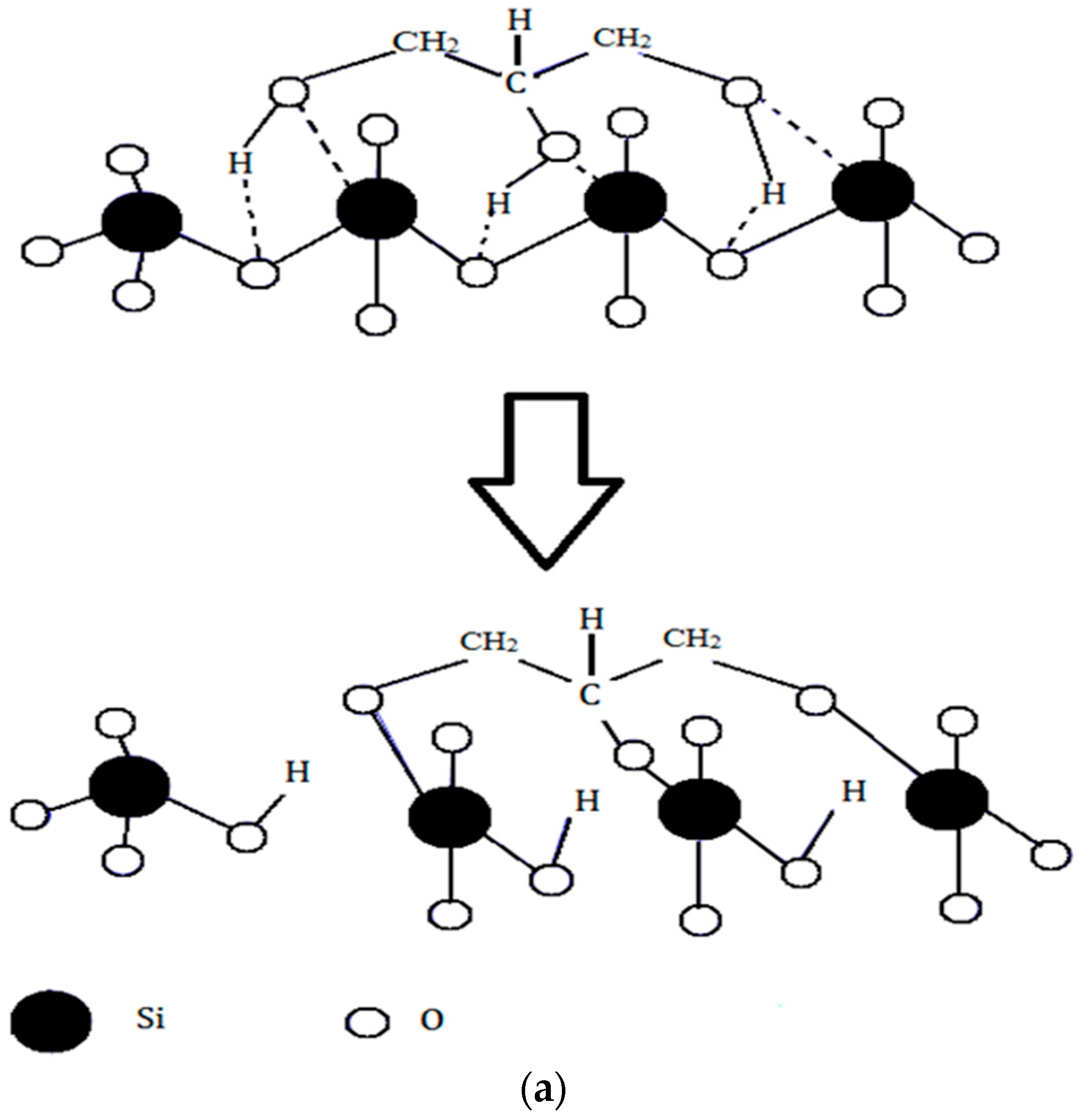
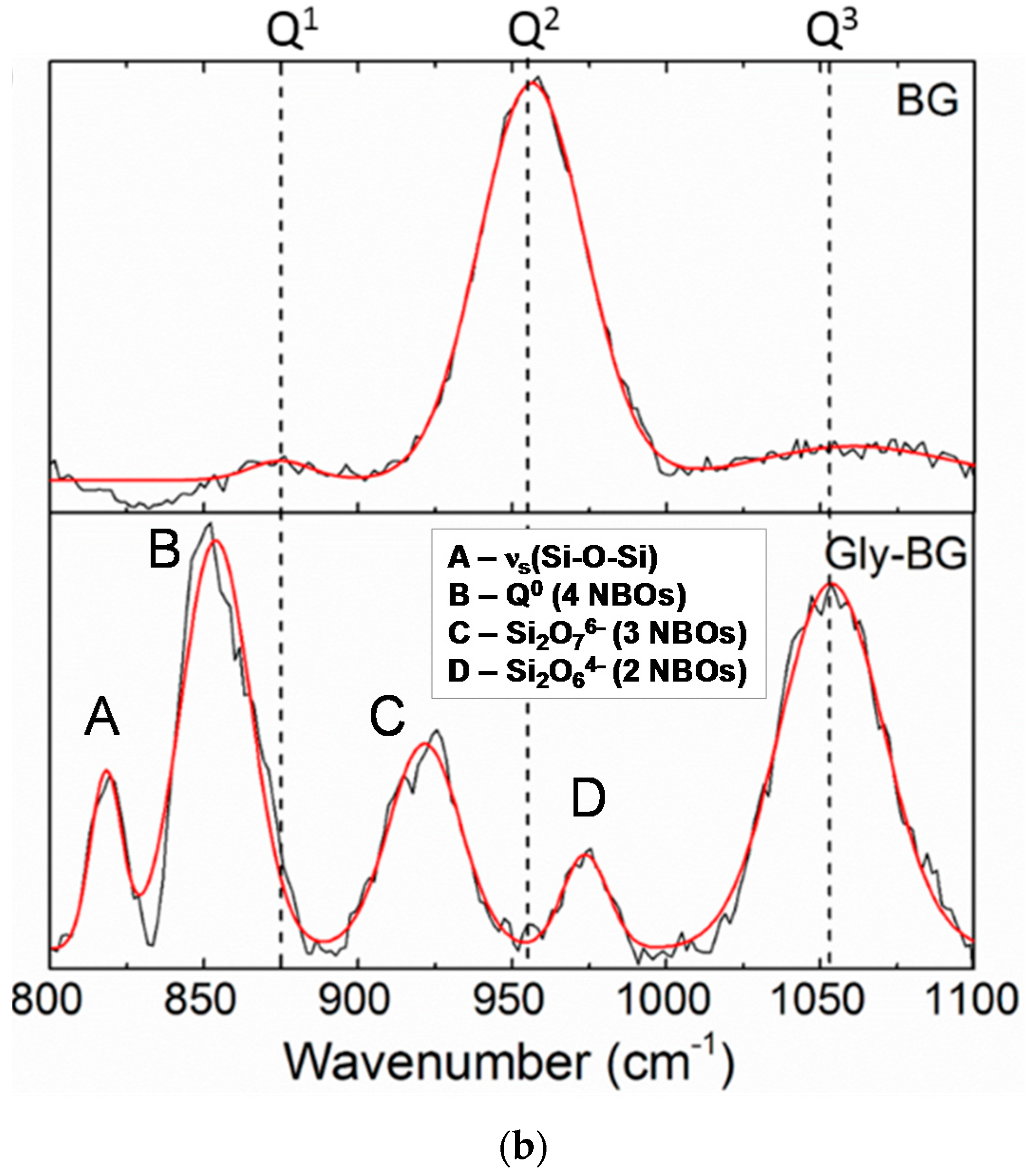
The difference in the in vitro behavior between BG and BG-gly can be explained by structural features of the BG-gly paste. The results of our previous spectroscopic study on BG-gly [
10] clearly indicate that glycerol chemically interacts with the surface of the BG particles. Typically, the reaction between gly and the BG surface can be described in a similar way as the reaction between siloxane bonds and alcohols, which was studied in the past by various researchers [
25,
26]. Thus, glycerol probably reacts with the BG surface to generate alkoxy-like, as well as silanol groups (
Figure 5a). Assuming a Q
2 site at the surface reacting with one molecule of gly, one can expect that through the alcoholysis of a Si-O bond a Q
3 site is created in addition to a silanol group. This is supported, for example, by Raman spectroscopic data of the BG-gly paste (
Figure 5b), which show significant differences between the BG-gly glass network and that of BG. Thus, the BG network consists predominantly of Q
2 sites (absorption band at ca. 950 cm
−1 [
10]) in addition to small amounts of Q
1 (ca. 875 cm
−1) and Q
3 (ca. 1050 cm
−1) sites. Unlike BG, the network in BG-gly shows the band at 1050 cm
−1 assigned to Q
3 species being significantly enhanced as compared to the as-prepared BG [
10]. Moreover, other intensive absorption bands at ca. 975 cm
−1 (Si
2O
64− units, possessing two non-bridging oxygens, NBOs), 925 cm
−1 (Si
2O
76− units with three NBOs), 875 cm
−1 (Q
0, i.e., four NBOs) and ~825 cm
−1 (symmetric ≡Si–O–Si≡ stretching vibration) [
27,
28] have been observed; whereas the band assigned to the Q
2 units of BG disappeared upon interaction between BG and glycerol (
Figure 5b). This indicates that the interaction of gly with BG leads to a significantly less polymerized glass network (at least at the glass surface) and to an increased number of silanol groups. As an obvious consequence, the bioactivity of the BG-gly sample is significantly improved as compared to that of the neat BG. This may also explain the faster leaching kinetics of Ca
2+ and Mg
+2 in BG-gly, as compared to the neat BG.
Considering the ion release of the samples upon exposure to SBF, as well as their mineralization behavior, it is very intriguing that the BG sample mineralizes calcite together with hydroxyapatite; whereas the BG-gly sample shows the intermediate precipitation of calcite, which is then consumed/dissolved and, after 172 h of immersion in SBF, only HA is present on the surface of BG-gly. Firstly, the precipitation of calcite is somehow surprising, as the composition of the simulated body fluid indicates supersaturation conditions with respect to HA and, in fact, undersaturation conditions concerning calcite. However, it was shown in various studies that a strong release of Ca
2+ from the bioactive glasses during the SBF test correlated with an abrupt PO
43− depletion may create supersaturation conditions for calcite [
23,
24,
29,
30,
31]. This may be the explanation also in the present case, as supported by the evolution of the ion concentrations of PO
43− and Ca
2+ (
Figure 2c,d, respectively). It is shown that the concentration of Ca
2+ in SBF strongly increases within the first 72 h for both samples, BG and BG-gly, that is, from 2.7 mmol/L to 3.6 and 3.9 mmol/L, respectively (
Figure 2c); at the same time, PO
43− ion concentration significantly decreases from 1 mmol/L to 0.22 and 0.21 mmol/L, respectively. Thus, it seems that the ion release behavior of both samples leads to favorable saturation conditions for calcite, which is observed to mineralize on their surface. Even more intriguing is the fact that BG-gly exhibits only HA on its surface after 168 h of immersion in SBF (
Figure 3b), whereas in the case of BG sample, calcite and HA coexist on its surface (
Figure 3a). The coexistence of calcite and HA on the surface of bioactive glasses exposed to SBF was reported in several studies. It was stated that calcite and HA co-precipitate in the very early stage of SBF immersion for glasses exhibiting high Ca
2+ release ability [
24], and thus both phases are present on their surface upon SBF exposure. This was shown for various bioactive glasses [
24,
29,
31] as well as for our BG sample in the present study. However, the BG-gly system showed the disappearance of calcite and the presence of only HA upon 168 h of SBF immersion. This is a rather intriguing behavior which has not been reported so far. Various studies in the literature reported on the conversion of calcium carbonate (aragonite or calcite) into hydroxyapatite, for example, [
32,
33]. However, this process was typically carried out under hydrothermal conditions, namely, temperatures of ca. 150–250 °C and pressures up to 100 MPa [
33]. Nevertheless, some studies can be found in literature reporting on the conversion of calcium carbonate into hydroxyapatite at room temperature in phosphate buffer solutions [
34,
35]. The conversion of calcium carbonate into HA is believed to rely mainly on the Ca ion release from the carbonate as well as on the presence of (hydrogen) phosphate ions in the solution. Moreover, the pH value of the solution seems to play a significant role: in a case study, it was shown that HA is generated from aragonite at pH values in the range from 7.4 to 8.0, whereas a lower pH values other calcium phosphate phases were generated [
35].
In the present case, it is intriguing that BG shows the coexistence of calcite and HA, whereas BG-gly shows only the presence of HA on its surface. Considering the evolution of the pH of the SBF solution for both samples, it can be concluded that, beside the first few hours of exposure, both solutions show similar pH values. Thus, the difference in the surface mineralization of the samples can rely only on differences with respect to the ion release behavior. Indeed, as shown in
Figure 2c, there is a clear difference between BG and BG-gly with respect to the release of PO
43−: the concentration of PO
43− in the SBF solution of BG-gly decreases continuously from 3 h to 168 h of exposure to SBF test, indicating a continuous deposition of phosphate-containing phases on the surface of BG. On the other hand, in the case of the BG sample, the concentration of PO
43− in the SBF solution drastically increases at exposure time higher than 72 h, indicating that the crystallization of phosphate phases on the surface of BG is suppressed.
Based on the obtained results, we assume that both samples, that is, BG and BG-gly, mineralize calcite on their surface in the early stage of their SBF exposure due to the strong release of Ca
2+ and PO
43− which probably creates (super)saturation conditions for calcite. At exposure times longer than 72 h, the BG and BG-gly behavior is different. Thus, the sample BG shows the coexistence of HA and calcite after 168 h of soaking in SBF; this can be explained by the conversion of calcite into HA, which is a dissolution and re-precipitation process [
34]. The evolution of the PO
43− concentration in the SBF solution from 72 h to 168 h of exposure time indicate that probably two concurrent processes occur, that is, PO
43− dissolution from the glass and phosphate phases/HA precipitation, the first being dominating. Unlike in BG, the phosphate depletion in the SBF solution of BG-gly is continuous over the time; consequently, the precipitation of phosphate phases/HA on the surface of BG-gly seems to be dominating and overlaps the dissolution of phosphate from the glass. It seems that the modification of the BG surface with glycerol induces the difference in its mineralization behavior, as discussed above. However, more detailed investigation is needed in order to elucidate the precise reason of the selective HA mineralization on the surface of BG-gly.