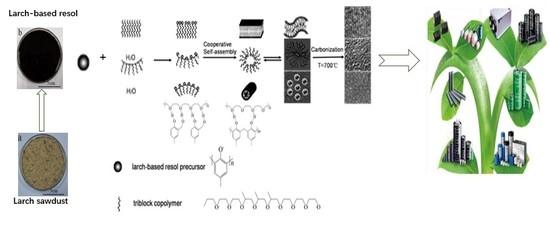
Facile Control of the Porous Structure of Larch-Derived Mesoporous Carbons via Self-Assembly for Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Ordered Larch-Based Mesoporous Carbons
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
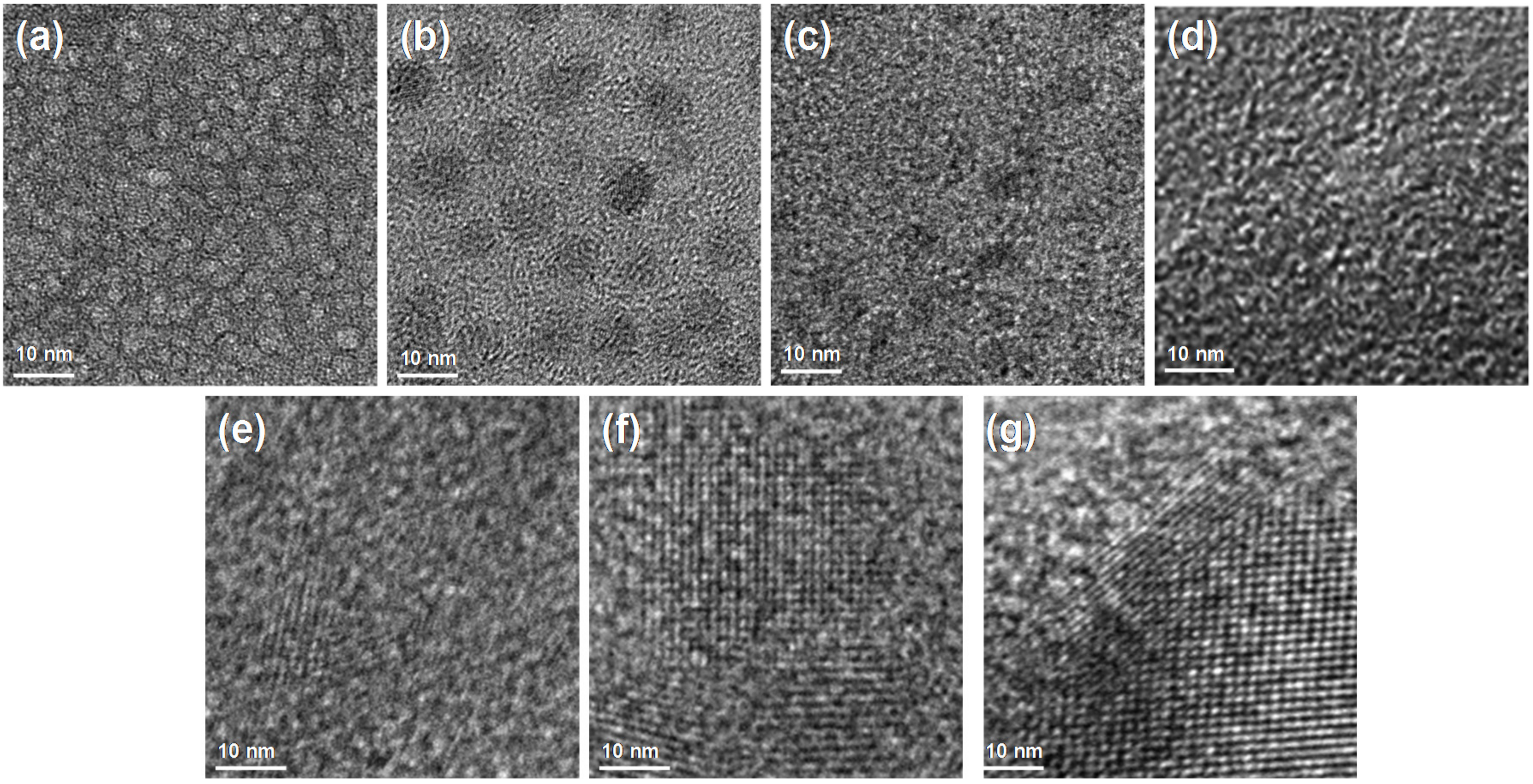
3.1. Structural and Textural Properties of Mesoporous Carbons
3.2. Formation Mechanism of Mesoporous Carbons
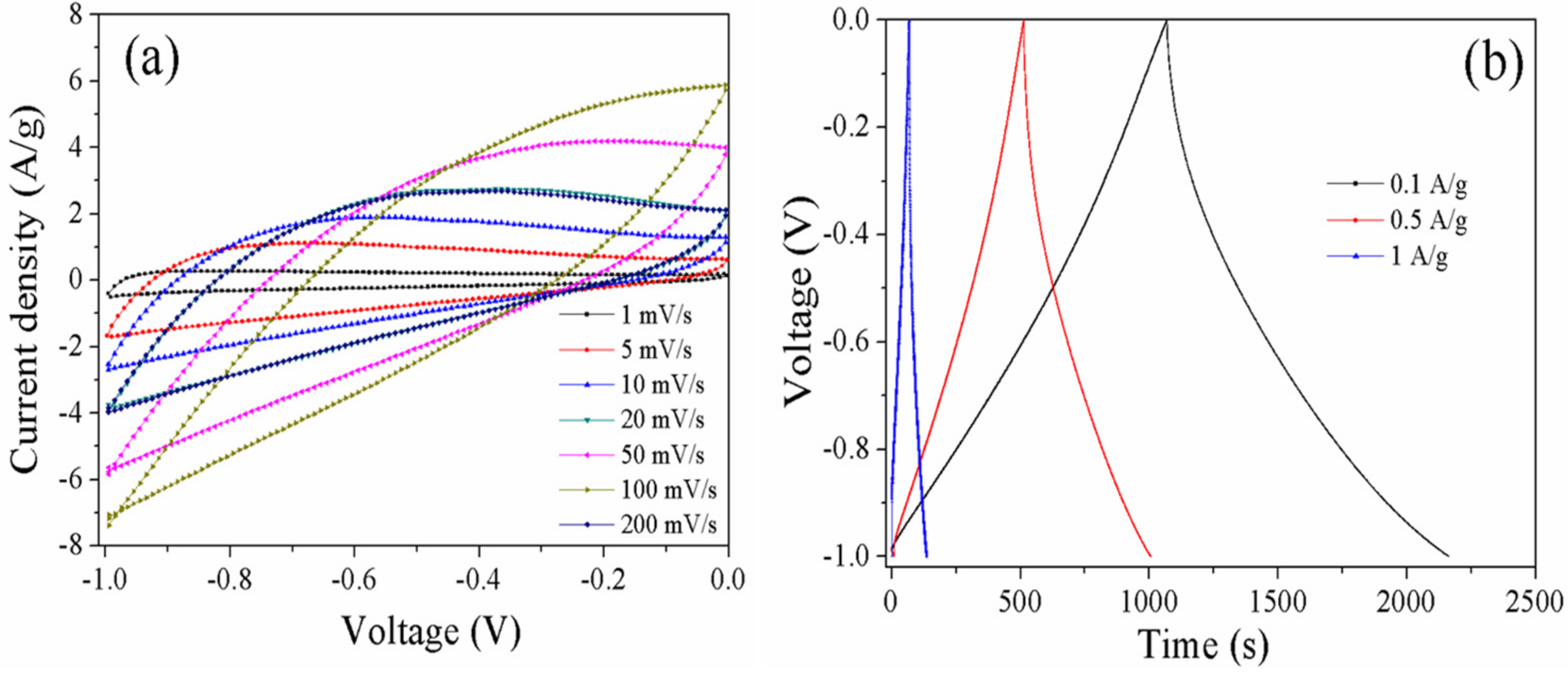
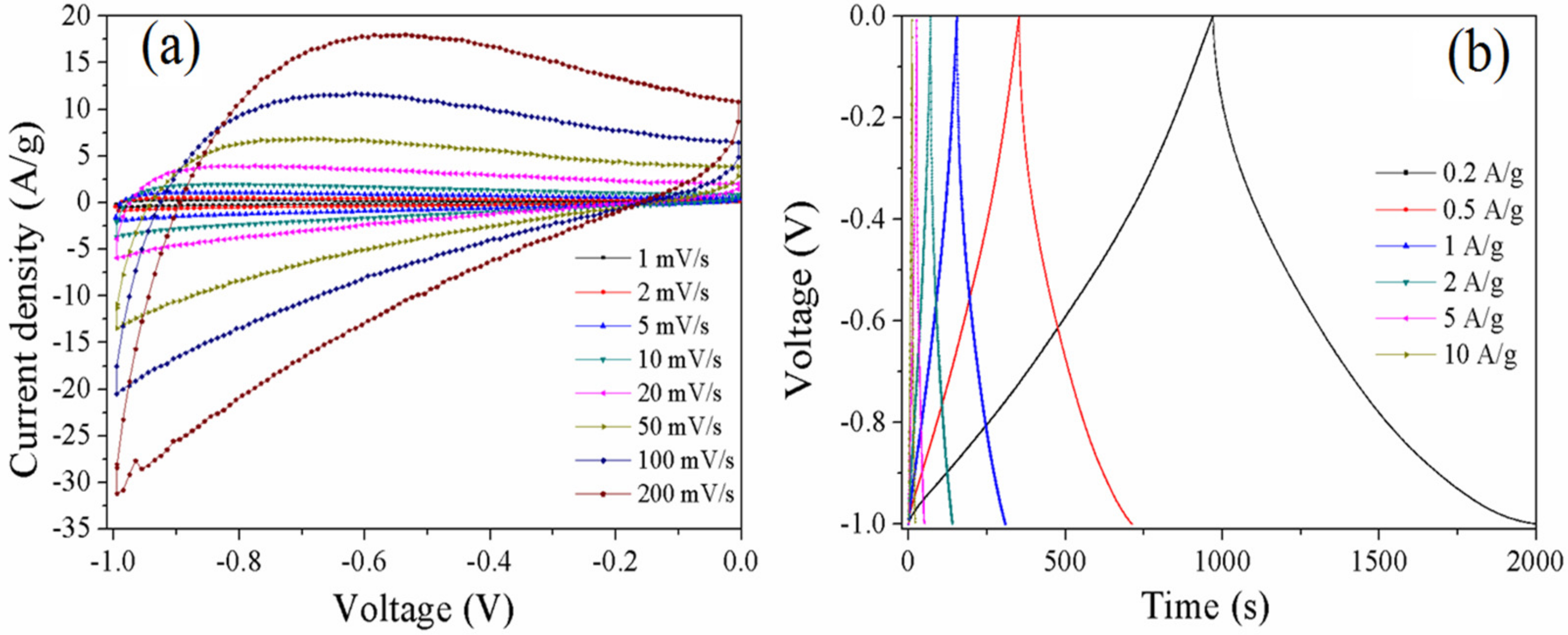
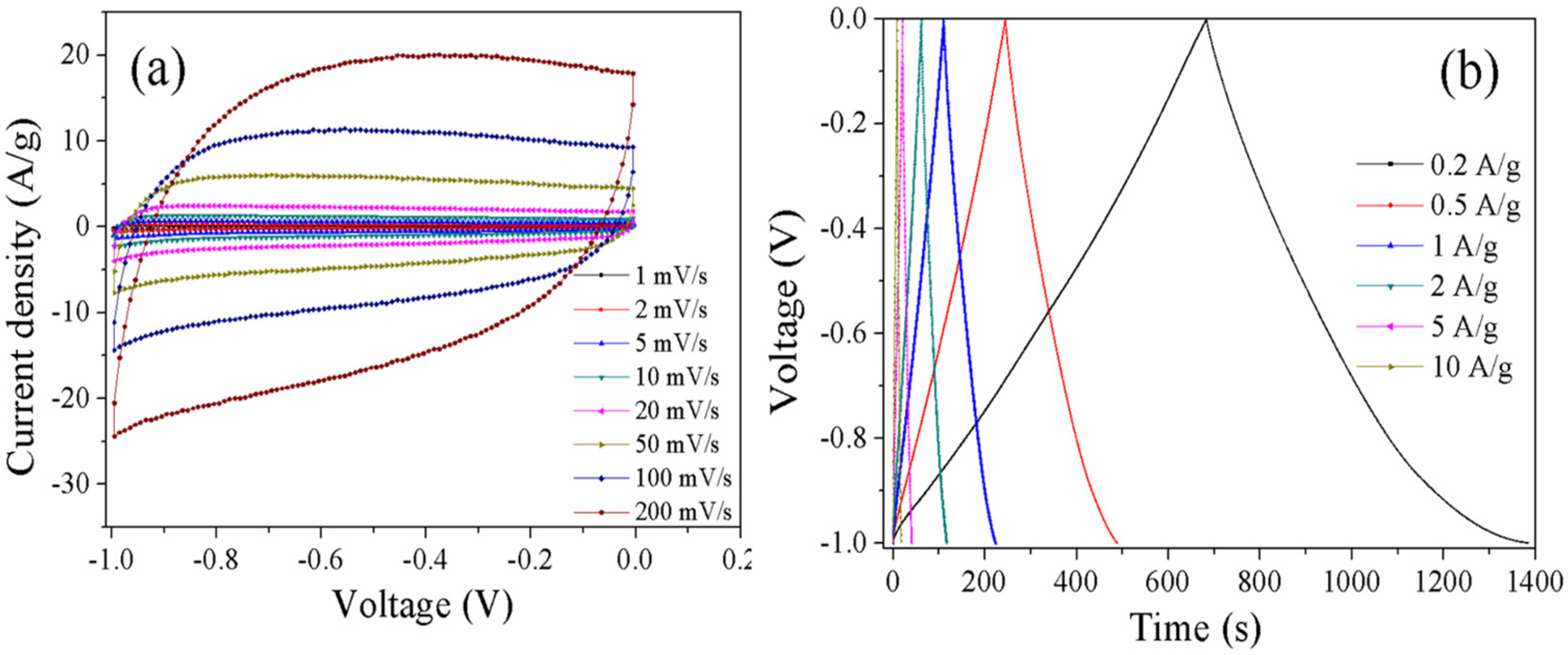
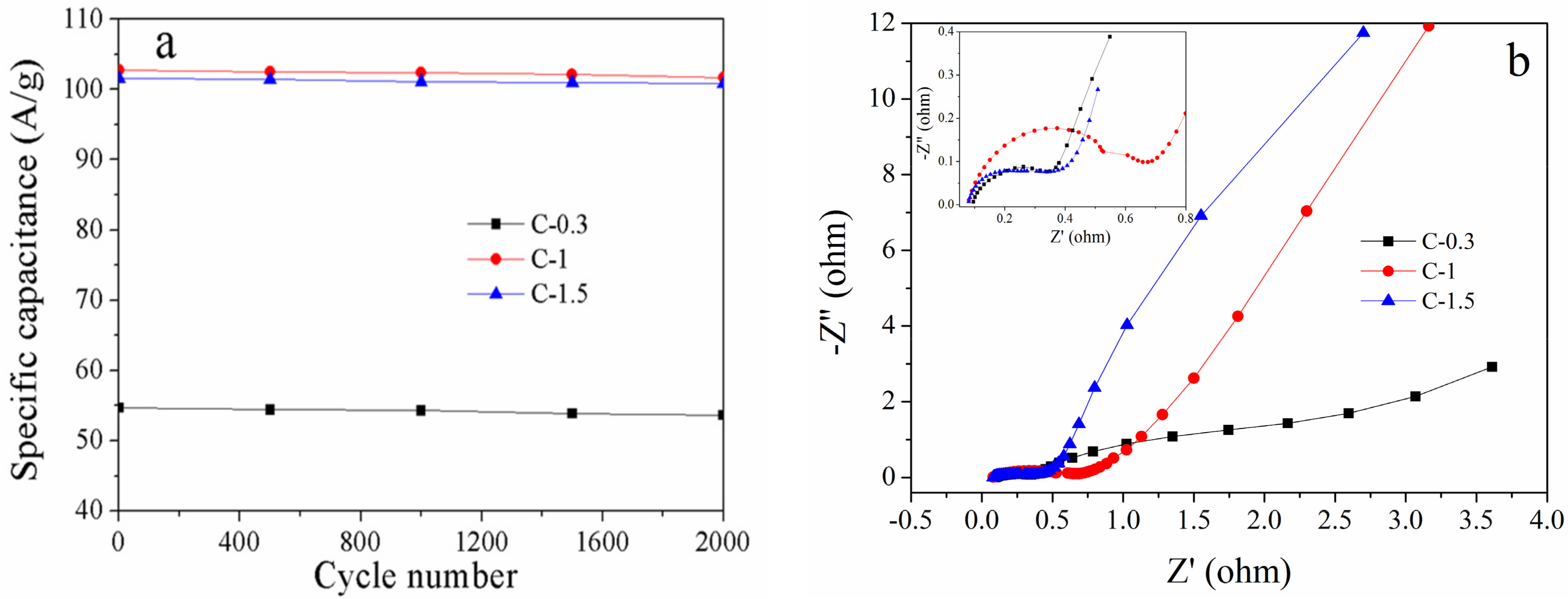
3.3. Electrochemical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.C.; Zhai, K.; Wang, G.; Li, Q.; Guo, P. Preparation and Electrocapacitive Properties of Hierarchical Porous Carbons Based on Loofah Sponge. Materials 2016, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Ma, H.; Wang, J.X.; Xie, Y.Y. Porous carbon nanosheets from coal tar from high-performance supercapacitors. J. Power Sources 2017, 357, 41–46. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Sun, L.X.; Xu, F.; Zou, Y.J.; Chu, H.L.; Zhu, M. Synthesis of N-doped hierarchical carbon spheres for CO2 capture and supercapacitors. RSC Adv. 2016, 6, 1422–1427. [Google Scholar] [CrossRef]
- Fletcher, S.; Kirkpatrick, I.; Dring, R.; Puttock, R.; Howroyd, S. The modelling of carbon-based supercapacitors: Distributions of time constants and Pascal Equivalent Circuits. J. Power Sources 2017, 345, 247–253. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Zou, L.; Wang, F.; Wang, X.; Yao, X. Nanocellulose-derived highly porous carbon aerogels for supercapacitors. Carbon 2016, 99, 203–211. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring porosity in carbon materials for supercapacitor applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.J.; Tong, X.; Zhong, L.X.; Sun, R.C. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Sevilla, M. High-surface area carbons from renewable sources with a bimodal micro-mesoporosity for high-performance ionic liquid-based supercapacitors. Carbon 2015, 94, 41–52. [Google Scholar] [CrossRef]
- Li, W.Q.; Liu, S.; Pan, N.; Zeng, F.J.; Liang, Y. Post-treatment-free synthesis of highly mesoporous carbon for high-performance supercapacitor in aqueous electrolytes. J. Power Sources 2017, 357, 138–143. [Google Scholar] [CrossRef]
- Ahmed, A.; Surjith, A.; Kateryna, B.; Mohan, V.J. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar]
- Bhattacharjya, D.; Kim, M.S.; Yu, J.S. High performance supercapacitor prepared from hollow mesoporous carbon capsules with hierarchical nanoarchitecture. J. Power Sources 2013, 244, 799–805. [Google Scholar] [CrossRef]
- Ma, X.; Gan, L.; Liu, M.; Tripathi, P.K.; Chen, L. Mesoporous size controllable carbon microspheres and their electrochemical performances for supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 8407–8415. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.M.; He, Y.; Liu, N.; Tan, T.; Liang, C. Biomass Derived Nitrogen-Doped Highly Porous Carbon Material with a Hierarchical Porous Structure for High-Performance Lithium/Sulfur Batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, F.; Dou, Y.; Tu, B.; Zhao, D. A comprehensive study on KOH activation of ordered mesoporous carbons and their supercapacitor application. J. Mater. Chem. 2012, 22, 93–99. [Google Scholar] [CrossRef]
- Ma, T.Y.; Liu, L.; Yuan, Z.Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.D.; Yang, Z.X.; Mokaya, R. Templated nanoscale porous carbons. Nanoscale 2010, 2, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent progress in soft-templating of porous carbon materials. Soft Matter 2012, 8, 10801–10812. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Celzard, A. Easy and eco-friendly synthesis of ordered mesoporous carbons by self-assembly of tannin with a block copolymer. Green Chem. 2016, 18, 3265–3271. [Google Scholar] [CrossRef]
- Wang, C.J.; Wu, D.P.; Wang, H.J.; Gao, Z.Y.; Jiang, K. Nitrogen-doped two-dimensional porous carbon sheets derived from clover biomass for high-performance supercapacitors. J. Power Sources 2017, 363, 375–383. [Google Scholar] [CrossRef]
- Nelson, K.M.; Mahurin, S.M.; Mayes, R.T.; Teague, C.M.; Dai, S. Preparation and CO2 adsorption properties of soft-templated mesoporous carbons derived from chestnut tannin precursors. Microporous Mesoporous Mater. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Xu, F.; Chen, Y.; Tang, M.; Wang, H.; Deng, J.; Wang, Y. Acid induced self-assembly strategy to synthesize ordered mesoporous carbons from biomass. ACS Sustain. Chem. Eng. 2016, 4, 4473–4479. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; Wu, Y.; Zhao, X.; Liu, S. Honeycomb carbon foams with tunable pore structures prepared from liquefied larch sawdust by self-foaming. Ind. Crops Prod. 2015, 64, 215–223. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Liu, S.X. Coupled soft-template/hydrothermal process synthesis of mesoporous carbon spheres from liquefied larch sawdust. Mater. Lett. 2013, 107, 5–8. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Zhang, S.; Liu, L.; Liu, S.X. Facile fabrication of hollow and honeycomb-like carbon spheres from liquefied larch sawdust via ultrasonic spray pyrolysis. Mater. Lett. 2015, 157, 135–138. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Liu, S. Ordered mesoporous carbon membrane prepared from liquefied larch by a soft method. Mater. Lett. 2014, 126, 174–177. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.Q.; Wan, Y.; Stein, A.; Zhao, D.Y. A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Meng, Y.; Gu, D.; Yan, Y.; Zhao, D.Y. An aqueous cooperative assembly route to synthesize ordered mesoporous carbons with controlled structures and morphology. Chem. Mater. 2006, 18, 5279–5288. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, W.; Liu, S.X. A porous carbon foam prepared from liquefied birch sawdust. J. Mater. Sci. 2012, 47, 1977–1984. [Google Scholar] [CrossRef]
- Jin, J.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Pore structure and pore size controls of ordered mesoporous carbons prepared from resorcinol/formaldehyde/triblock polymers. Microporous Mesoporous Mater. 2009, 118, 218–223. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous electrode materials for supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Tak, J.K.; Holt, C.M.B.; Stephenson, T.; Mitlin, D. Supercapacitors based on carbons with tuned porosity derived from paper pulp mill sludge biowaste. Carbon 2013, 57, 317–328. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Wang, Y.; Wei, T.; Jing, X. Three-dimensional flower-like and hierarchical porous carbon materials as high-rate performance electrodes for supercapacitors. Carbon 2014, 67, 119–127. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, H.; Lin, Z.; Yin, J.; Lu, H.; Zhao, M. 3D hierarchical porous carbon for supercapacitors prepared from lignin through a facile template-free method. ChemSusChem 2015, 8, 2114–2122. [Google Scholar] [CrossRef] [PubMed]

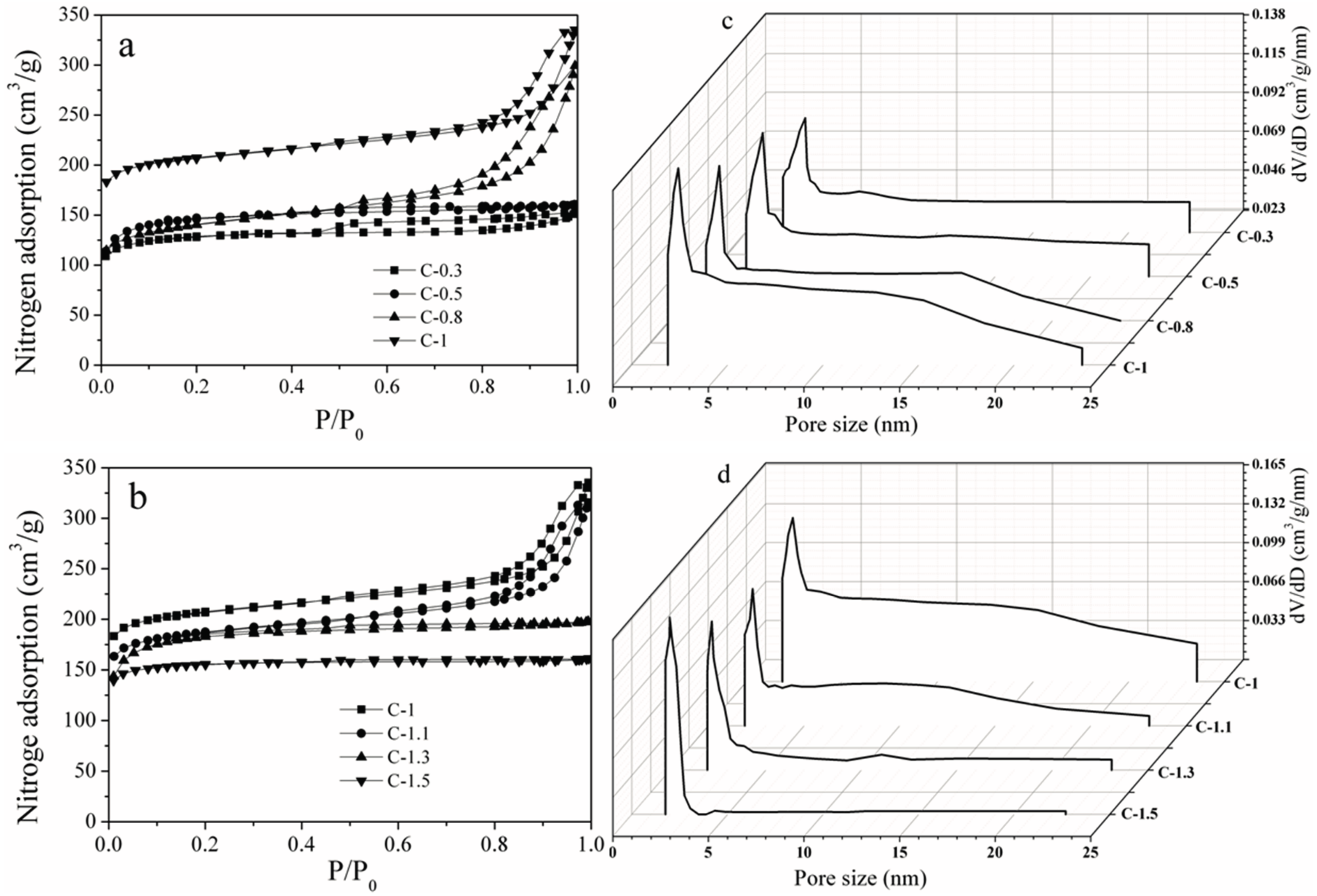

| Sample | SBET (m2/g) | Smeso/SBET (%) | Smicro/SBET (%) |
|---|---|---|---|
| C-0.3 | 393 | 14 | 86 |
| C-0.5 | 410 | 17 | 83 |
| C-0.8 | 421 | 20 | 80 |
| C-1 | 634 | 20 | 80 |
| C-1.1 | 601 | 18 | 82 |
| C-1.3 | 569 | 14 | 86 |
| C-1.5 | 475 | 12 | 88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, W.; Chen, H.; Wang, S.; Kong, F.; Liu, S. Facile Control of the Porous Structure of Larch-Derived Mesoporous Carbons via Self-Assembly for Supercapacitors. Materials 2017, 10, 1330. https://doi.org/10.3390/ma10111330
Zhao X, Li W, Chen H, Wang S, Kong F, Liu S. Facile Control of the Porous Structure of Larch-Derived Mesoporous Carbons via Self-Assembly for Supercapacitors. Materials. 2017; 10(11):1330. https://doi.org/10.3390/ma10111330
Chicago/Turabian StyleZhao, Xin, Wei Li, Honglei Chen, Shoujuan Wang, Fangong Kong, and Shouxin Liu. 2017. "Facile Control of the Porous Structure of Larch-Derived Mesoporous Carbons via Self-Assembly for Supercapacitors" Materials 10, no. 11: 1330. https://doi.org/10.3390/ma10111330