Enhancement of Osteoblastic-Like Cell Activity by Glow Discharge Plasma Surface Modified Hydroxyapatite/β-Tricalcium Phosphate Bone Substitute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
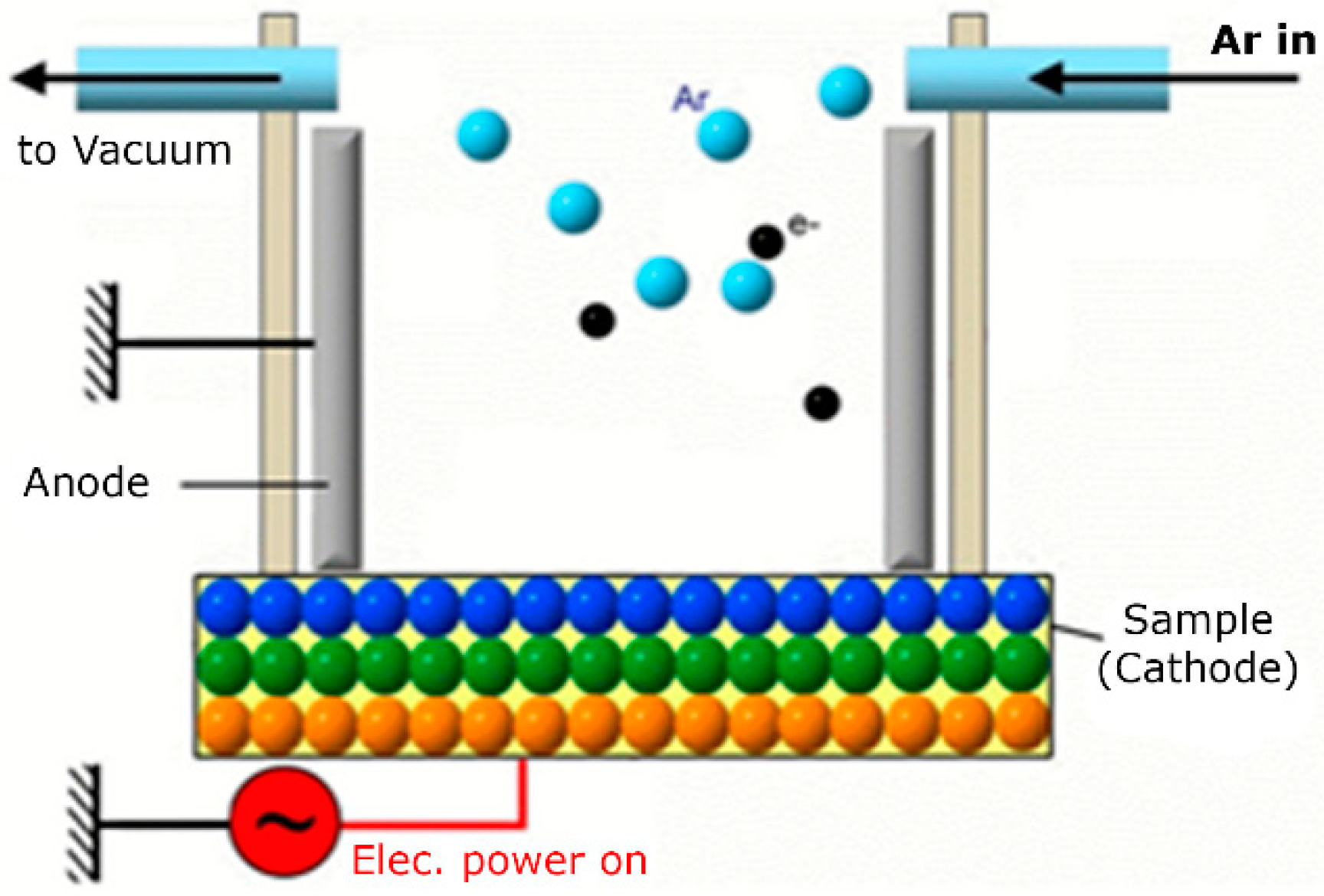
2.2. GDP Sputtering
2.3. Surface Topography Evaluations
2.4. Energy Dispersive Spectrometry
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. X-ray Diffraction Analyses
2.7. Cell Viability Assays
2.8. Cell Morphology
2.9. Alkaline Phosphatase Assays
2.10. Statistical Analyses
3. Results
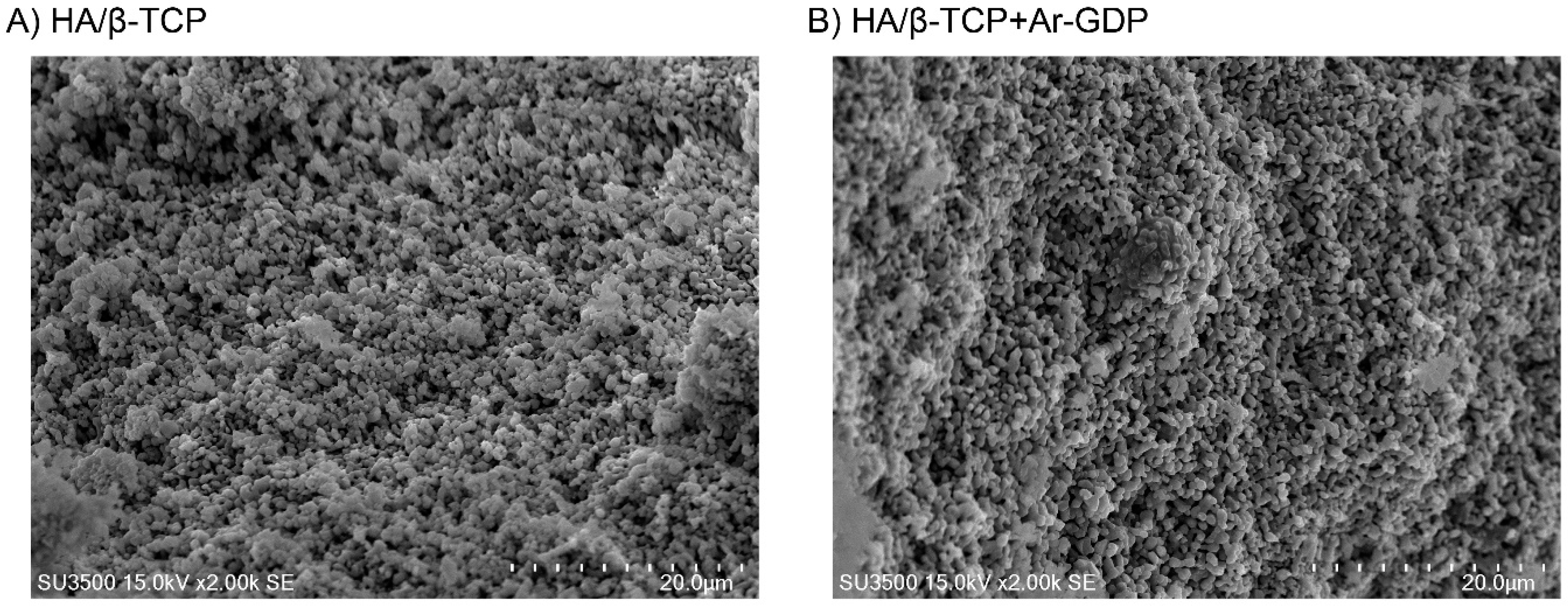
3.1. SEM Observations
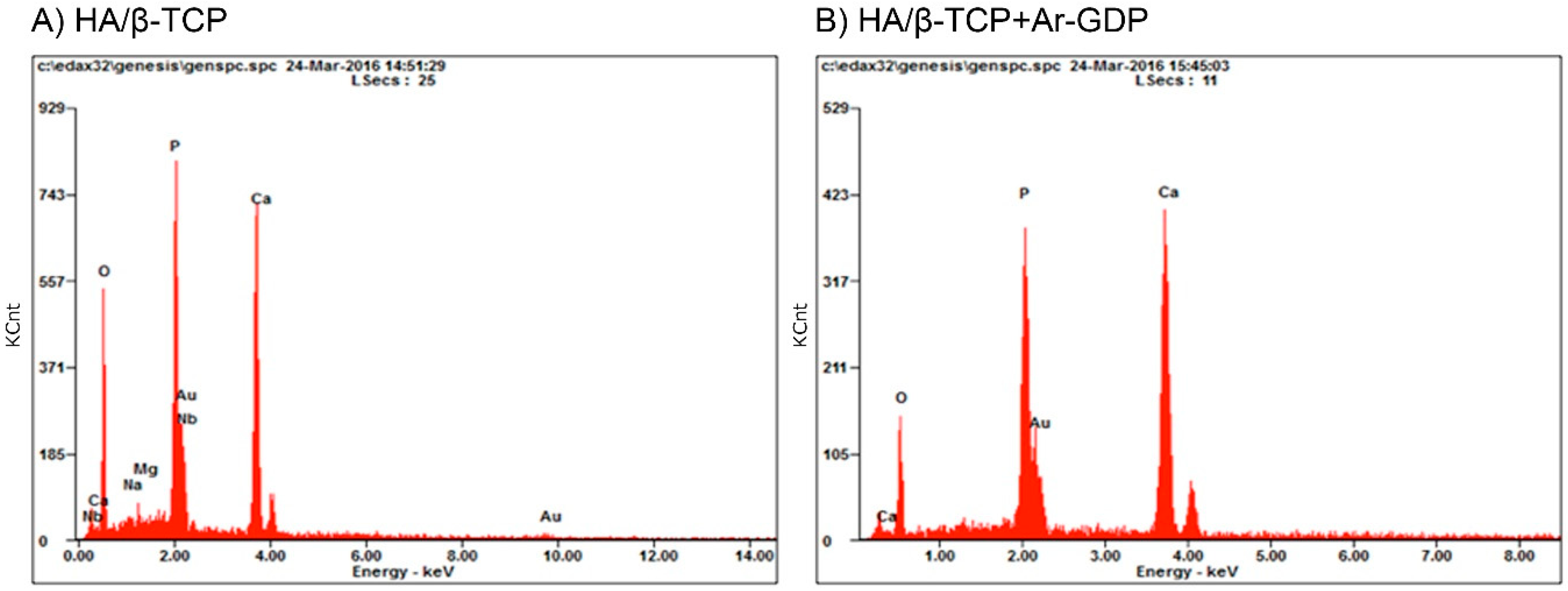
3.2. EDS Analysis
3.3. XPS
3.4. Cell Viability and Morphology Assessments
3.5. Cell Morphology
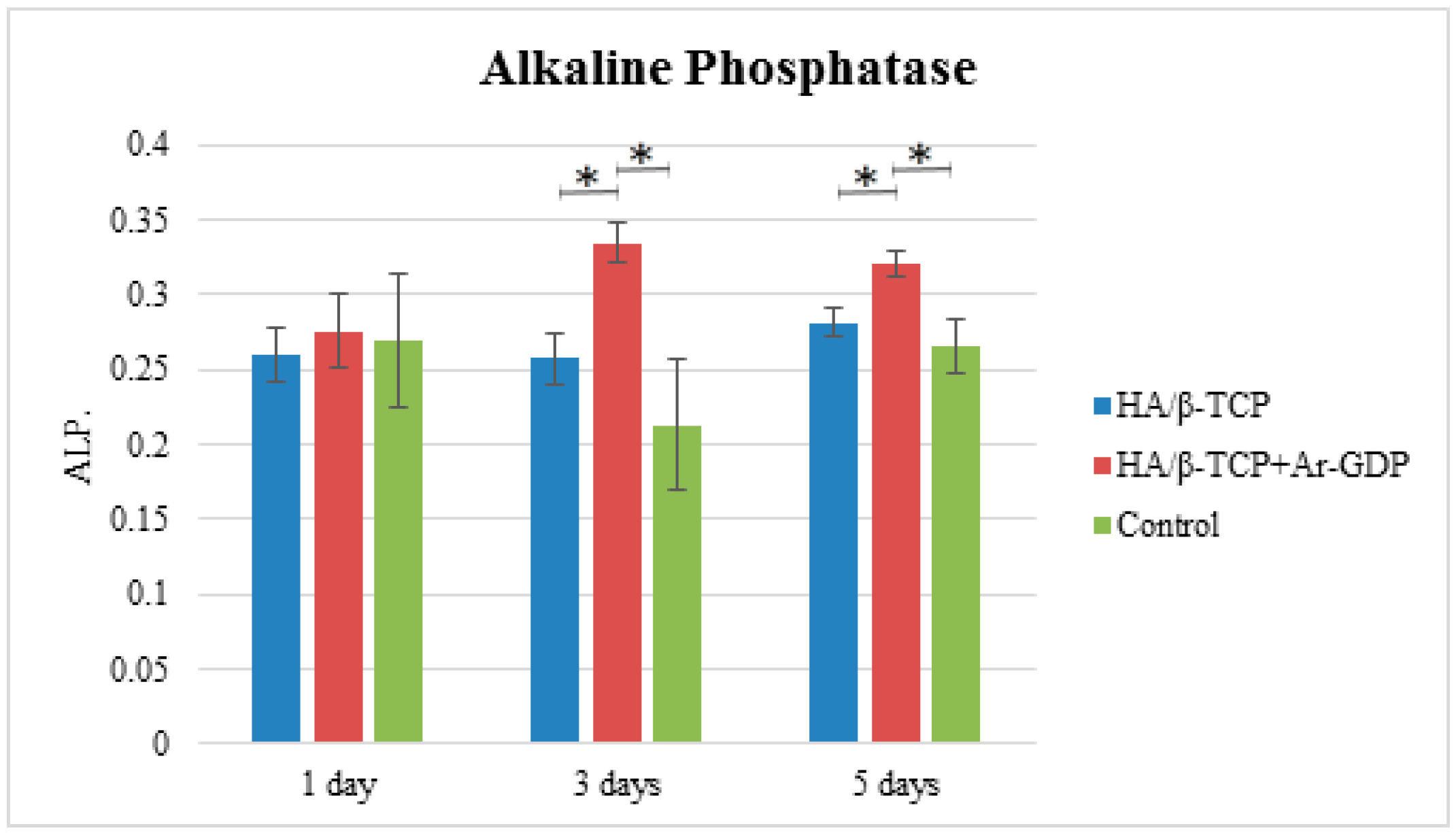
3.6. Alkaline Phosphatase Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Funding Sources
References
- Susin, C.; Wikesjö, U.M. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontology 2000 2013, 62, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L. Which reconstructive procedures are effective for treating the periodontal intraosseous defect? Periodontology 2000 2005, 37, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontology 2000 2012, 59, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.; Layrolle, P.; Clergeau, L.P.; Enckel, B.; Pilet, P.; Amouriq, Y.; Daculsi, G.; Giumelli, B. The safety and efficacy of an injectable bone substitute in dental sockets demonstrated in a human clinical trial. Biomaterials 2007, 28, 3295–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, A.; Piattelli, A.; Perrotti, V.; Manzon, L.; Iezzi, G. Maxillary sinus augmentation in humans using cortical porcine bone: A histological and histomorphometrical evaluation after 4 and 6 months. Clin. Implant Dent. Relat. Res. 2011, 13, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; Gunsolley, J.C. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann. Periodontol. 2003, 8, 227–265. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Scarano, A.; Piattelli, M.; Piccirilli, M.; Caputi, S.; Piattelli, A. Histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone–derived biomaterial. J. Periodontol. 2006, 77, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Fernández, M.P.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Maté-Sánchez del Val, J.E.; Vicente-Ortega, V.; Meseguer-Olmos, L. Bone response to hydroxyapatites with open porosity of animal origin (porcine [OsteoBiol® mp3] and bovine [Endobon®]): A radiological and histomorphometric study. Clin. Oral Implants Res. 2011, 22, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Heitz-Mayfield, L.J.; Needleman, I.; Moles, D.; Scabbia, A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J. Clin. Periodontol. 2002, 29, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.B.; Legeros, R.Z.; Lynch, K.L.; Lee, K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/βTCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.M.; Torres, J.; Tresguerres, I.; Clemente, C.; López-Cabarcos, E.; Blanco, L.J. Bone augmentation in rabbit calvariae: Comparative study between Bio-Oss® and a novel β-TCP/DCPD granulate. J. Clin. Periodontol. 2006, 33, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, T.J.; Termaat, M.F.; den Boer, F.C.; Patka, P.; Bakker, F.C.; Henk, J.T.M. Properties of calcium phosphate ceramics in relation to their in vivo behavior. J. Trauma Acute Care Surg. 2000, 48, 179–186. [Google Scholar] [CrossRef]
- Garrido, C.A.; Lobo, S.E.; Turíbio, F.M.; LeGeros, R.Z. Biphasic calcium phosphate bioceramics for orthopaedic reconstructions: Clinical outcomes. Int. J. Biomater. 2011, 2011, 129727. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Huffer, W.E.; Benedict, J.J.; Turner, A.S.; Briest, A.; Rettenmaier, R.; Springer, M.; Walboomers, X.F. Repair of sheep long bone cortical defects filled with COLLOSS®, COLLOSS® E, OSSAPLAST®, and fresh iliac crest autograft. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, B.-O. Preparation and Characterization of Glow Discharge Modified Titanium Surfaces. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 1995. [Google Scholar]
- Aronsson, B.O.; Lausmaa, J.; Kasemo, B. Glow discharge plasma treatment for surface cleaning and modification of metallic biomaterials. J. Biomed. Mater. Res. A 1997, 35, 49–73. [Google Scholar] [CrossRef]
- Gombotz, W.; Hoffman, A. Gas-discharge techniques for biomaterial modification. CRC Crit. Rev. Biocompat. 1987, 4, 1–42. [Google Scholar]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: On the role of cleanliness, contamination, and preparation procedures. J. Biomed. Mater. Res. A 1988, 22, 145–158. [Google Scholar] [CrossRef]
- Smith, D.; Pilliar, R.; Metson, J.; McIntyre, N. Dental implant materials. II. Preparative procedures and surface spectroscopic studies. J. Biomed. Mater. Res. 1991, 25, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the microstructure and properties of titanium alloys through nitriding and other surface engineering methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; Geyter, N.D.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, A.; Yeung, K.W.; Kao, R.Y.T.; Wu, G.; Hu, T.; Xu, Z.; Chu, P.K. Plasma-modified biomaterials for self-antimicrobial applications. ACS Appl. Mater. Interfaces 2011, 3, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, Y.; Qin, L.; Guo, Z.; Li, D. Effect of composition and macropore percentage on mechanical and in vitro cell proliferation and differentiation properties of 3D printed HA/β-TCP scaffolds. RSC Adv. 2017, 7, 43186–43196. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity; ISO 10993-5:2009; International Organization for Standardization: Geneva, Switzerland, 2009.
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int. J. Oral Maxillofac. Implants 2002, 17, 771–777. [Google Scholar] [PubMed]
- Huang, H.M.; Hsieh, S.C.; Teng, N.C.; Feng, S.W.; Ou, K.L.; Chang, W.J. Biological surface modification of titanium surfaces using glow discharge plasma. Med. Biol. Eng. Comput. 2011, 49, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Feng, S.W.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Lin, H.K.; Wang, P.D.; Chang, W.J. Surface analysis of titanium biological modification with glow discharge. Clin. Implant Dent. Relat. Res. 2015, 17, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Wang, H.T.; Nelea, V.; Luo, L.; Antoniou, J.; Wertheimer, M.R. The effect of glow discharge plasma surface modification of polymers on the osteogenic differentiation of committed human mesenchymal stem cells. Biomaterials 2006, 27, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Chim, H.; Ong, J.L.; Schantz, J.T.; Hutmacher, D.W.; Agrawal, C. Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (d,l-lactide) scaffolds. J. Biomed. Mater. Res. A 2003, 65, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.; Annaz, B.; Saeed, S.; Revell, P.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. Mater. Med. 2005, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- LeGeros, R.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Tzaphlidou, M.; Zaichick, V. Calcium, phosphorus, calcium-phosphorus ratio in rib bone of healthy humans. Biol. Trace Element Res. 2003, 93, 63–74. [Google Scholar] [CrossRef]
- França, R.; Samani, T.D.; Bayade, G.; Yahia, L.H.; Sacher, E. Nanoscale surface characterization of biphasic calcium phosphate, with comparisons to calcium hydroxyapatite and β-tricalcium phosphate bioceramics. J. Colloid Interface Sci. 2014, 420, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lee, W.F.; Feng, S.W.; Huang, H.M.; Lin, C.T.; Teng, N.C.; Chang, W.J. In vitro analysis of fibronectin-modified titanium surfaces. PLoS ONE 2016, 11, e0146219. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Carranza, F., Jr.; Kenney, E. Calcium phosphate ceramics in dentistry: A review of the literature. J. West. Soc. Periodontol. Periodontal Abstr. 1984, 32, 88–108. [Google Scholar] [PubMed]
- Bensaha, T.; El Mjabber, H. Evaluation of new bone formation after sinus augmentation with two different methods. Int. J. Oral Maxillofac. Surg. 2016, 45, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 97–108. [Google Scholar] [CrossRef]
- Daculsi, G.; Durand, M.; Fabre, T.; Vogt, F.; Uzel, A.P.; Rouvillain, J.L. Development and clinical cases of injectable bone void filler used in orthopaedic. IRBM 2012, 33, 254–262. [Google Scholar] [CrossRef]
- Seong, K.C.; Cho, K.S.; Daculsi, C.; Seris, E.; Guy, D. Eight-year clinical follow-up of sinus grafts with micro-macroporous biphasic calcium phosphate granules. In Key Engineering Materials; Trans Tech Publications: Zürich, Switzerland, 2014; pp. 321–324. [Google Scholar]




| Materials | HA/β-TCP | HA/β-TCP + Ar-GDP | ||
|---|---|---|---|---|
| Element | Weight % | Atomic % | Weight % | Atomic % |
| O | 33.81 | 58.15 | 25.02 | 48.13 |
| P | 18.19 | 16.16 | 19.19 | 19.07 |
| Au | 13.34 | 1.86 | 16.43 | 2.57 |
| Ca | 31.64 | 21.72 | 39.36 | 30.23 |
| Mg | 0.66 | 0.75 | 0 | 0 |
| Nb | 1.62 | 0.48 | 0 | 0 |
| Na | 0.73 | 0.87 | 0 | 0 |
| Total | 100 | 100 | 100 | 100 |
| Materials | C1s | O1s | Na1s | P2p | Ca2p |
|---|---|---|---|---|---|
| HA/β-TCP | 10.72 | 60.5 | 0.46 | 12.53 | 15.79 |
| HA/β-TCP + Ar-GDP | 12.06 | 59.79 | 0.2 | 12.06 | 16.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca, E.; Pan, Y.-H.; Tsai, A.I.; Lin, P.-Y.; Lin, C.-K.; Huang, H.-M.; Teng, N.-C.; Wang, P.D.; Chang, W.-J. Enhancement of Osteoblastic-Like Cell Activity by Glow Discharge Plasma Surface Modified Hydroxyapatite/β-Tricalcium Phosphate Bone Substitute. Materials 2017, 10, 1347. https://doi.org/10.3390/ma10121347
Salamanca E, Pan Y-H, Tsai AI, Lin P-Y, Lin C-K, Huang H-M, Teng N-C, Wang PD, Chang W-J. Enhancement of Osteoblastic-Like Cell Activity by Glow Discharge Plasma Surface Modified Hydroxyapatite/β-Tricalcium Phosphate Bone Substitute. Materials. 2017; 10(12):1347. https://doi.org/10.3390/ma10121347
Chicago/Turabian StyleSalamanca, Eisner, Yu-Hwa Pan, Aileen I. Tsai, Pei-Ying Lin, Ching-Kai Lin, Haw-Ming Huang, Nai-Chia Teng, Peter D. Wang, and Wei-Jen Chang. 2017. "Enhancement of Osteoblastic-Like Cell Activity by Glow Discharge Plasma Surface Modified Hydroxyapatite/β-Tricalcium Phosphate Bone Substitute" Materials 10, no. 12: 1347. https://doi.org/10.3390/ma10121347





