Optimization and Characterization of Paper-Made Surface Enhanced Raman Scattering (SERS) Substrates with Au and Ag NPs for Quantitative Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. SERS Substrates Preparation and Characterization
2.3. Analytes Preparation for SERS Measurements
2.4. Instrumentation, SERS Spectra, and Images Acquisition
2.5. Data Preprocessing, Analysis, and Plotting
3. Results and Discussion
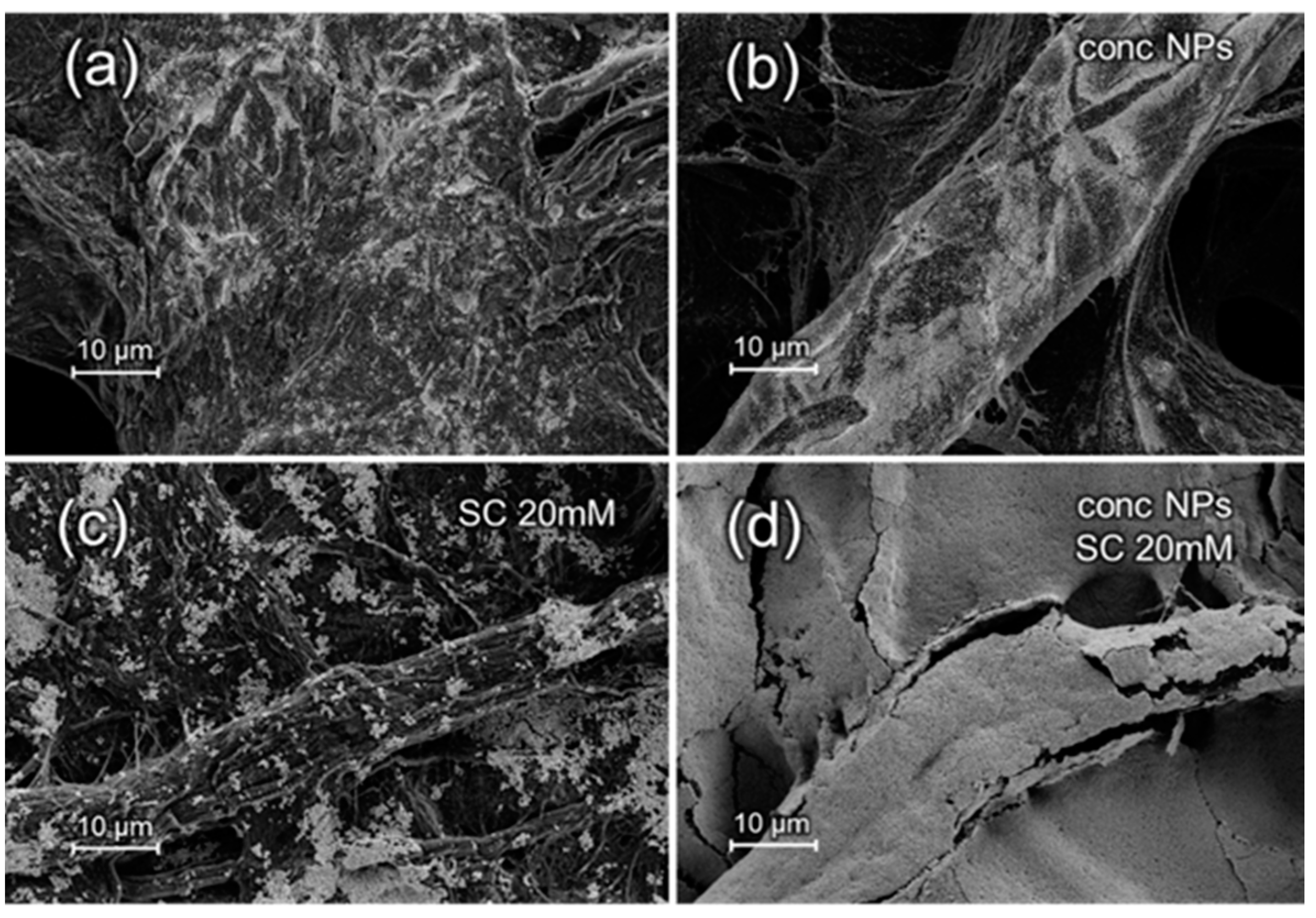
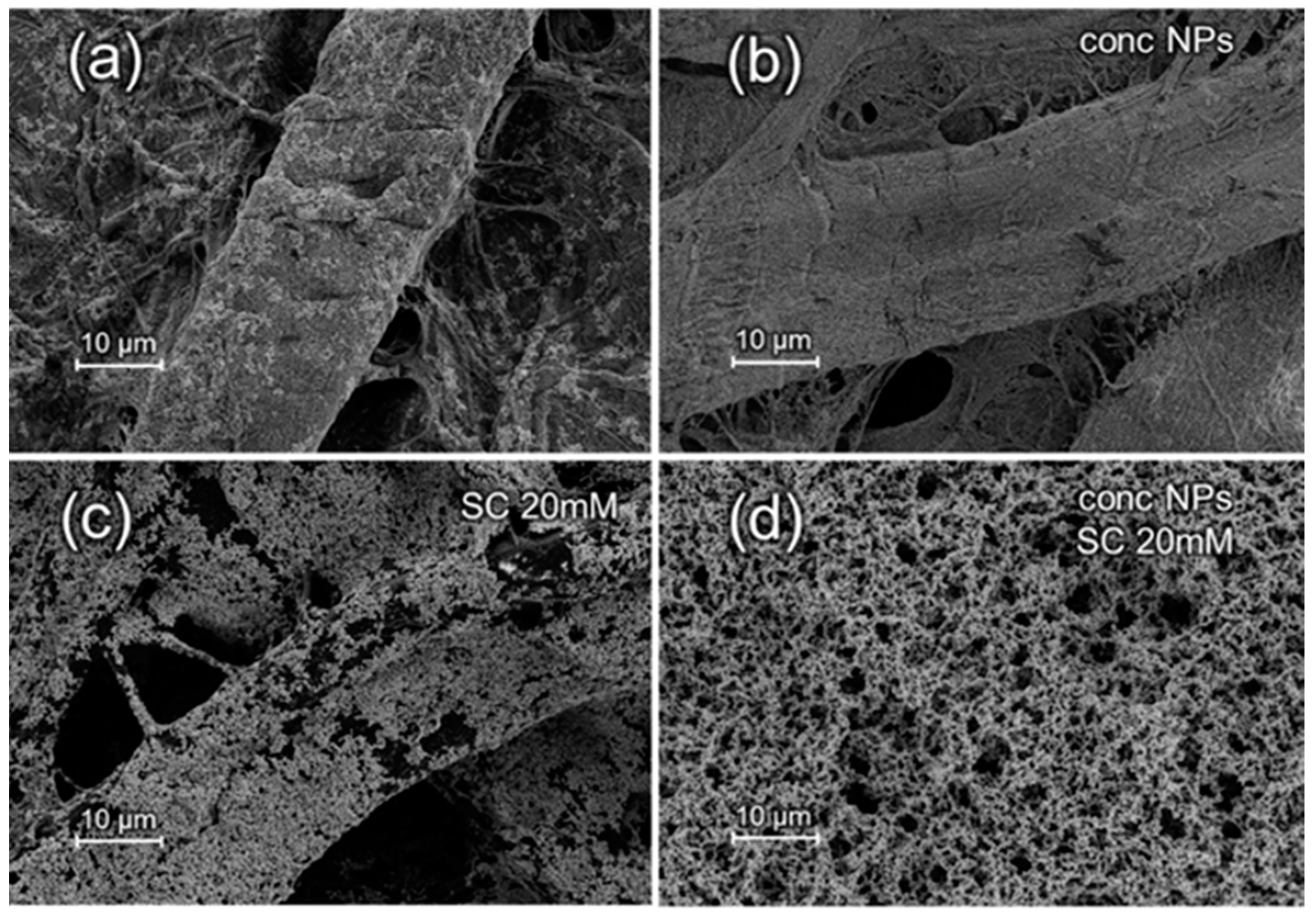
3.1. SEM Characterization of Substrates
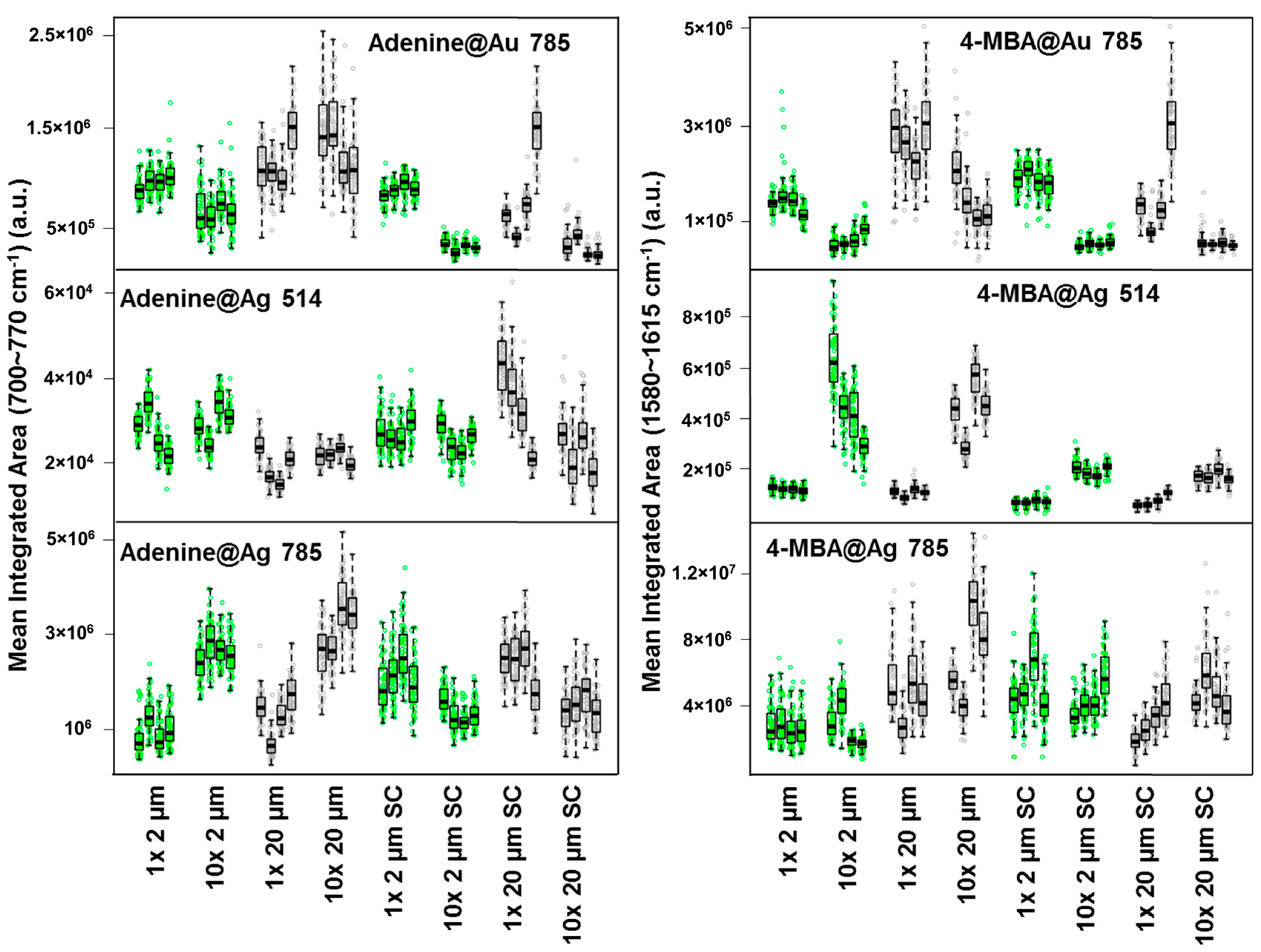
3.2. Effect of Paper Porosity, Aggregating Agents and Nanoparticles Concentration on Substrates Repeatability
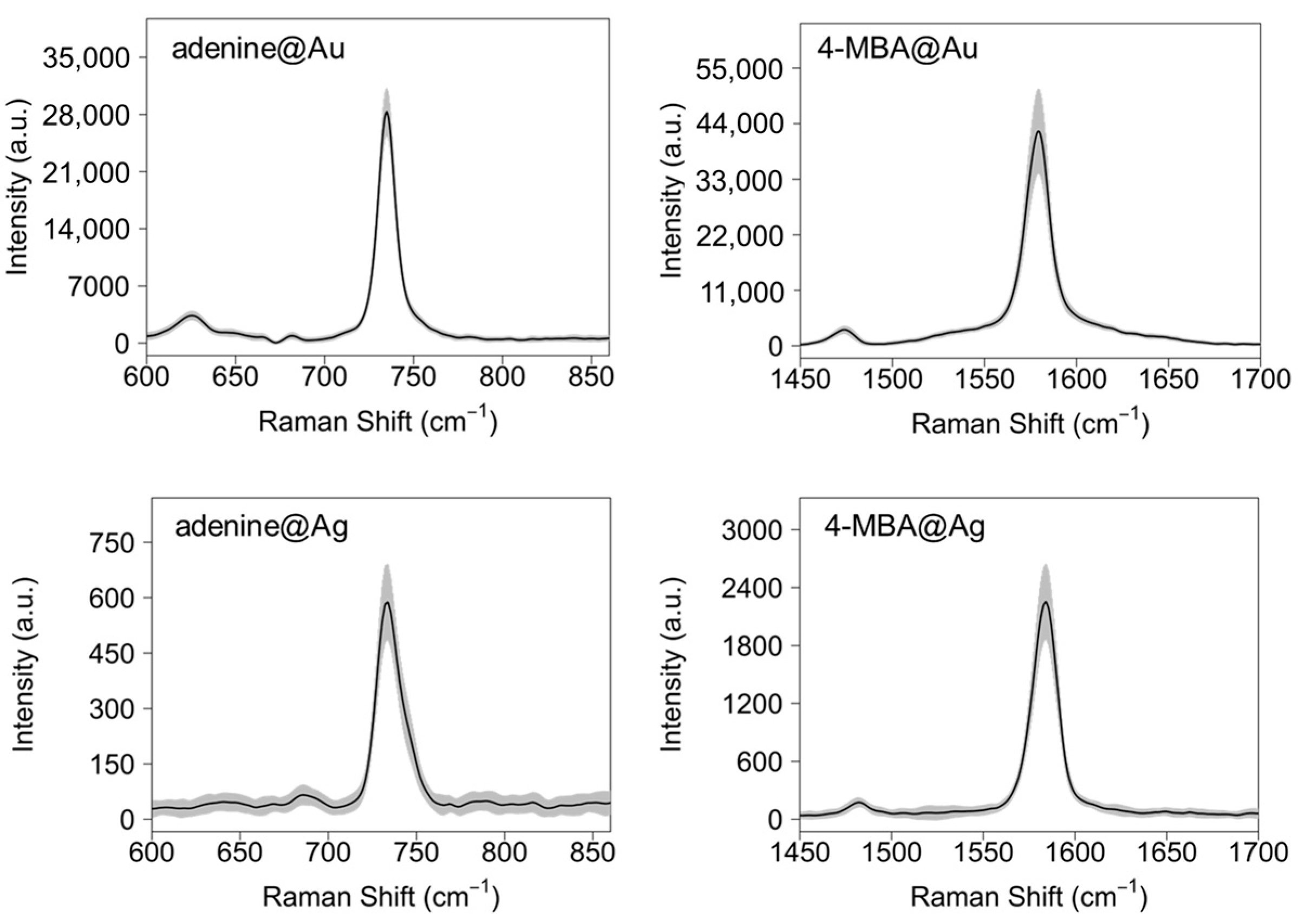
3.3. Estimation of the Raman Enhancement Factor (EF)
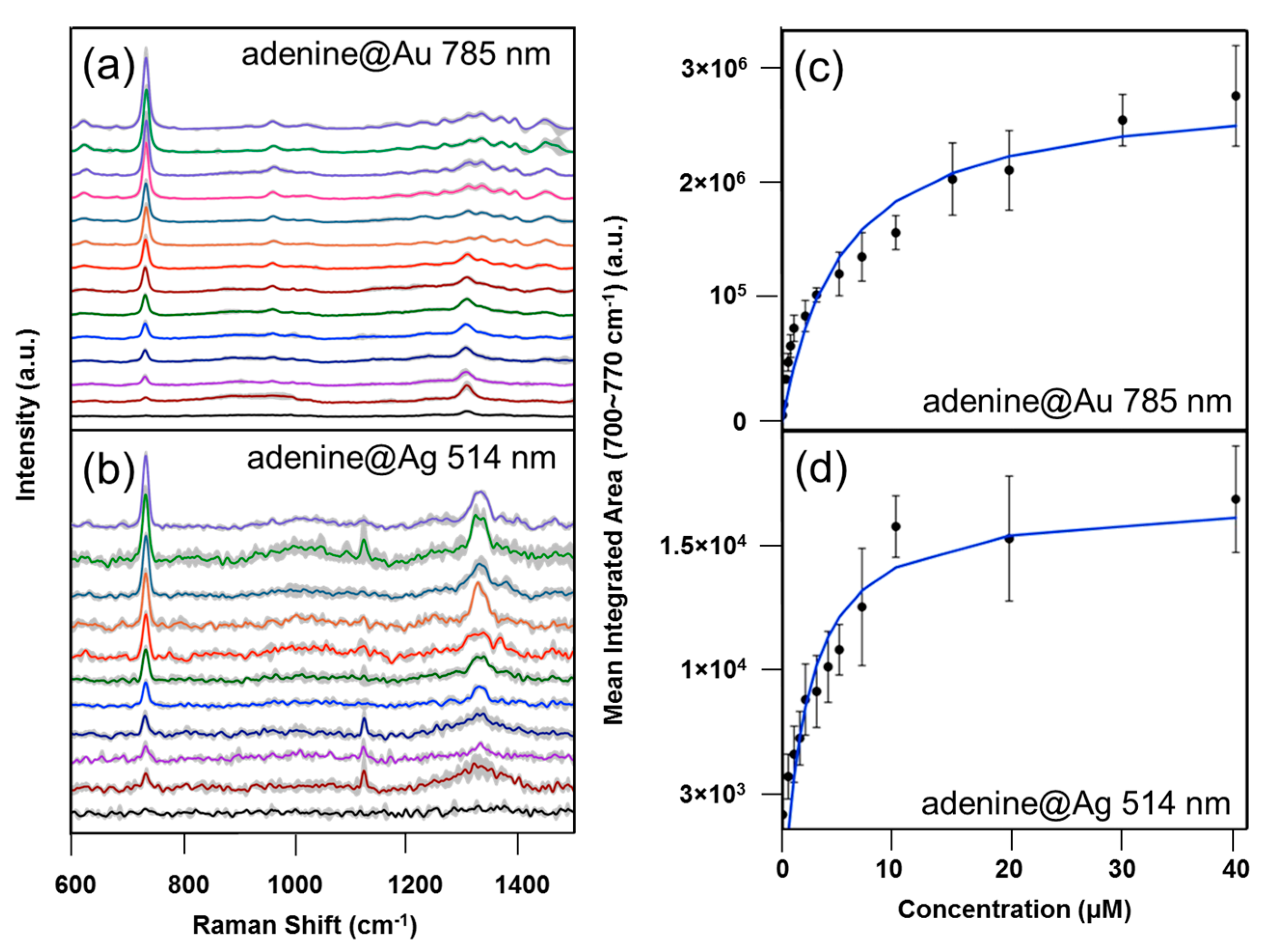
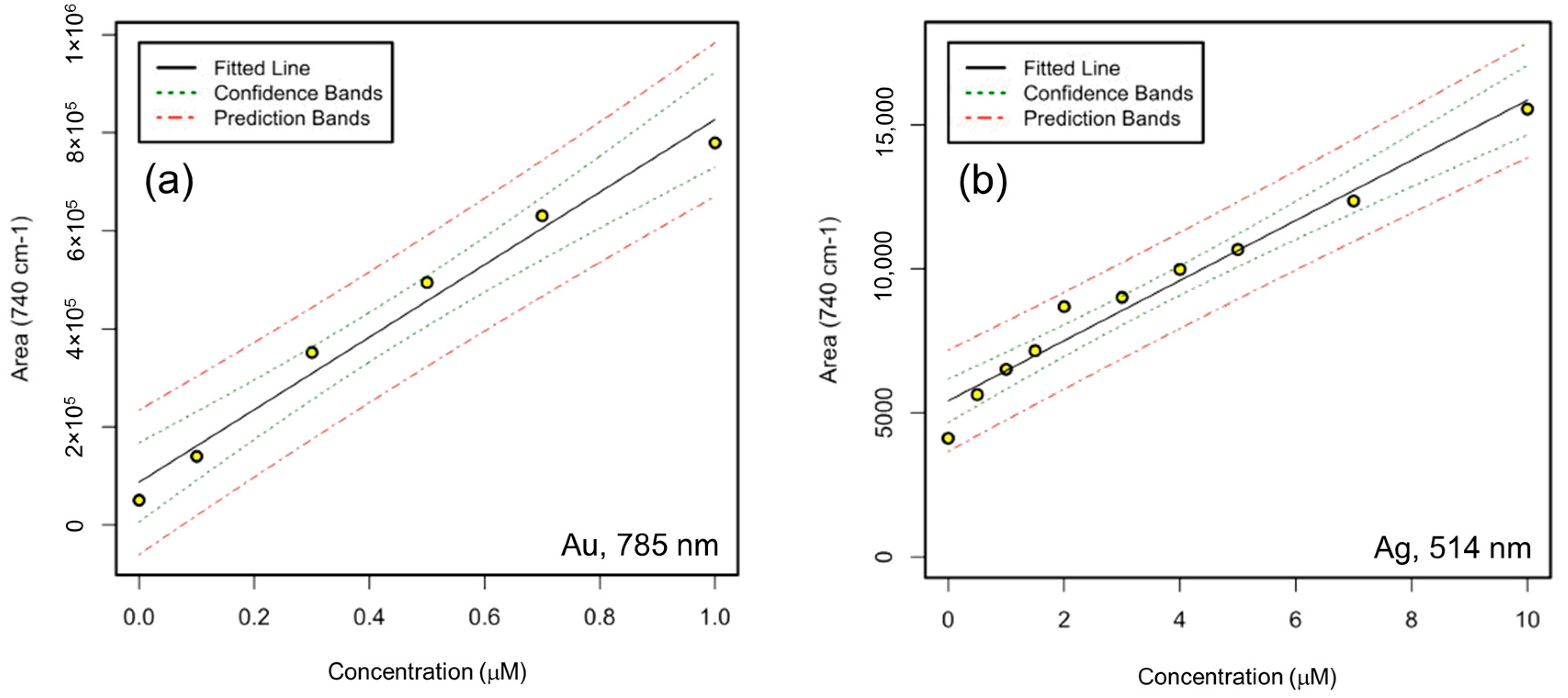
3.4. Adenine and 4-MBA Quantification
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4-MBA | 4-Mercaptobenzoic acid |
| Au | gold |
| Ag | silver |
| NPs | nanoparticles |
| PBS | Phosphate Buffered Saline |
| RSD | relative standard deviation |
| LOD | lower limit of detection |
| LOQ | lower limit of quantification |
References
- Betz, J.F.; Yu, W.W.; Cheng, Y.; White, I.M.; Rubloff, G.W. Simple SERS substrates: Powerful, portable, and full of potential. Phys. Chem. Chem. Phys. 2014, 16, 2224–2239. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Schlücker, S. Medical applications of surface-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2013, 15, 5329–5344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Sensing using localised surface plasmon resonance sensors. Chem. Commun. 2012, 48, 8999–9010. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Hiromoto, M.; Begun, G.; Moody, R. Surface-enhanced Raman spectrometry for trace organic analysis. Anal. Chem. 1984, 56, 1667–1670. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Harris, R.A.; Swart, H.C. Embedded plasmonic nanostructures: Synthesis, fundamental aspects and their surface enhanced Raman scattering applications. Int. Rev. Phys. Chem. 2016, 35, 353–398. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Kim, D.-H. Nanoparticle polymer composites on solid substrates for plasmonic sensing applications. Nano Today 2016, 11, 415–434. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Surface-enhanced Raman spectroscopy using metallic nanostructures. Trends Anal. Chem. 1998, 17, 557–582. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive Chemical Analysis by Raman Spectroscopy. Chem. Rev. (Wash. DC U.S.) 1999, 99, 2957–2976. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Fornasaro, S.; Dalla Marta, S.; Rabusin, M.; Bonifacio, A.; Sergo, V. Toward SERS-based point-of-care approaches for therapeutic drug monitoring: The case of methotrexate. Faraday Discuss. 2016, 187, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, U.; Rösch, P.; Popp, J. Raman spectroscopy towards clinical application: Drug monitoring and pathogen identification. Int. J. Antimicrob. Agents 2015, 46 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Wang, B.; Li, Z.; Huang, H.; Li, C.; Yu, X.; Chu, P.K. Paper-based plasmonic platform for sensitive, noninvasive, and rapid cancer screening. Biosens. Bioelectron. 2014, 54, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, B.-B.; Liu, X.-Q.; Zhang, Y.-L.; Xu, Y.; Chen, Q.-D.; Sun, H.-B. Highly efficient SERS test strips. Chem. Commun. 2012, 48, 5913–5915. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Tian, L.; Singamaneni, S. Paper-based SERS swab for rapid trace detection on real-world surfaces. ACS Appl. Mater. Interfaces 2010, 2, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 11301. [Google Scholar] [CrossRef] [PubMed]
- Lahr, R.H.; Wallace, G.C.; Vikesland, P.J. Raman characterization of nanoparticle transport in microfluidic paper-based analytical devices (uPADs). ACS Appl. Mater. Interfaces 2015, 7, 9139–9146. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Yu, W.W. A simple filter-based approach to surface enhanced Raman spectroscopy for trace chemical detection. Analyst 2012, 137, 1168–1173. [Google Scholar]
- Hoppmann, E.P.; Yu, W.W.; White, I.M. Highly sensitive and flexible inkjet printed SERS sensors on paper. Methods 2013, 63, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.H.; Li, D.; Simon, G.P.; Garnier, G. Gold nanoparticle-paper as a three-dimensional surface enhanced Raman scattering substrate. Langmuir 2012, 28, 8782–8790. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Liz-Marzán, L.M. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys. Chem. Chem. Phys. 2013, 15, 5288–5300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Tsai, B.C.; Yang, J. Silver nanoparticle-treated filter paper as a highly sensitive surface-enhanced Raman scattering (SERS) substrate for detection of tyrosine in aqueous solution. Anal. Chim. Acta 2011, 708, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, L.; Yang, L. Designing of the functional paper-based surface-enhanced Raman spectroscopy substrates for colorants detection. Mater. Res. Bull. 2015, 63, 199–204. [Google Scholar] [CrossRef]
- Hasi, W.L.J.; Lin, X.; Lou, X.T.; Lin, S.; Yang, F.; Lin, D.Y.; Lu, Z.W. Chloride ion-assisted self-assembly of silver nanoparticles on filter paper as SERS substrate. Appl. Phys. A Mater. Sci. Process. 2014, 118, 799–807. [Google Scholar] [CrossRef]
- Mehn, D.; Morasso, C.; Vanna, R.; Bedoni, M.; Prosperi, D.; Gramatica, F. Immobilised gold nanostars in a paper-based test system for surface-enhanced Raman spectroscopy. Vib. Spectrosc. 2013, 68, 45–50. [Google Scholar] [CrossRef]
- Lee, C.H.; Hankus, M.E.; Tian, L.; Pellegrino, P.M.; Singamaneni, S. Highly Sensitive Surface Enhanced Raman Scattering Substrates. Anal. Chem. 2011, 83, 8953–8958. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Duarte, R.M.B.O.; Duarte, A.C. Immobilization strategies and analytical applications for metallic and metal-oxide nanomaterials on surfaces. TrAC Trends Anal. Chem. 2012, 40, 90–105. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. Inkjet printed surface enhanced raman spectroscopy array on cellulose paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Santhanam, V. Paper-based SERS Active Substrates on Demand. RSC Adv. 2016, 6, 68545–68552. [Google Scholar] [CrossRef]
- Kim, W.S.; Shin, J.H.; Park, H.K.; Choi, S. A low-cost, monometallic, surface-enhanced Raman scattering-functionalized paper platform for spot-on bioassays. Sens. Actuators B Chem. 2015, 222, 1112–1118. [Google Scholar] [CrossRef]
- Hasi, W.-L.-J.; Lin, S.; Lin, X.; Lou, X.-T.; Yang, F.; Lin, D.-Y.; Lu, Z.-W. Rapid fabrication of self-assembled interfacial film decorated filter paper as an excellent surface-enhanced Raman scattering substrate. Anal. Methods 2014, 6, 9547–9553. [Google Scholar] [CrossRef]
- Rajapandiyan, P.; Yang, J. Photochemical method for decoration of silver nanoparticles on filter paper substrate for SERS application. J. Raman Spectrosc. 2014, 45, 574–580. [Google Scholar] [CrossRef]
- Webb, J.A.; Aufrecht, J.; Hungerford, C.; Bardhan, R. Ultrasensitive analyte detection with plasmonic paper dipsticks and swabs integrated with branched nanoantennas. J. Mater. Chem. C 2014, 2, 10446–10454. [Google Scholar] [CrossRef]
- Desmonda, C.; Kar, S.; Tai, Y. Formation of gold nanostructures on copier paper surface for cost effective SERS active substrate—Effect of halide additives. Appl. Surf. Sci. 2016, 367, 362–369. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hiller, J. Synthesis of Gold Nanoparticles Turkevich method. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley: Chichester, UK, 2006; ISBN 9780470035641. [Google Scholar]
- Larmour, I.A.; Faulds, K.; Graham, D. SERS activity and stability of the most frequently used silver colloids. J. Raman Spectrosc. 2012, 43, 202–206. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Giese, B.; Mcnaughton, D. Surface-Enhanced Raman Spectroscopic and Density Functional Theory Study of Adenine Adsorption to Silver Surfaces Surface-Enhanced Raman Spectroscopic and Density Functional Theory Study of Adenine Adsorption to Silver Surfaces. J. Phys. Chem. B 2002, 106, 101–112. [Google Scholar] [CrossRef]
- Pagliai, M.; Caporali, S.; Muniz-Miranda, M.; Pratesi, G.; Schettino, V. SERS, XPS, and DFT study of adenine adsorption on silver and gold surfaces. J. Phys. Chem. Lett. 2012, 3, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Belaites, C.; Sergo, V. “hyperSpec: A Package to Handle Hyperspectral Data Sets in R”, R Package Version 0.98-20161118. Available online: http://hyperspec.r-forge.r-project.org (accessed on 30 September 2017).
- Liland, K.H.; Mevik, B.; Canteri, R. Baseline: Baseline Correction of Spectra. R Package Version 1.2-1. 2015. Available online: https://CRAN.R-project.org/package=baseline (accessed on 30 September 2017).
- Ranke, J. chemCal: Calibration Functions for Analytical Chemistry. R Package Version 0.1-37. 2015. Available online: https://CRAN.R-project.org/package=chemCal (accessed on 30 September 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 30 September 2017).
- Novara, C.; Dalla Marta, S.; Virga, A.; Lamberti, A.; Angelini, A.; Chiadò, A.; Rivolo, P.; Geobaldo, F.; Sergo, V.; Bonifacio, A.; et al. SERS-Active Ag Nanoparticles on Porous Silicon and PDMS Substrates: A Comparative Study of Uniformity and Raman Efficiency. J. Phys. Chem. C 2016, 120, 16946–16953. [Google Scholar] [CrossRef]
| Au Substrates—785 nm Excitation | RSD% Intra Sample (Inter-Sample) | |||
|---|---|---|---|---|
| Average Pore Size | NPs Concentration | w/o Citrate | with Citrate | |
| 4-MBA | 2 µm | 1× | 10.8, 27.3, 14.1, 14.3 (13.4) | 12, 13.7, 16.2, 15.6 (6.2) |
| 10× | 27.9, 14.3, 26.9, 19.4 (23.7) | 14.7, 16.0, 10.4, 17.9 (7.3) | ||
| 20 µm | 1× | 27.5, 19.8, 20.4, 23.7 (14.4) | 17.9, 22.7, 18.2, 23.7 (60.6) | |
| 10× | 27.5, 32.0, 25.3, 26.6 (34.1) | 32.0, 11.3, 21.1, 11.5 (6.4) | ||
| Adenine | 2 µm | 1× | 13.4, 12.6, 12.5, 14.8 (6.1) | 12.5, 9.3, 12.8, 9.8 (6.0) |
| 10× | 34.3, 24.5, 19.4, 31.5 (10.7) | 14.9, 21.6, 11.7, 9.8 (11.7) | ||
| 20 µm | 1× | 21.8, 17.0, 18.4, 18.5 (19.3) | 13.8, 13.4, 17.3, 18.5 (57.6) | |
| 10× | 26.6, 24.2, 24.0, 31.5 (17.0) | 35.3, 25.6, 18.9, 24.5 (32.3) | ||
| Ag Substrates—514 nm Excitation | RSD% Intra Sample (Inter-Sample) | |||
|---|---|---|---|---|
| Average Pore Size | NPs Concentration | w/o Citrate | with Citrate | |
| 4-MBA | 2 µm | 1× | 11.0, 9.8, 12.1, 12.4 (4.4) | 16.7, 15.9, 15.7, 18.3 (6.6) |
| 10× | 21.0, 17.4, 24.0, 15.4 (31.6) | 13.9, 11.6, 9.6, 9.4 (9.1) | ||
| 20 µm | 1× | 12.4, 11.6, 14.1, 9.5 (12.1) | 17.8, 16.9, 16.8, 9.5 (26.8) | |
| 10× | 12.8, 12.1, 12.8, 11.3 (25.6) | 12.8, 13.3, 15.5, 12.5 (9.4) | ||
| Adenine | 2 µm | 1× | 8.4, 9.7, 12.6, 11.8 (20.0) | 16.7, 13.7, 12.8, 13.4 (7.4) |
| 10× | 9.7, 9.1, 9.8, 8.5 (14.7) | 10.4, 13.0, 12.2, 8.4 (12.5) | ||
| 20 µm | 1× | 12.9, 12.3, 10.4, 10.7 (21.8) | 15.5, 17.2, 17.0, 10.7 (29.0) | |
| 10× | 11.4, 8.2, 7.2, 8.8 (7.6) | 19.0, 31.1, 21.4, 26.4 (21.7) | ||
| Ag Substrates—785 nm excitation | RSD% Intra Sample (Inter-Sample) | |||
|---|---|---|---|---|
| Average Pore Size | NPs Concentration | w/o Citrate | with Citrate | |
| 4-MBA | 2 µm | 1× | 38.1, 41.3, 41.2, 36.0 (6.9) | 29.1, 23.3, 27.9, 31.3 (26.9) |
| 10× | 31.3, 24.8, 17.5, 21.1 (42.6) | 21.5, 20.7, 22.9, 23.3 (23.4) | ||
| 20 µm | 1× | 34.5, 32.7, 35.3, 30.1 (28.4) | 33.4, 29.5, 28.1, 30.1 (35.6) | |
| 10× | 15.0, 18.2, 19.3 15.0 (40.0) | 20.0, 29.0, 31.1, 35.3 (20.2) | ||
| Adenine | 2 µm | 1× | 47.3, 27.8, 38.8, 37.8 (24.7) | 26.4, 24.1, 22.5, 28.0 (15.2) |
| 10× | 15.8, 16.9, 12.6, 14.9 (7.4) | 16.5, 25.0, 19.3, 22.1 (15.6) | ||
| 20 µm | 1× | 21.9, 39.0, 21.7, 27.2 (35.2) | 17.1, 19.6, 19.6, 27.2 (18.6) | |
| 10× | 22.4, 13.3, 16.3, 14.4 (16.3) | 33.1, 32.4, 30.0, 34.1 (14.1) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Marta, S.; Novara, C.; Giorgis, F.; Bonifacio, A.; Sergo, V. Optimization and Characterization of Paper-Made Surface Enhanced Raman Scattering (SERS) Substrates with Au and Ag NPs for Quantitative Analysis. Materials 2017, 10, 1365. https://doi.org/10.3390/ma10121365
Dalla Marta S, Novara C, Giorgis F, Bonifacio A, Sergo V. Optimization and Characterization of Paper-Made Surface Enhanced Raman Scattering (SERS) Substrates with Au and Ag NPs for Quantitative Analysis. Materials. 2017; 10(12):1365. https://doi.org/10.3390/ma10121365
Chicago/Turabian StyleDalla Marta, Silvia, Chiara Novara, Fabrizio Giorgis, Alois Bonifacio, and Valter Sergo. 2017. "Optimization and Characterization of Paper-Made Surface Enhanced Raman Scattering (SERS) Substrates with Au and Ag NPs for Quantitative Analysis" Materials 10, no. 12: 1365. https://doi.org/10.3390/ma10121365




