Comparative Studies on Ultraviolet-Light-Derived Photoresponse Properties of ZnO, AZO, and GZO Transparent Semiconductor Thin Films
Abstract
:1. Introduction
2. Materials and Methods
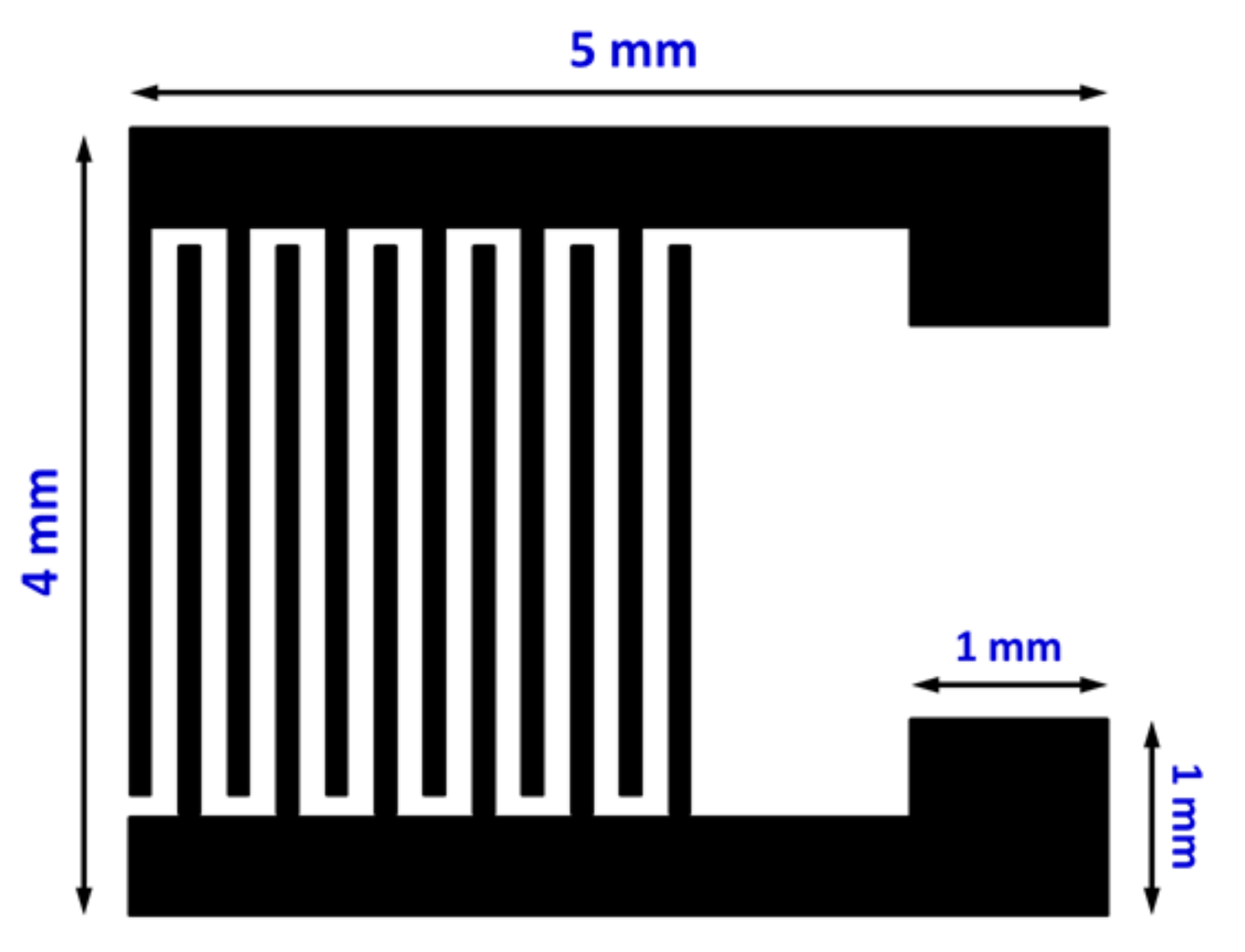
2.1. Thin Film Preparation and UV Photodetector Fabrication
2.2. Property Characterization
3. Results and Discussion
3.1. Structural Features, Optical and Electrical Properties of Oxide Thin Films
3.2. UV Sensing and Photoswitching Studies of Photodetectors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alaie, Z.; Nejad, S.M.; Yousefi, M.H. Recent advances in ultraviolet photodetectors. Mater. Sci. Semicond. Process. 2015, 29, 16–55. [Google Scholar] [CrossRef]
- Sang, L.; Liao, M.; Sumiya, M. A comprehensive review of semiconductor ultraviolet photodetectors: From thin film to one-dimensional nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.A.; Meher, S.R.; Alex, Z.C.; Balakrishnan, L.; Shambavi, K. Structural, optical and photodetection characteristics of Cd alloyed ZnO thin film by spin coating. J. Alloys Compd. 2017, 695, 3753–3759. [Google Scholar] [CrossRef]
- Inamdar, S.I.; Rajpure, K.Y. High-performance metal-semiconductor-metal UV photodetector based on spray deposited ZnO thin films. J. Alloys Compd. 2014, 595, 55–59. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Z.; Wang, M.; Cao, M.; Sun, Z.; Shao, J. Low temperature annealed ZnO film UV photodetector with fast photoresponse. Sens. Actuators A Phys. 2017, 253, 173–180. [Google Scholar]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Srinatha, N.; Raghu, P.; Mahesh, H.M.; Angadj, B. Spin-coated Al-doped ZnO thin film for optical applications: Structural, micro-structural, optical and luminescence studies. J. Alloys Compd. 2017, 722, 888–895. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Lee, W.C. Effect of dopants on the structural, optical and electrical properties of sol-gel derived ZnO semiconductor thin films. Curr. Appl. Phys. 2013, 13, 60–65. [Google Scholar] [CrossRef]
- Gabás, M.; Landa-Cánovas, A.; Costa-Krämer, J.L.; Agulló-Rueda, F.; González-Elipe, A.R.; Díaz-Carrasco, P.; Hernandez-Moro, J.; Lorite, I.; Herrero, P.; Castillero, P.; et al. Differences in n-type doping efficiency between Al- and Ga-ZnO films. J. Appl. Phys. 2013, 113, 163709. [Google Scholar] [CrossRef]
- Lung, C.; Toma, M.; Pop, M.; Pop, A.; Marconi, D. Characterization of the structural and optical properties of ZnO thin films doped with Ga, Al and (Al + Ga). J. Alloys Compd. 2017, 725, 1238–1243. [Google Scholar] [CrossRef]
- Ng, Z.N.; Chan, K.Y.; Low, C.Y.; Kamaruddin, S.A.; Sahdan, M.Z. Al and Ga doped ZnO films prepared by a sol-gel spin coating technique. Ceram. Int. 2015, 41, S254–S258. [Google Scholar] [CrossRef]
- Ebrahimifard, R.; Golobostanfard, M.R.; Abdizadeh, H. Sol-gel derived Al and ZnO thin film: An optoelectronic study. Appl. Surf. Sci. 2014, 290, 252–259. [Google Scholar] [CrossRef]
- Jun, M.C.; Park, S.U.; Koh, J.H. Comparative studies of Al-doped ZnO and Ga-doped ZnO transparent. Nanoscale Res. Lett. 2012, 7, 639. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tian, N.; Deng, Y.F.; Zhang, H.H. Ultraviolet photodetector based on sol-gel synthesized MgZnO nanoparticle with photoconductive gain. J. Alloys Compd. 2016, 667, 359–362. [Google Scholar] [CrossRef]
- Shaikh, S.K.; Ganbavle, V.V.; Inamdar, S.I.; Rajpure, K.Y. Multifunctional zinc oxide thin films for high-performance UV photodetectors and nitrogen dioxide gas sensors. RSC Adv. 2016, 6, 25641–25650. [Google Scholar] [CrossRef]
- Shinde, S.S.; Rajpure, K.Y. High-performance UV detector based on Ga-doped zinc oxide thin films. Appl. Surf. Sci. 2011, 257, 9595–9599. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Al-Dossary, O.; Umar, A. ZnO nanostructured thin films: Depositions, properties and applications—A review. Mater. Express 2015, 5, 3–23. [Google Scholar] [CrossRef]
- Zak, A.K.; Majia, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Salah, M.; Azizi, S.; Boukhachem, A.; Khaldi, C.; Amlouk, M.; Lamloumi, J. Structural, morphological, optical and photodetector properties of sprayed Li-doped ZnO thin films. J. Mater. Sci. 2017, 52, 10439–10454. [Google Scholar] [CrossRef]
- Kim, Y.S.; Tai, W.P. Electrical and optical properties of Al-doped ZnO thin films by sol-gel process. Appl. Surf. Sci. 2007, 253, 4911–4916. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y. Influence of dopant concentration on the optical properties of ZnO: In films by sol-gel method. Thin Solid Films 2009, 517, 5023–5028. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Yu, S.H. Optoelectronic characteristics of UV photodetectors based on sol-gel synthesized GZO semiconductor thin films. J. Alloys Compd. 2014, 596, 145–150. [Google Scholar] [CrossRef]
- Musat, V.; Teixeira, B.; Fortunato, E.; Monteiro, R.C.C.; Vilarinho, P. Al-doped ZnO thin films by sol-gel method. Surf. Coat. Technol. 2004, 180–181, 659–662. [Google Scholar] [CrossRef]
- Postica, V.; Hoppe, M.; Grottrup, J.; Hayes, P.; Robisch, V.; Smazna, D.; Adelung, R.; Viana, B.; Aschehoug, P.; Pauporte, T.; et al. Morphology dependent UV photoresponse of Sn-doped ZnO microstructures. Solid State Sci. 2017, 71, 75–86. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Wu, P.H. Properties of solution-processed MgInZnO semiconductor thin films and photodetectors fabricated at a low temperature using UV-assisted thermal annealing. Ceram. Int. 2017, 43, 11874–11878. [Google Scholar] [CrossRef]
- Kim, S.J.; Saravanakumar, B.; Mohan, R.; Thiyagarajan, K. Investigation of UV photoresponse property of Al, N co-doped ZnO film. J. Alloys Compd. 2013, 580, 538–543. [Google Scholar]
- Su, Y.K.; Peng, S.M.; Ji, L.W.; Wu, C.Z.; Cheng, W.B.; Liu, C.H. Ultraviolet ZnO nanorod photosensors. Langmuir 2010, 26, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.W.; Ma, J.G.; Zhang, J.Y.; Lu, Y.M.; Jiang, D.Y.; Li, B.H.; Zhao, D.X.; Zhang, Z.Z.; Yao, B.; Shen, D.Z. Ultraviolet photoconductive detector with high visible rejection and fast photoresponse based on ZnO thin film. Solid-State Electron. 2007, 51, 757–761. [Google Scholar] [CrossRef]
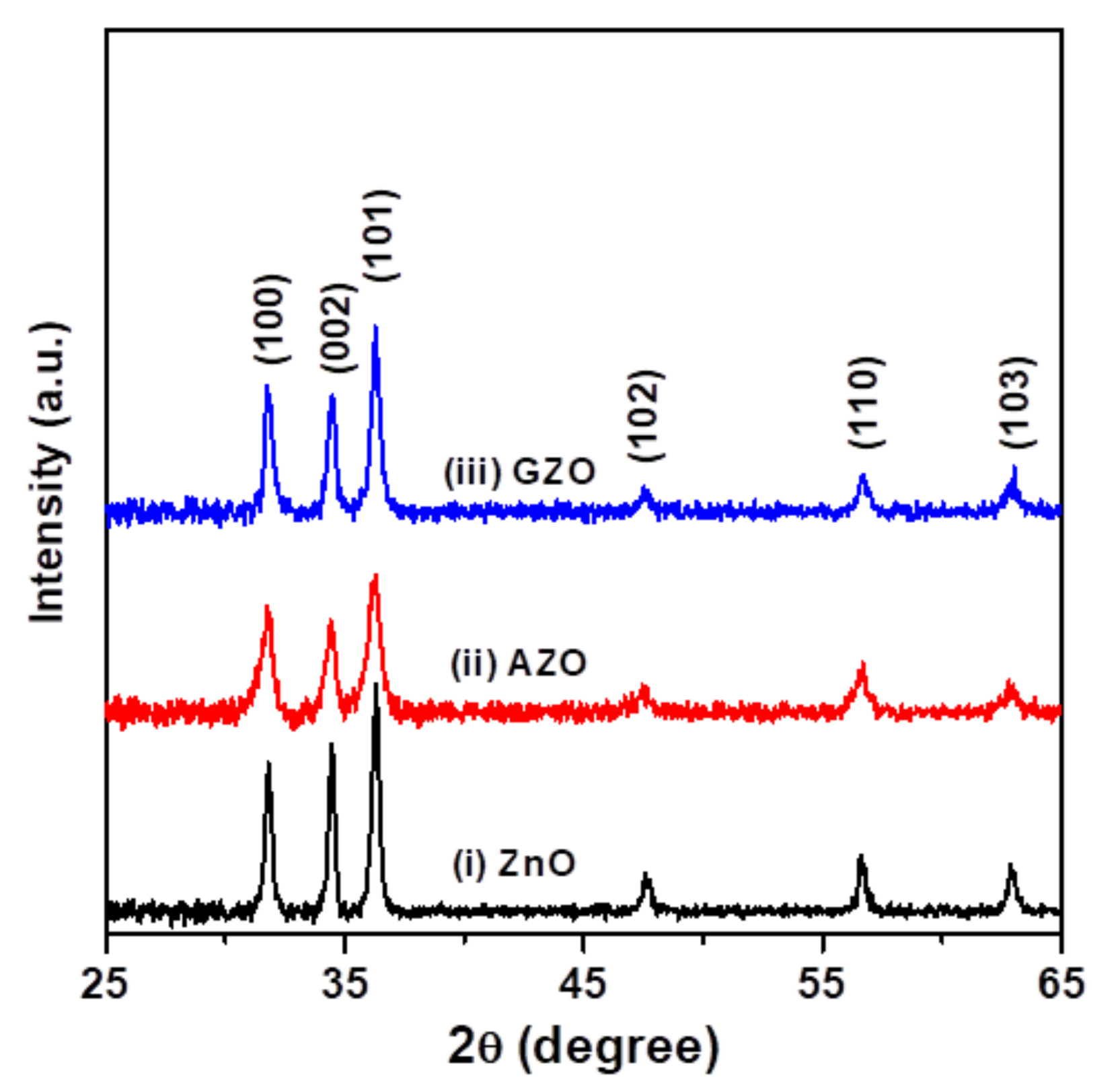
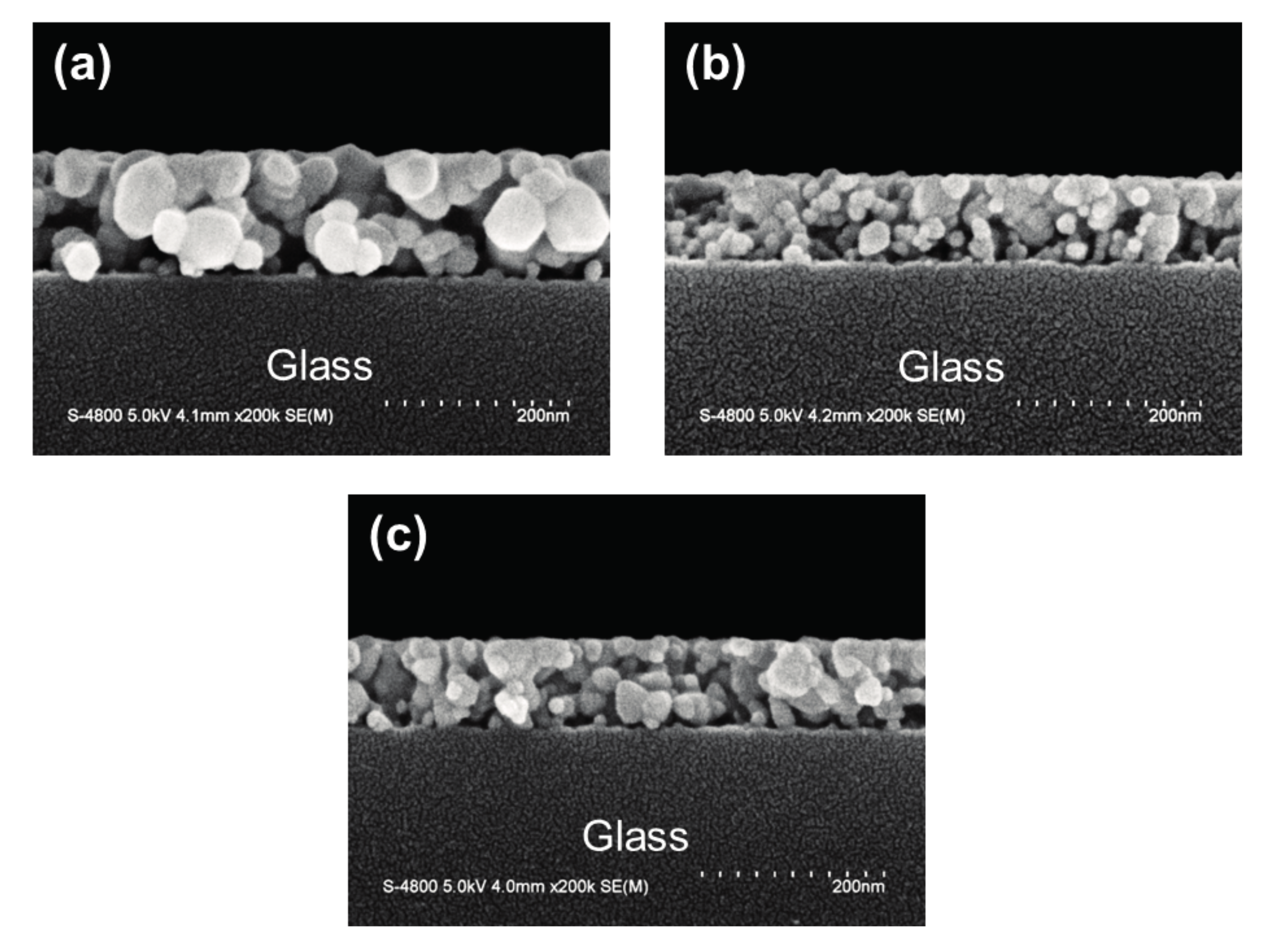
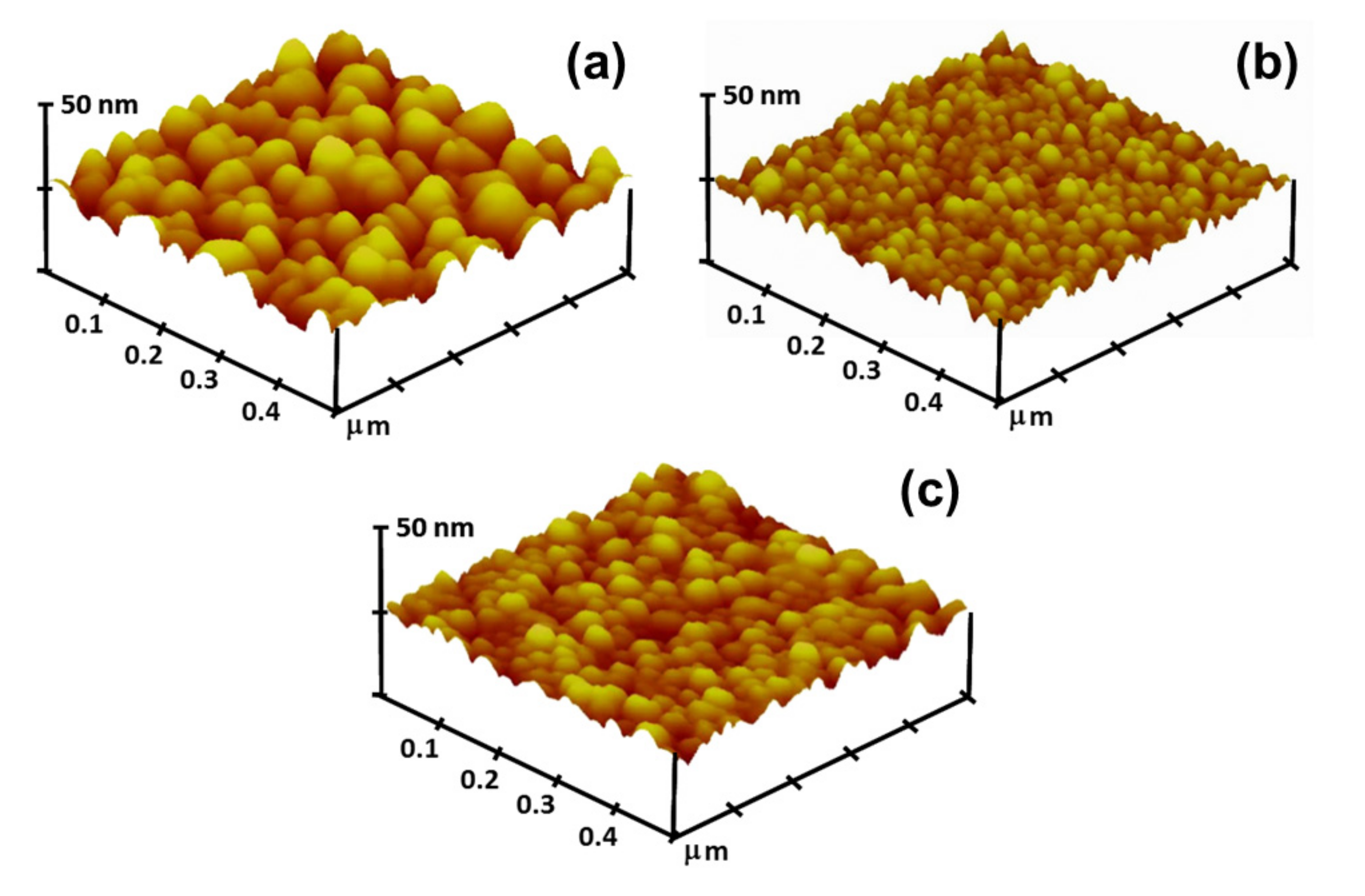
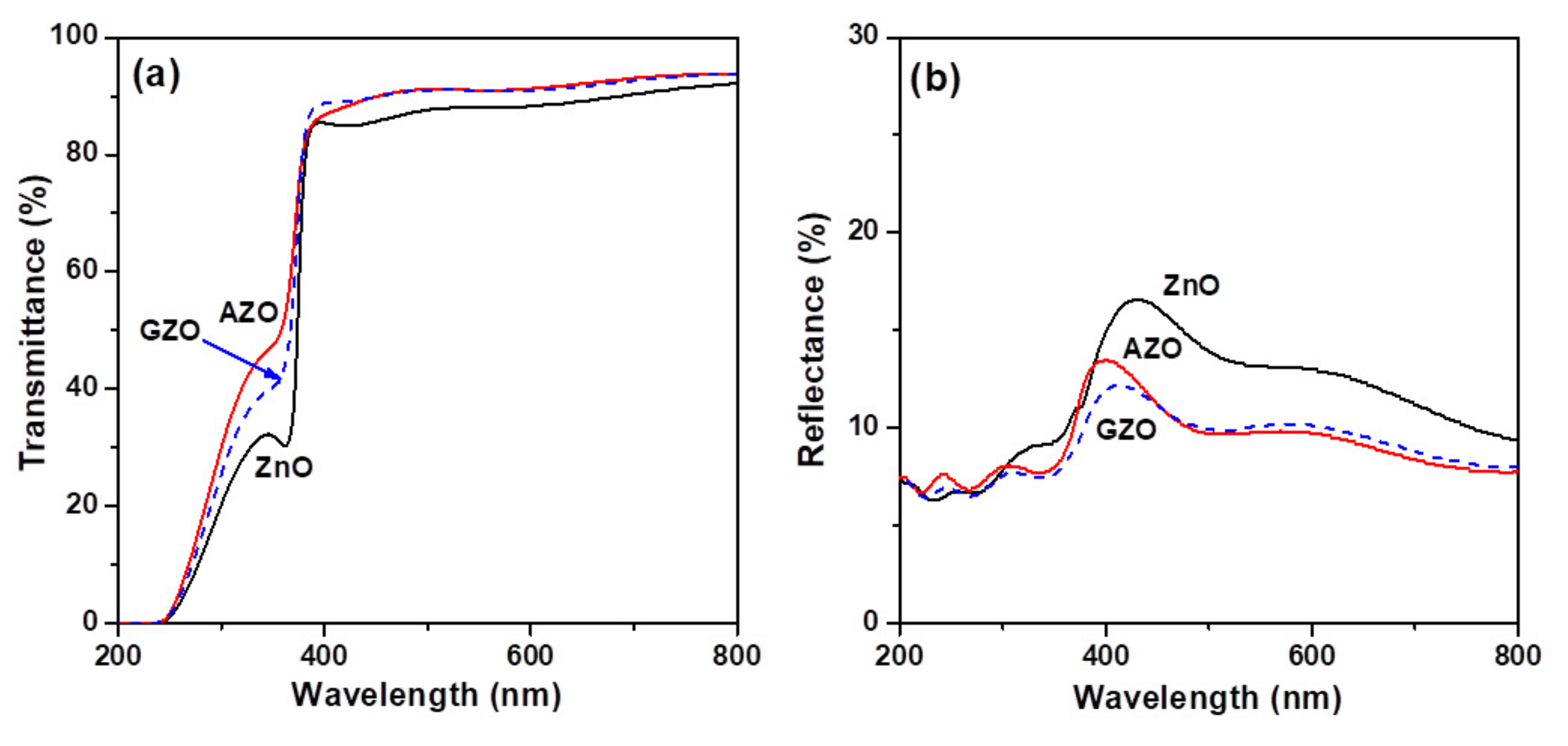
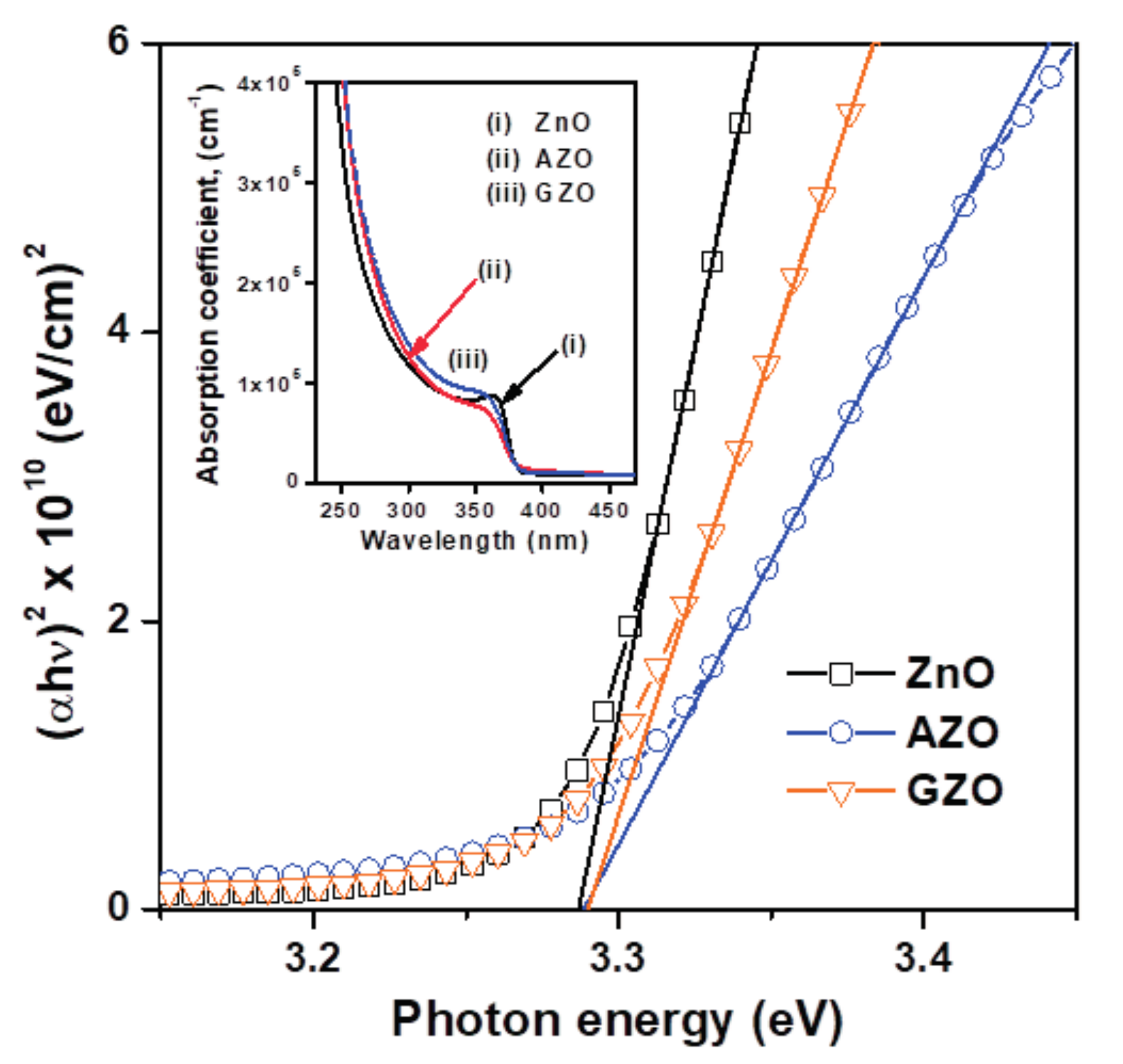
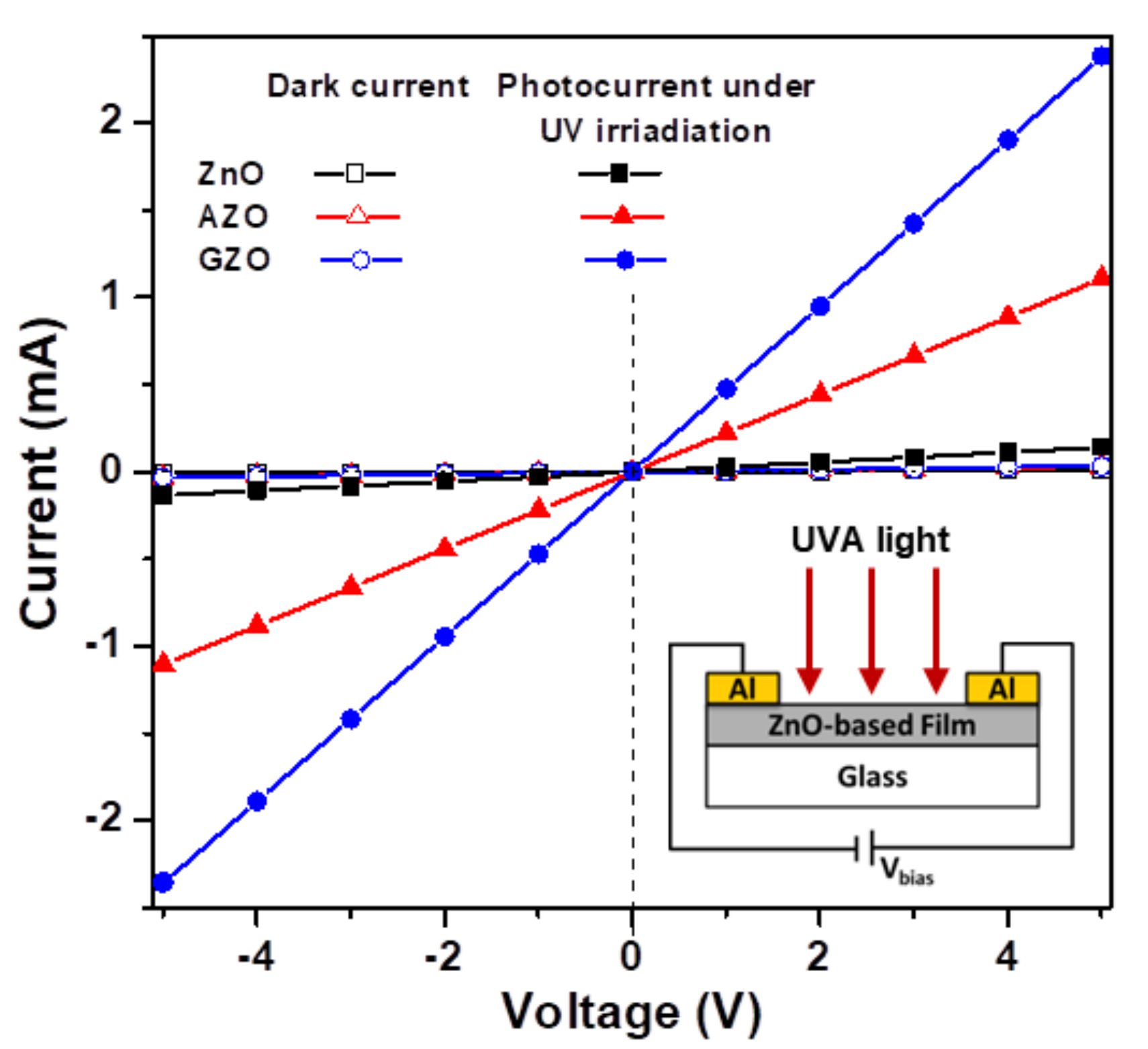
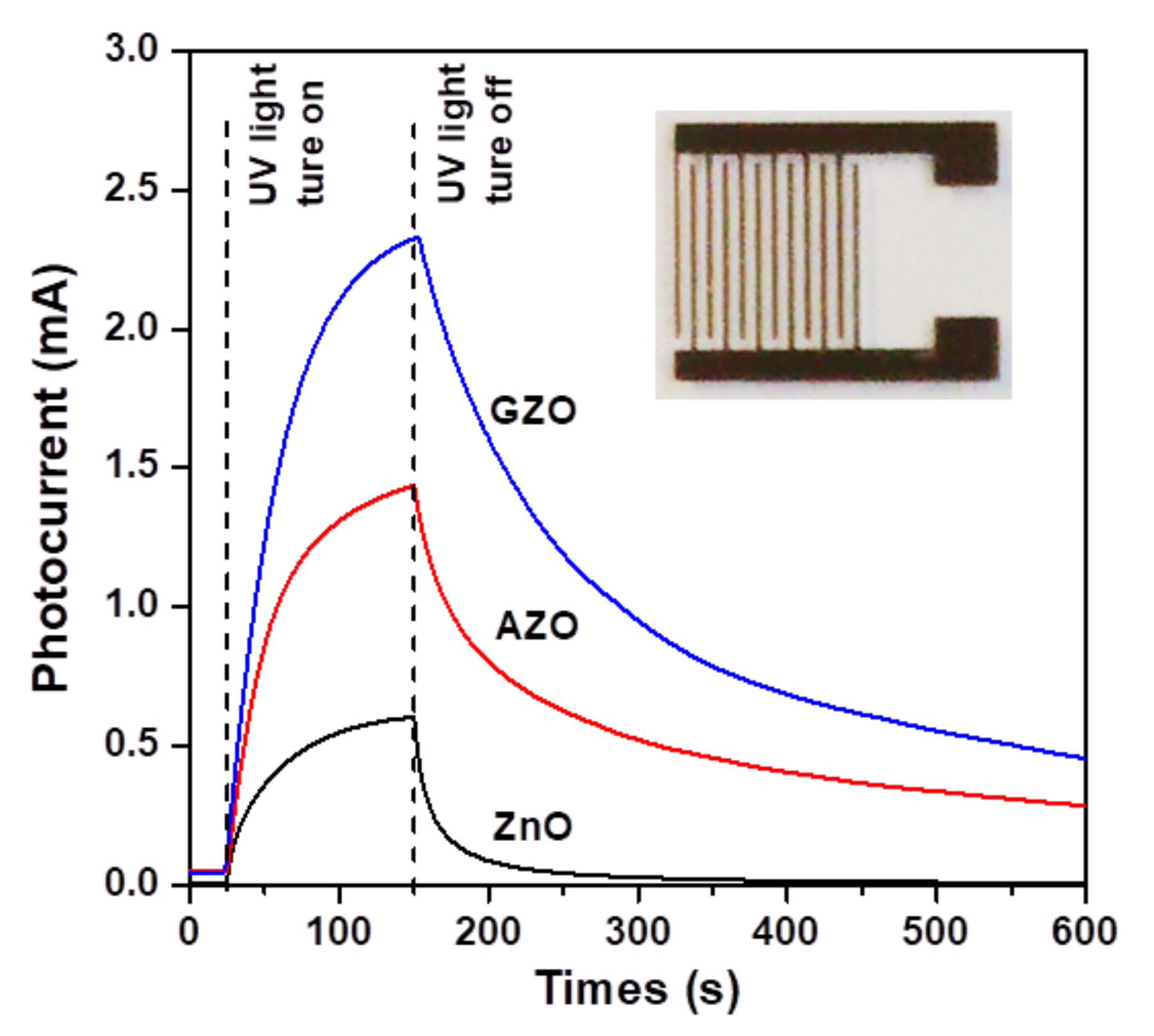
| Thin Film Sample | Average Crystallite Size (nm) | Root Mean Square (RMS) Roughness (nm) | Average Transmittance (%) a | Average Reflectance (%) b | Mean Electron Concentration (cm−3) | Mean Hall Mobility (cm2/Vs) | Mean Resistivity (Ω-cm) |
|---|---|---|---|---|---|---|---|
| ZnO | 26.1 | 5.13 | 88.6 | 12.8 | 1.84 × 1014 | 4.76 | 7.71 × 103 |
| AZO | 16.2 | 2.86 | 91.6 | 9.55 | 2.52 × 1015 | 6.09 | 4.14 × 102 |
| GZO | 22.5 | 3.08 | 91.4 | 9.75 | 5.09 × 1015 | 11.0 | 1.22 × 102 |
| Sensing Layer | UV Response (IUVA/Idark) | Photoconductivity Gain (Ion/Ioff) | Sensitivity (%) | Photocutrrent Responsivity (A/W) |
|---|---|---|---|---|
| ZnO | 15.3 | 67.0 | 66.0 | 12.0 |
| AZO | 35.5 | 28.7 | 27.7 | 28.0 |
| GZO | 73.3 | 51.8 | 50.8 | 46.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsay, C.-Y.; Hsu, W.-T. Comparative Studies on Ultraviolet-Light-Derived Photoresponse Properties of ZnO, AZO, and GZO Transparent Semiconductor Thin Films. Materials 2017, 10, 1379. https://doi.org/10.3390/ma10121379
Tsay C-Y, Hsu W-T. Comparative Studies on Ultraviolet-Light-Derived Photoresponse Properties of ZnO, AZO, and GZO Transparent Semiconductor Thin Films. Materials. 2017; 10(12):1379. https://doi.org/10.3390/ma10121379
Chicago/Turabian StyleTsay, Chien-Yie, and Wei-Tse Hsu. 2017. "Comparative Studies on Ultraviolet-Light-Derived Photoresponse Properties of ZnO, AZO, and GZO Transparent Semiconductor Thin Films" Materials 10, no. 12: 1379. https://doi.org/10.3390/ma10121379





