Co-Precipitation, Strength and Electrical Resistivity of Cu–26 wt % Ag–0.1 wt % Fe Alloy
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
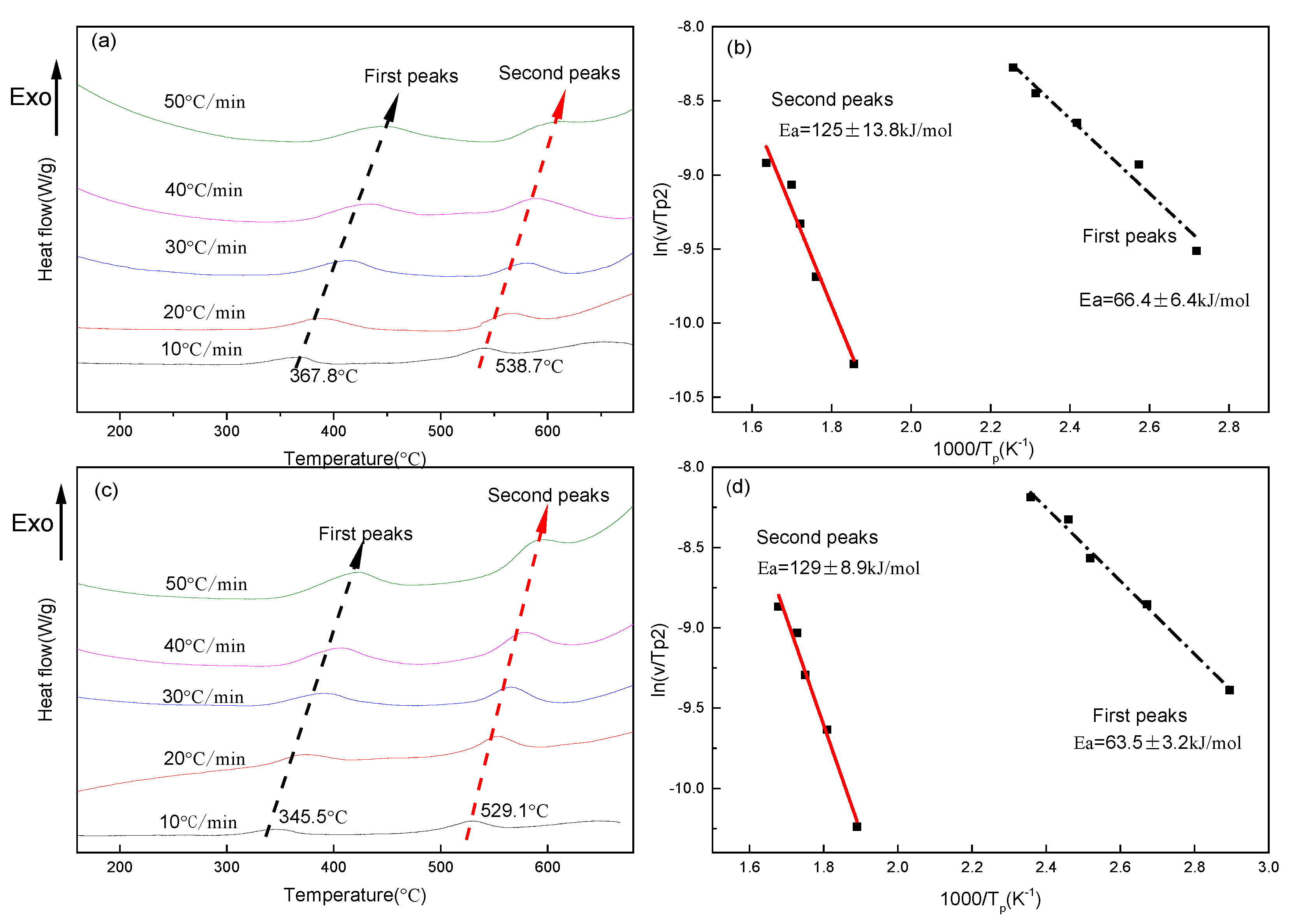
3.1. Precipitation Kinetics of Ag and Cu
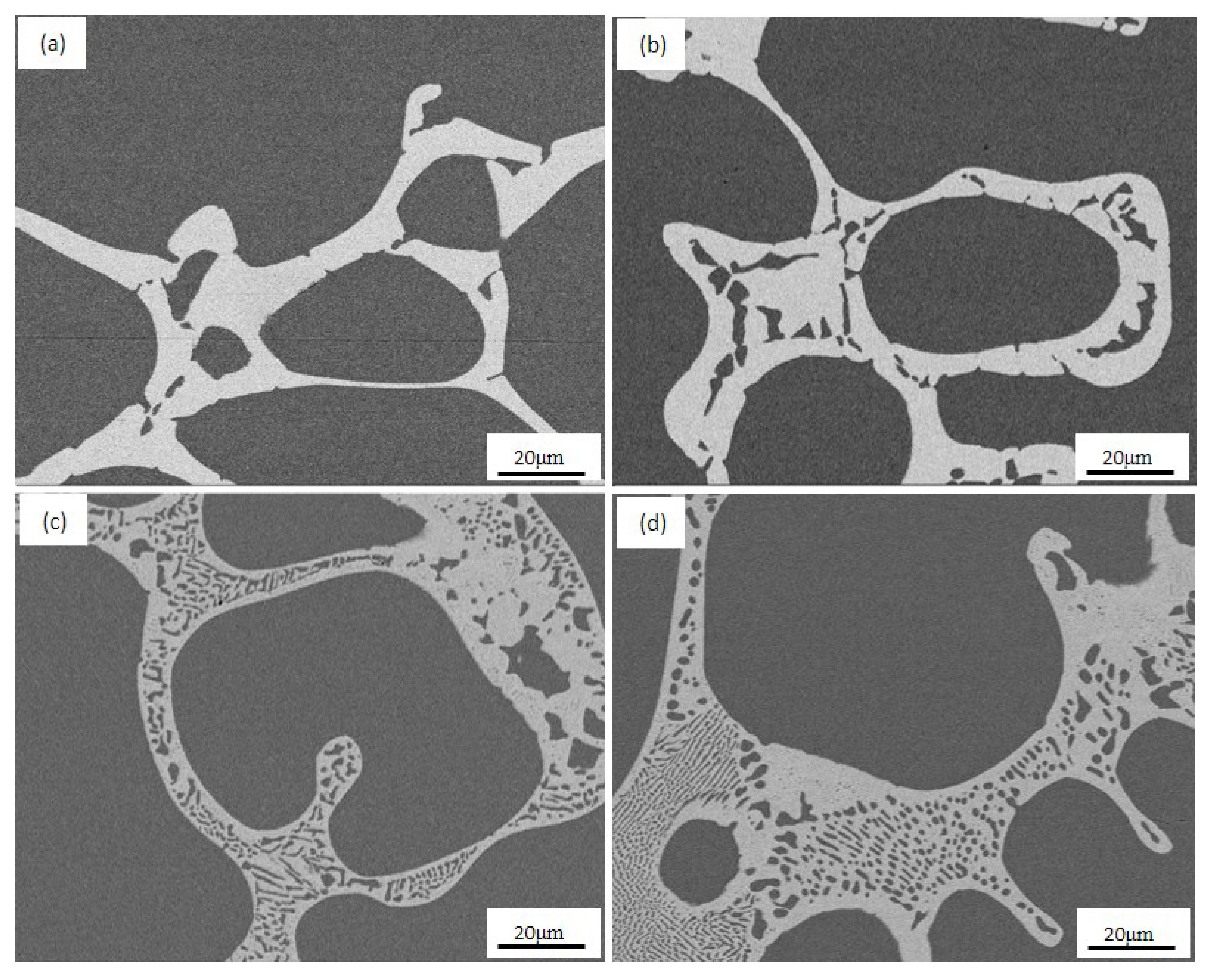
3.2. Morphology of Alloys
3.2.1. Morphology of Eutectic Colonies
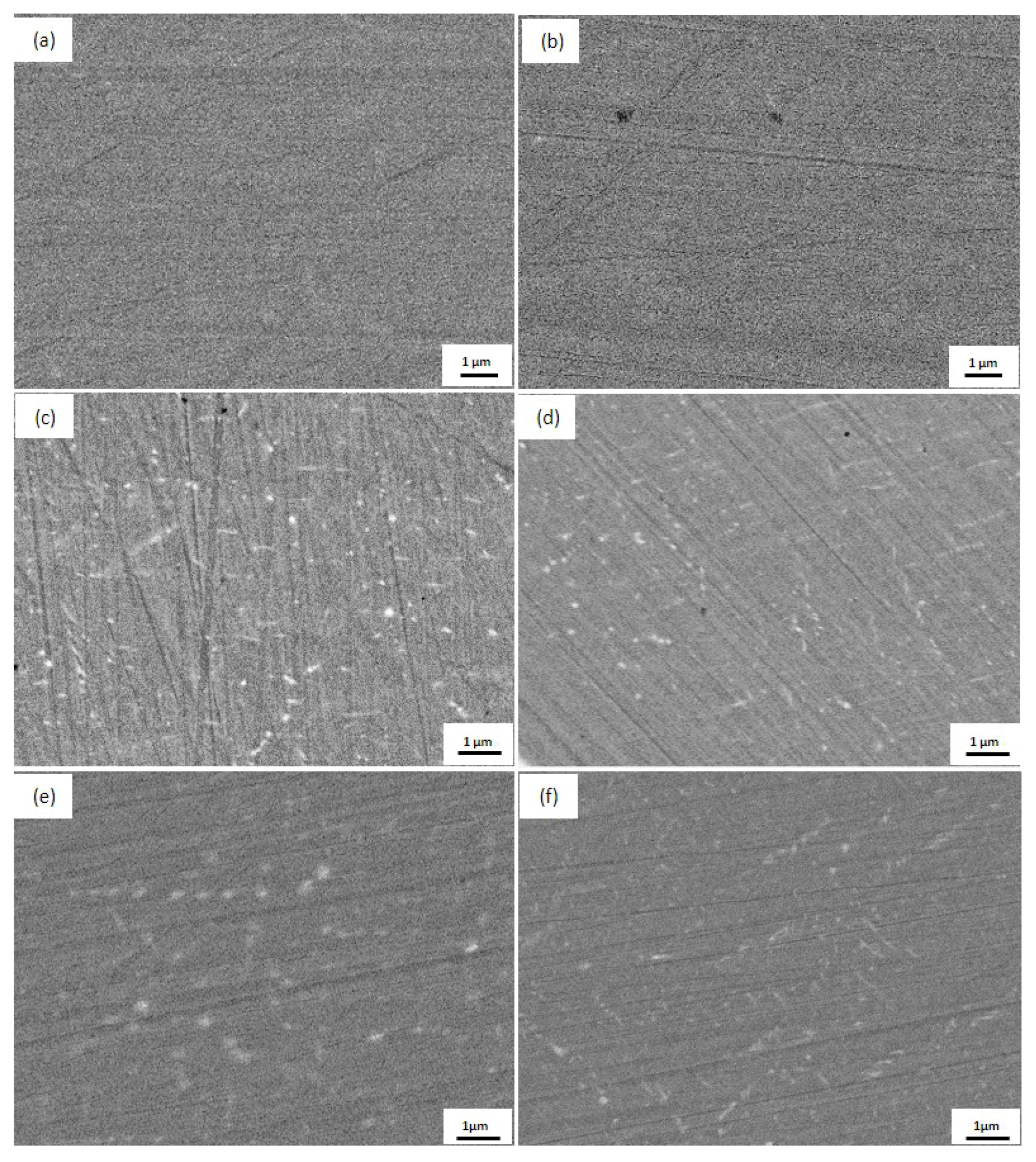
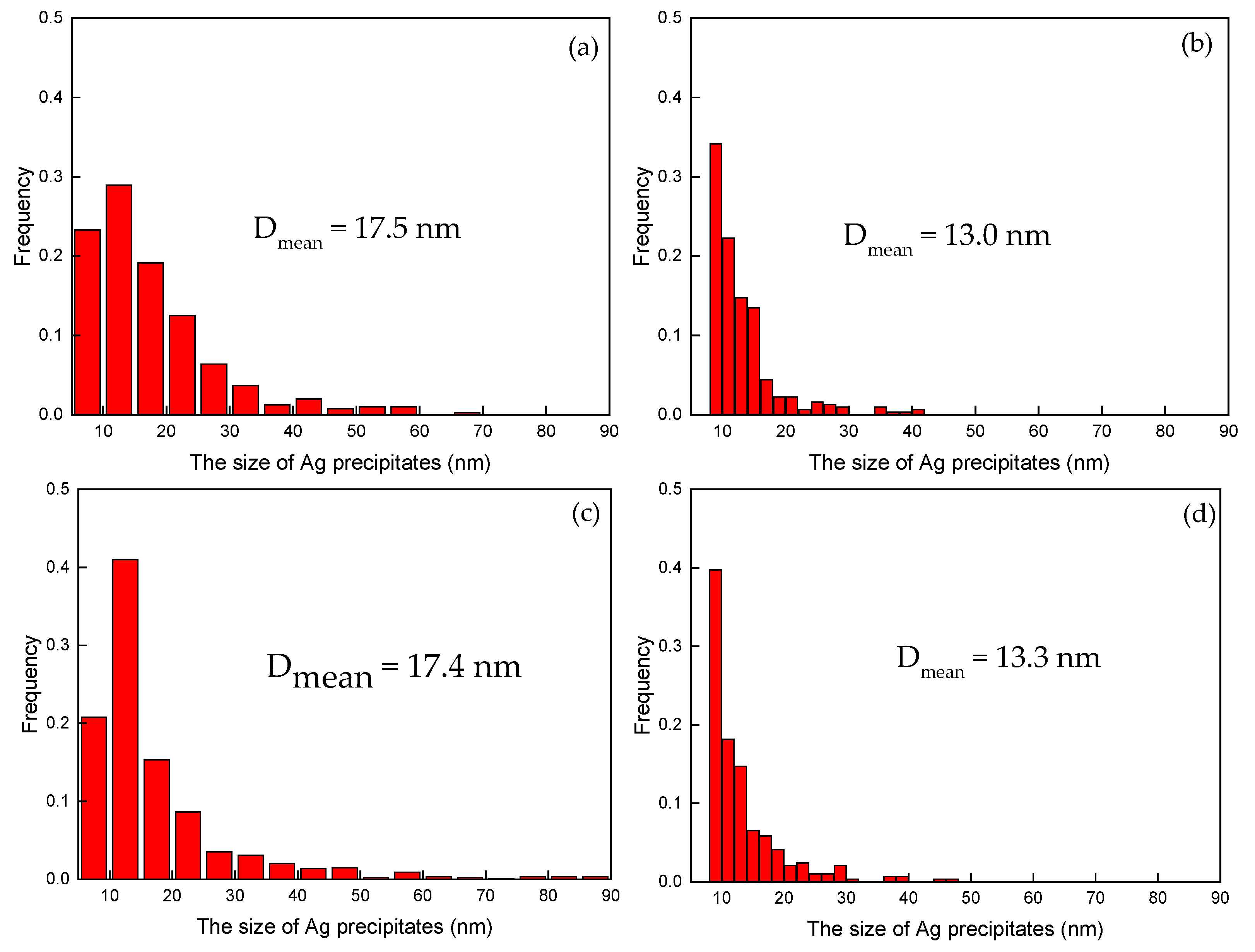
3.2.2. Morphology of Ag Precipitates in the Cu Matrix
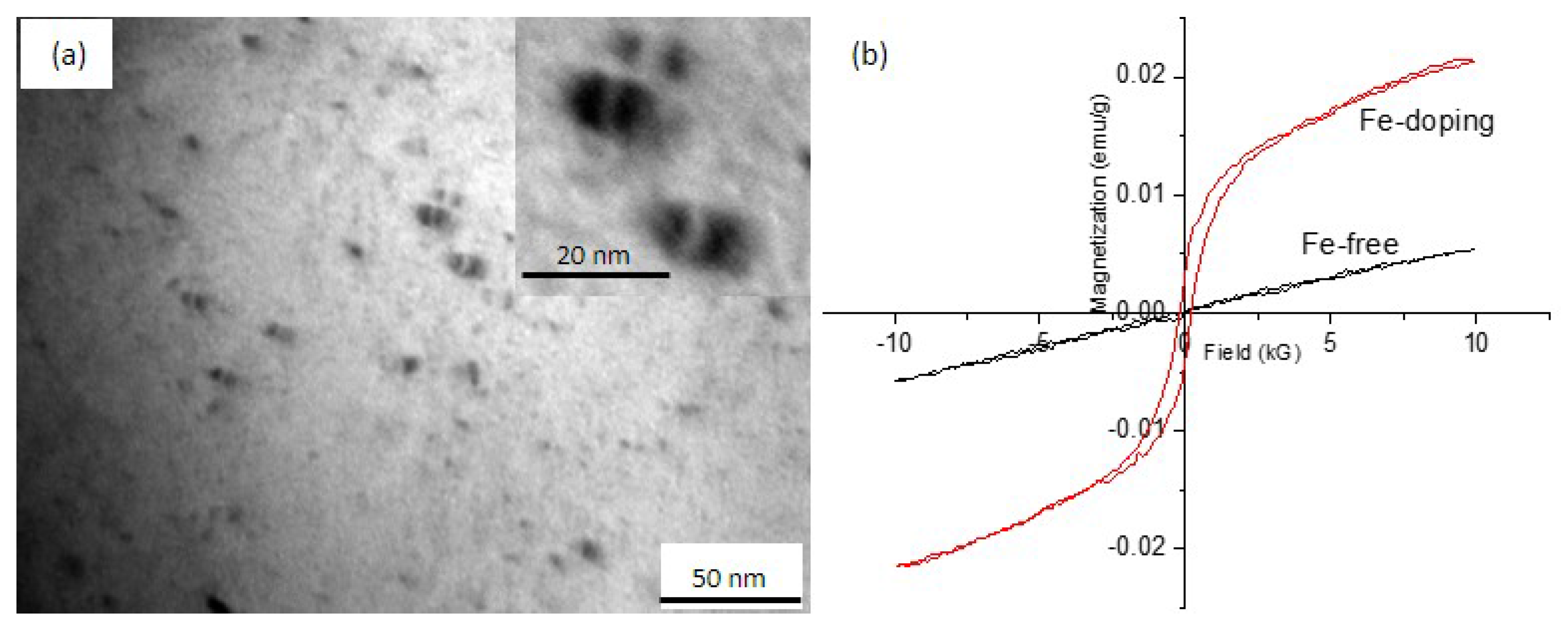
3.2.3. Morphology of Fe Precipitates in the Cu Matrix
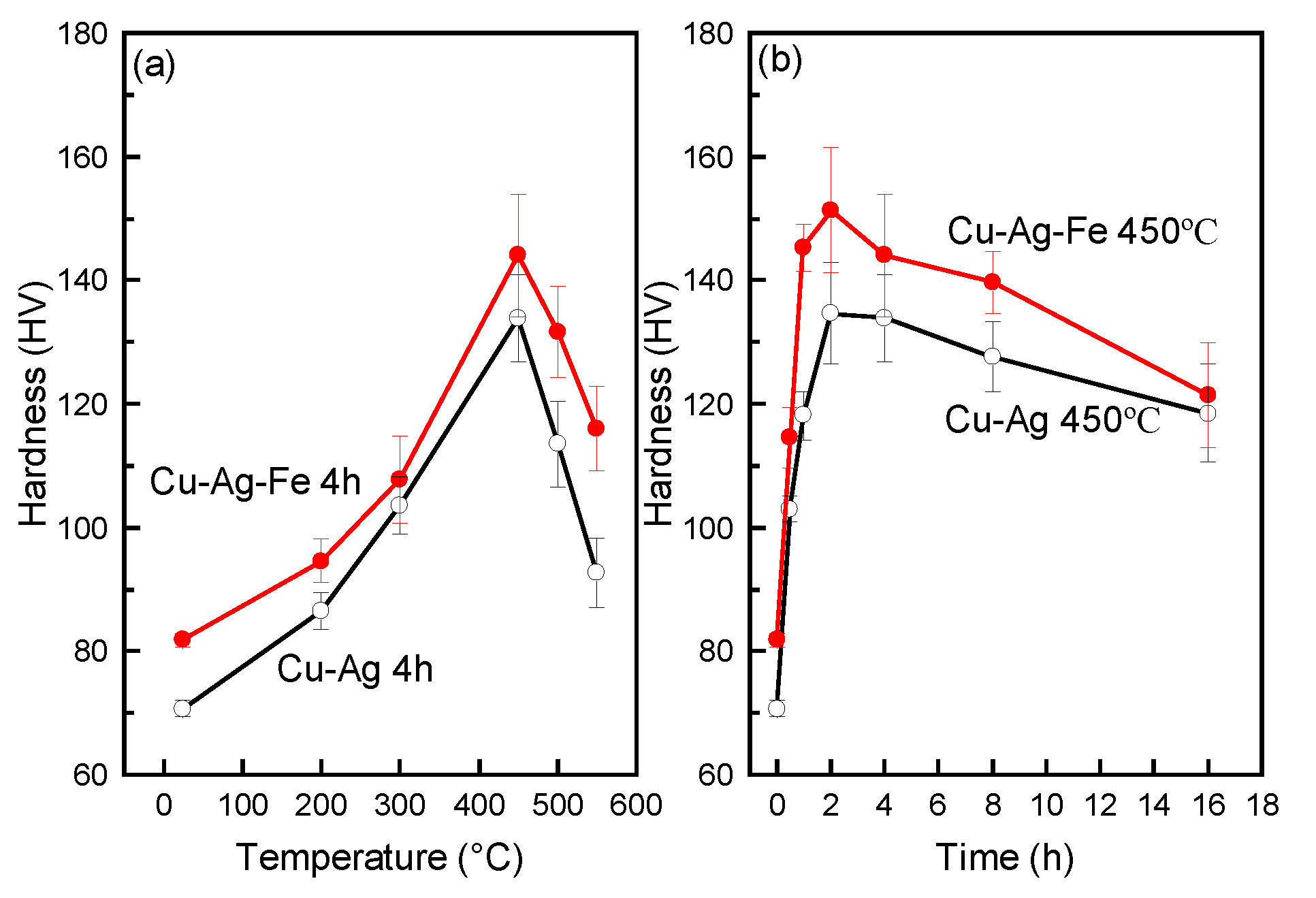
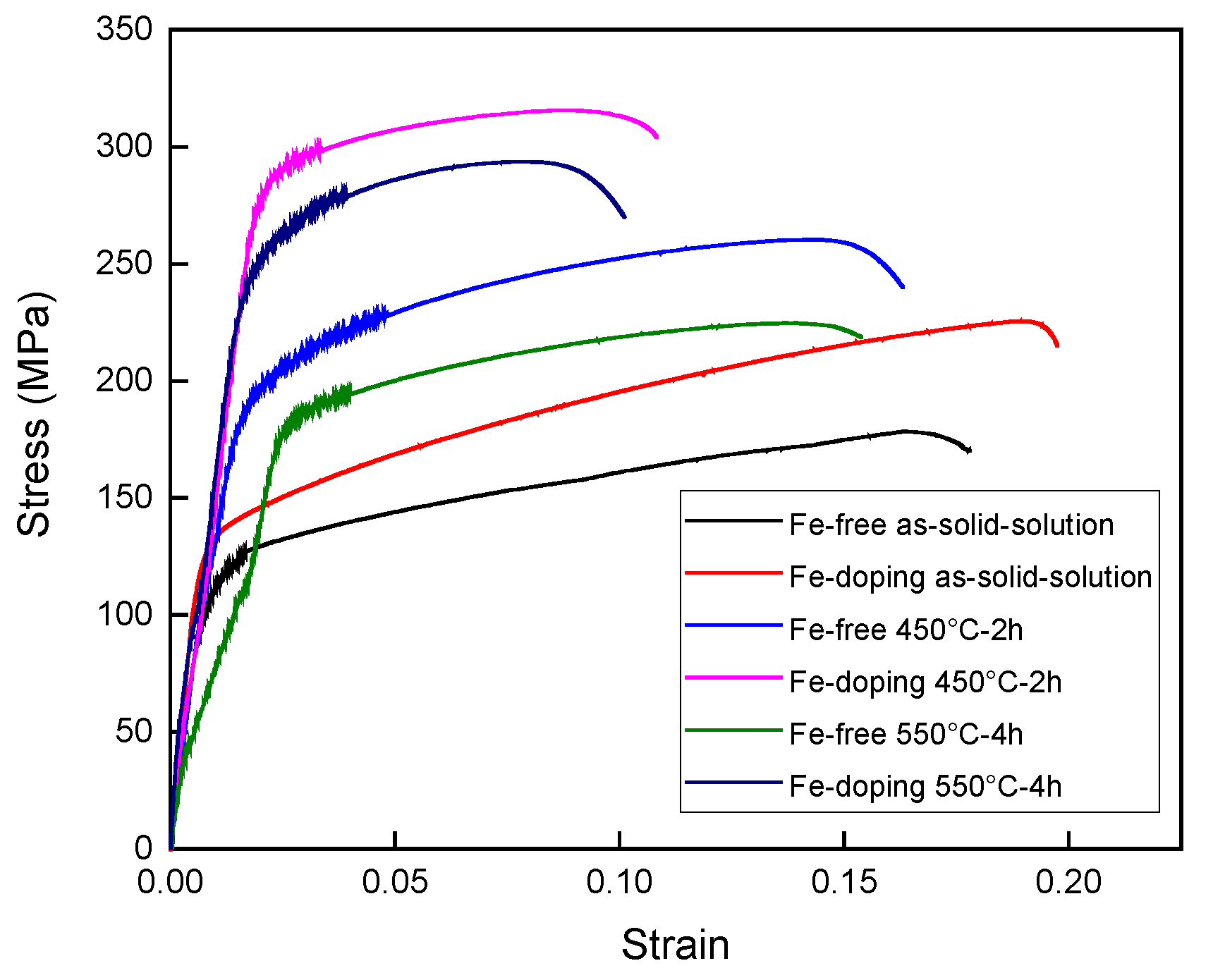
3.3. Hardness and Tensile Strength of Alloys
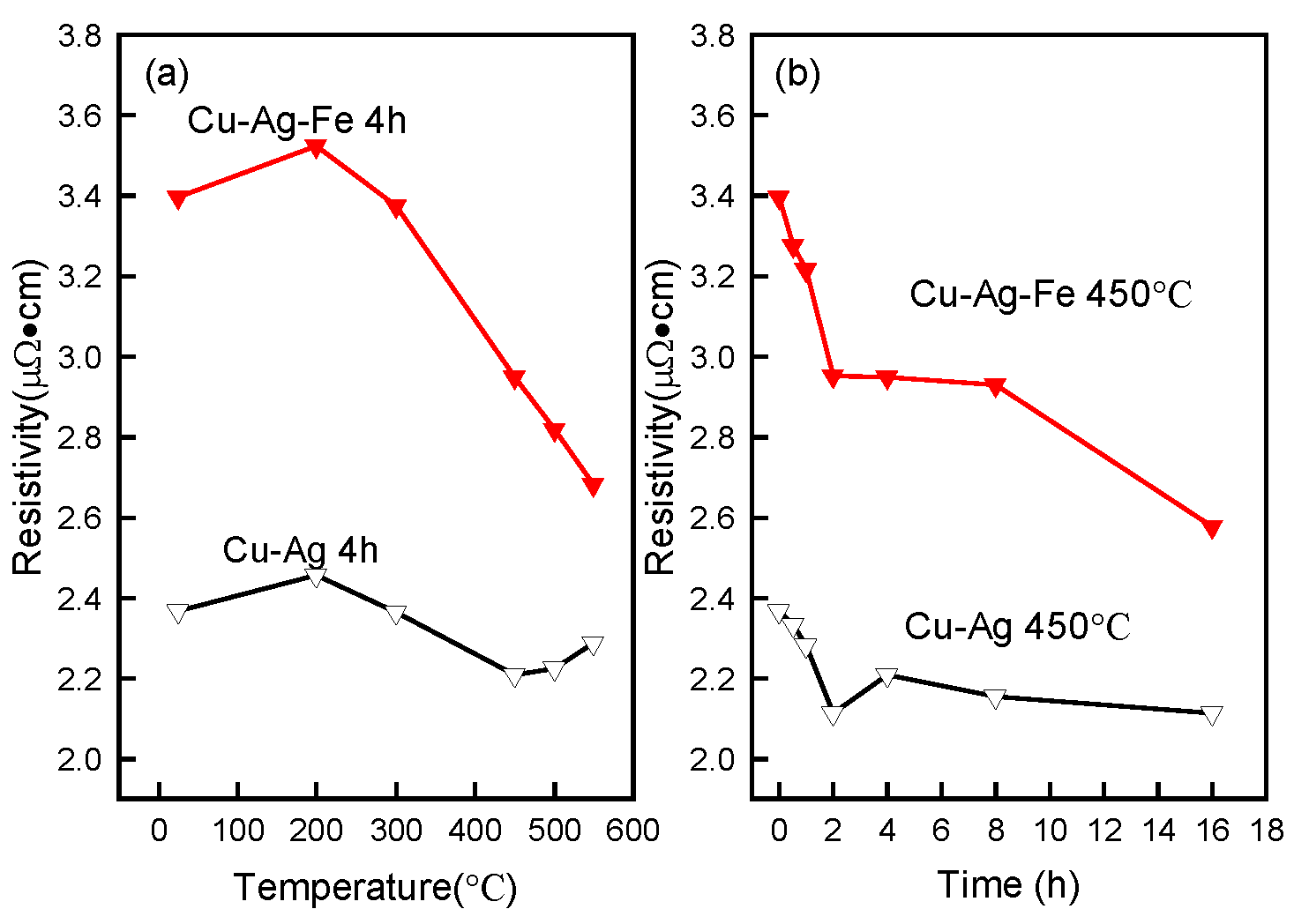
3.4. Electrical Resistivity
4. Discussion
4.1. The Effect of Fe-Doping on the Precipitation of Ag Precipitates
4.2. The Effect of Fe-Doping on Strength
4.3. The Effect of Fe-Doping on Electrical Resistivity
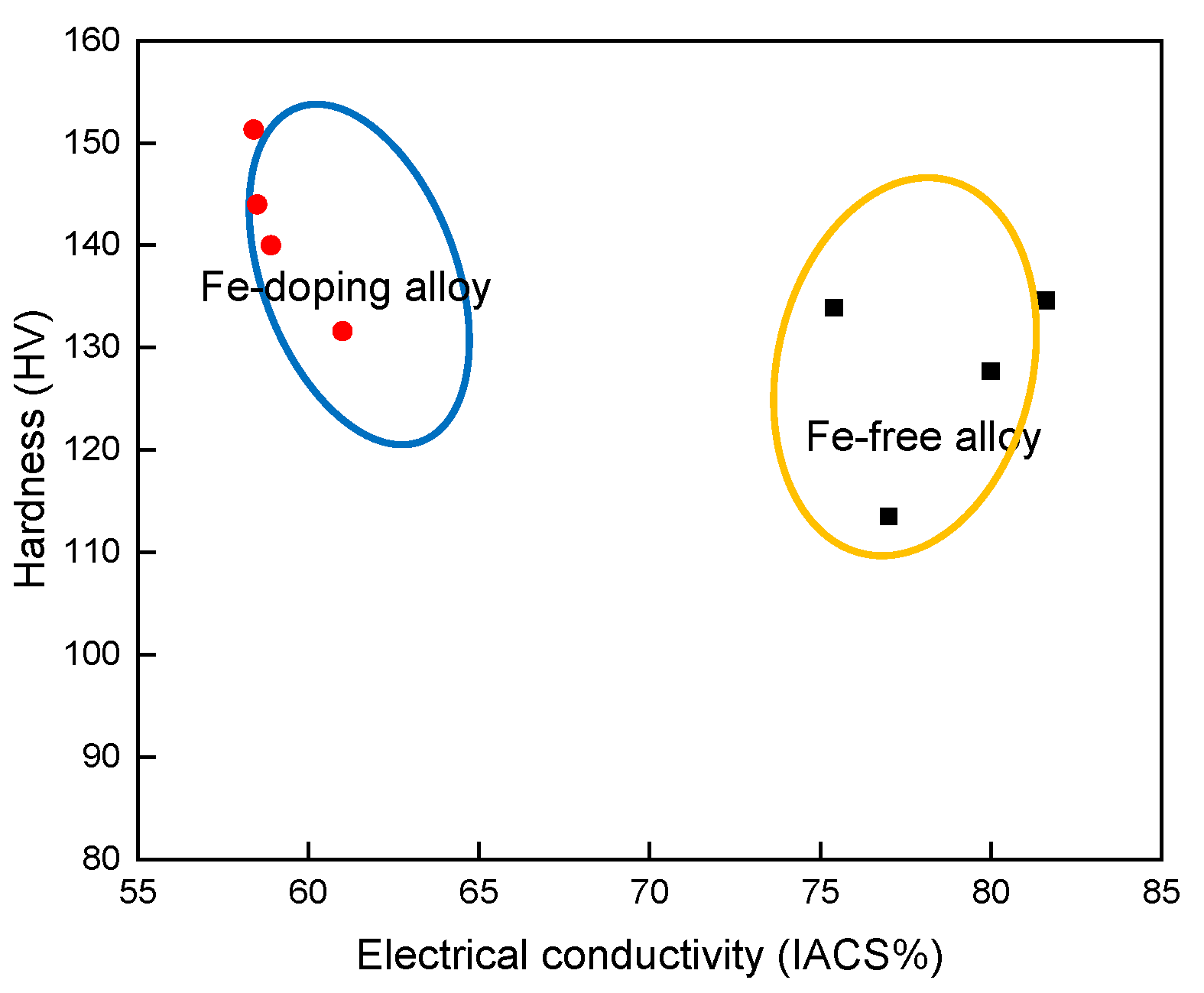
4.4. The Diagram between Electrical Conductivity and Strength
5. Conclusions
- (1)
- In a Cu–26 wt % Ag alloy, the activation energy of Ag precipitated out of the Cu matrix was 66.4 ± 6.4 kJ/mol, and the activation energy of Cu precipitated out of the Ag matrix was 125 ± 13.8 kJ/mol. With respect to the Cu–26 wt % Ag–0.1 wt % Fe alloy, these two energies were 63.5 ± 3.2 kJ/mol and 129 ± 8.9 kJ/mol, respectively. Fe-doping had a negligible influence on the activation energies of Ag and Cu precipitates.
- (2)
- The continuous rod-shaped Ag precipitates precipitated out of the Cu matrix of both the Fe-free alloy as well as the Fe-doping alloy after ageing treatments. The Fe addition decreased the size and the spacing of the continuous rod-shaped Ag precipitates, because the elastic strain field caused by the presence of the overlapped Fe diffusion field inhibited the nucleation of Ag precipitates.
- (3)
- The electrical resistivity of the Fe-free alloy slightly increased after ageing at 550 °C, due to the dissolution of Ag into the Cu matrix. Fe-doping alloy aged at 550 °C-4 h indicated that γ-Fe did precipitate from the Cu matrix, and that the solubility of Fe in Cu was about 0.09%. Thus, the electrical resistivity of Fe-doping alloy decreased after ageing.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Embury, J.D.; Han, K. Conductor materials for high field magnets. Curr. Opin. Solid State Mater. Sci. 1998, 3, 304–308. [Google Scholar] [CrossRef]
- Sakai, Y.; Inoue, K.; Maeda, H. New High-Strength, High-Conductivity Cu-Ag Alloy Sheets. Acta Metall. Mater. 1995, 43, 1517–1522. [Google Scholar] [CrossRef]
- Hong, S.I.; Hill, M.A. Mechanical stability and electrical conductivity of Cu-Ag filamentary microcomposites. Mater. Sci. Eng. A 1999, 264, 151–158. [Google Scholar] [CrossRef]
- Verhoeven, J.; Downing, H.; Chumbley, L.S.; Gibson, E. The resistivity and microstructure of heavily drawn Cu-Nb alloys. J. Appl. Phys. 1989, 65, 1293–1301. [Google Scholar] [CrossRef]
- Biselli, C.; Morris, D.G. Microstructure and strength of Cu-Fe in situ composites after very high drawing strains. Acta Mater. 1996, 44, 493–504. [Google Scholar] [CrossRef]
- Islamgaliev, R.K.; Nesterov, K.M.; Valiev, R.Z. Structure, strength, and electric conductivity of a Cu-Cr copper-based alloy subjected to severe plastic deformation. Phys. Met. Metallogr. 2015, 116, 209–218. [Google Scholar] [CrossRef]
- Sakai, Y.; Inoue, K.; Maeda, H. High-strength and high-conductivity Cu-Ag alloy sheets: New promising conductor for high-fieId Bitter coils. IEEE Trans. Magn. 1994, 30, 2114–2117. [Google Scholar] [CrossRef]
- Zuo, X.W.; Zhao, C.C.; Niu, R.M.; Wang, E.G.; Han, K. Microstructural dependence of magnetoresistance in CuAg alloy solidified with high magnetic field. J. Mater. Process. Technol. 2015, 224, 208–212. [Google Scholar] [CrossRef]
- Benghalem, A.; Morris, D.G. Microstructure and strength of wire-drawn Cu-Ag filamentary composites. Acta Mater. 1997, 45, 397–406. [Google Scholar] [CrossRef]
- Han, K.; Vasquez, A.A.; Xin, Y.; Kalu, P.N. Microstructure and tensile properties of nanostructured Cu-25 wt % Ag. Acta Mater. 2003, 51, 767–780. [Google Scholar] [CrossRef]
- Liu, J.B.; Meng, L.; Zeng, Y.W. Microstructure evolution and properties of Cu-Ag microcomposites with different Ag content. Mater. Sci. Eng. A 2006, 435, 237–244. [Google Scholar] [CrossRef]
- Zuo, X.W.; Han, K.; Zhao, C.C.; Niu, R.M.; Wang, E.G. Microstructure and properties of nanostructured Cu-28 wt % Ag microcomposite deformed after solidifying under a high magnetic field. Mater. Sci. Eng. A 2014, 619, 319–327. [Google Scholar] [CrossRef]
- Zhao, C.; Zuo, X.; Wang, E.; Niu, R.; Han, K. Simultaneously increasing strength and electrical conductivity in nanostructured Cu-Ag composite. Mater. Sci. Eng. A 2016, 652, 296–304. [Google Scholar] [CrossRef]
- Zuo, X.W.; Han, K.; Zhao, C.C.; Niu, R.M.; Wang, E.G. Precipitation and dissolution of Ag in ageing hypoeutectic alloys. J. Alloys Compd. 2015, 622, 69–72. [Google Scholar] [CrossRef]
- Zhao, C.C.; Zuo, X.W.; Wang, E.G.; Han, K. Strength of Cu-28 wt % Ag Composite Solidified Under High Magnetic Field Followed by Cold Drawing. Met. Mater. Int. 2017, 23, 369–377. [Google Scholar] [CrossRef]
- Zuo, X.W.; Guo, R.; Zhao, C.C.; Zhang, L.; Wang, E.G.; Han, K. Microstructure and properties of Cu-6 wt % Ag composite thermomechanical-processed after directionally solidifying with magnetic field. J. Alloys Compd. 2016, 676, 46–53. [Google Scholar] [CrossRef]
- Nestorovic, S.; Markovic, I.; Markovic, D. Influence of thermomechanical treatment on the hardening mechanisms and structural changes of a cast Cu-6.6 wt % Ag alloy. Mater. Des. 2010, 31, 1644–1649. [Google Scholar] [CrossRef]
- Piyawit, W.; Xu, W.Z.; Mathaudhu, S.N.; Freudenberger, J.; Rigsbee, J.M.; Zhu, Y.T. Nucleation and growth mechanism of Ag precipitates in a CuAgZr alloy. Mater. Sci. Eng. A 2014, 610, 85–90. [Google Scholar] [CrossRef]
- Gaganov, A.; Freudenberger, J.; Botcharova, E.; Schultz, L. Effect of Zr additions on the microstructure, and the mechanical and electrical properties of Cu-7 wt % Ag alloys. Mater. Sci. Eng. A 2006, 437, 313–322. [Google Scholar] [CrossRef]
- Raabe, D.; Hangen, U. Correlation of microstructure and type II superconductivity of a heavily cold rolled Cu-20 mass % Nb in situ composite. Acta Mater. 1996, 44, 953–961. [Google Scholar] [CrossRef]
- Zuo, X.W.; Qu, L.; Zhao, C.C.; An, B.L.; Wang, E.G.; Niu, R.M.; Xin, Y.; Lu, J.; Han, K. Nucleation and growth of gamma-Fe precipitate in Cu-2% Fe alloy aged under high magnetic field. J. Alloys Compd. 2016, 662, 355–360. [Google Scholar] [CrossRef]
- Wu, Z.W.; Chen, Y.; Meng, L. Microstructure and properties of Cu-Fe microcomposites with prior homogenizing treatments. J. Alloys Compd. 2009, 481, 236–240. [Google Scholar] [CrossRef]
- Wu, Z.W.; Meng, L. Influences of different prior heat treatments on the microstructural and mechanical properties of Cu-Fe filamentary composites. J. Alloys Compd. 2011, 509, 8917–8921. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Handbook Volume 3: Alloy Phase Diagrams, 10th ed.; ASM International: Almere, The Netherlands, 1992; p. 734. [Google Scholar]
- Easterling, K.E.; Weatherly, G.C. On the nucleation of martensite in iron precipitates. Acta Metall. 1969, 17, 845–852. [Google Scholar] [CrossRef]
- Saji, S.; Hori, S.; Mima, G. Ageing Characteristics of Copper-Iron Alloys. Mater. Trans. JIM 1973, 14, 82–88. [Google Scholar] [CrossRef]
- Nakagawa, Y. Liquid immiscibility in copper-iron and copper-cobalt systems in the supercooled state. Acta Metall. 1958, 6, 704–711. [Google Scholar] [CrossRef]
- Verhoeven, J.; Chueh, S.; Gibson, E. Strength and conductivity ofin situ Cu-Fe alloys. J. Mater. Sci. 1989, 24, 1748–1752. [Google Scholar] [CrossRef]
- Go, Y.; Spitzig, W. Strengthening in deformation-processed Cu-20% Fe composites. J. Mater. Sci. 1991, 26, 163–171. [Google Scholar] [CrossRef]
- Smith, D.R.; Fickett, F. Low-temperature properties of silver. J. Res. Natl. Inst. Stand. Technol. 1995, 100, 119. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, H.Y.; Lu, Q.; Wang, J.; Sun, B.D. Effect of Ag addition on the as-cast microstructure of Cu-8 wt % Fe in situ composites. J. Alloys Compd. 2010, 508, 320–323. [Google Scholar] [CrossRef]
- Song, J.S.; Ahn, J.H.; Kim, H.S.; Hong, S.I. Comparison of microstructure and strength in wire-drawn and rolled Cu-9 Fe-1.2 Ag filamentary microcomposite. J. Mater. Sci. 2001, 36, 5881–5884. [Google Scholar] [CrossRef]
- Wang, Y.F.; Gao, H.Y.; Wang, J.; Han, Y.F.; Dai, Y.B.; Sun, B.D. First-principles calculations of Ag addition on the diffusion mechanisms of Cu-Fe alloys. Solid State Commun. 2014, 183, 60–63. [Google Scholar] [CrossRef]
- Liu, K.M.; Lu, D.P.; Zhou, H.T.; Chen, Z.B.; Atrens, A.; Lu, L. Influence of a high magnetic field on the microstructure and properties of a Cu-Fe-Ag in situ composite. Mater. Sci. Eng. A 2013, 584, 114–120. [Google Scholar] [CrossRef]
- Gao, H.Y.; Wang, J.; Shu, D.; Sun, B.D. Effect of Ag on the aging characteristics of Cu-Fe in situ composites. Scr. Mater. 2006, 54, 1931–1935. [Google Scholar] [CrossRef]
- Sun, B.D.; Gao, H.Y.; Wang, J.; Shu, D. Strength of deformation processed Cu-Fe-Ag in situ composites. Mater. Lett. 2007, 61, 1002–1006. [Google Scholar] [CrossRef]
- Song, J.S.; Hong, S.I.; Kim, H.S. Heavily drawn Cu-Fe-Ag and Cu-Fe-Cu microcomposites. J. Mater. Process. Technol. 2001, 113, 610–616. [Google Scholar] [CrossRef]
- Gayler, M.; Carrington, W. Metallographic study of the precipitation of copper from a silver-rich silver-copper alloy. J. Inst. Met. 1947, 73, 625–639. [Google Scholar]
- Li, R.; Zuo, X.W.; Wang, E.G. Microstructure, resistivity, and hardness of aged Ag-7 wt % Cu alloy. Acta Phys. Sin. 2017, 66, 311–321. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Hamana, D.; Hachouf, M.; Boumaza, L.; Biskri, Z.E.A. Precipitation Kinetics and Mechanism in Cu-7 wt % Ag Alloy. Mater. Sci. Appl. 2011, 2, 899–910. [Google Scholar] [CrossRef]
- Colombo, S.; Battaini, P.; Airoldi, G. Precipitation kinetics in Ag-7.5 wt %Cu alloy studied by isothermal DSC and electrical-resistance measurements. J. Alloys Compd. 2007, 437, 107–112. [Google Scholar] [CrossRef]
- Hamana, D.; Boumaza, L. Precipitation mechanism in Ag-8 wt %Cu alloy. J. Alloys Compd. 2009, 477, 217–223. [Google Scholar] [CrossRef]
- Bizjak, M.; Kosec, L.; Kosec, B.; Anzel, I. The characterization of phase transformations in rapidly solidified Al-Fe and Cu-Fe alloys through measurements of the electrical resistance and DSC. Metalurgija 2006, 45, 281–286. [Google Scholar]
- Watanabe, Y.; Murakami, J.-I.; Miura, H. Effect of annealing on saturation magnetization in deformed Cu-Fe alloys with transformed Fe particles. Mater. Sci. Eng. A 2002, 338, 299–304. [Google Scholar] [CrossRef]
- Nabarro, F.R.N. The Strains Produced by Precipitation in Alloys. Proc. R. Soc. A 1940, 175, 519–538. [Google Scholar] [CrossRef]
- Bacher, P.; Wynblatt, P.; Foiles, S.M. A Monte Carlo study of the structur and composition of (001) semicoherent interphase boundaries in Cu Ag Au alloys. Acta Metall. Mater. 1991, 39, 2681–2691. [Google Scholar] [CrossRef]
- Tomellini, M. Impact of soft impingement on the kinetics of diffusion-controlled growth of immiscible alloys. Comput. Mater. Sci. 2011, 50, 2371–2379. [Google Scholar] [CrossRef]
- Hao, C.; Zwaag, S.V.D. Modeling of soft impingement effect during solid-state partitioning phase transformations in binary alloys. J. Mater. Sci. 2011, 46, 1328–1336. [Google Scholar]
- Perez, M. Gibbs-Thomson effects in phase transformations. Scr. Mater. 2005, 52, 709–712. [Google Scholar] [CrossRef]
- Gubicza, J.; Hegedűs, Z.; Lábár, J.L.; Kauffmann, A.; Freudenberger, J.; Subramanya Sarma, V. Solute redistribution during annealing of a cold rolled Cu-Ag alloy. J. Alloys Compd. 2015, 623, 96–103. [Google Scholar] [CrossRef]
- Subramanian, P.; Perepezko, J. The Ag-Cu (silver-copper) system. J. Phase Equilib. 1993, 14, 62–75. [Google Scholar] [CrossRef]
- Kaptay, G. Nano-Calphad: extension of the Calphad method to systems with nano-phases and complexions. J. Mater. Sci. 2012, 47, 8320–8335. [Google Scholar] [CrossRef]
- Freudenberger, J.; Lyubimova, J.; Gaganov, A.; Witte, H.; Hickman, A.L.; Jones, H.; Nganbe, M. Non-destructive pulsed field CuAg-solenoids. Mater. Sci. Eng. A 2010, 527, 2004–2013. [Google Scholar] [CrossRef]
- Gottstein, G. Physikalische Grundlagen der Materialkunde, 3rd ed.; Springer Pulications Ltd.: Berlin, Germany, 2007; pp. 265–269. [Google Scholar]

| Alloy | Temperature | Mean Diameter d (nm) | Spacing λ (nm) | Measured Solute Ag Concentration in Cu, CM.Ag (at %, wt %) | Measured Volume Fraction of Ag Out of Cu, VM.f (%) |
|---|---|---|---|---|---|
| Fe-free | As-solid-solution | -- | -- | 3.94 (6.49) | 3.2 |
| 450 °C-4 h | 17.5 | 35.7 ± 1.2 | 3.0 (4.98) | 4.2 | |
| 500 °C-4 h | 17.4 | 15.8 ± 1.4 | 4.53 (7.46) | 2.7 | |
| 550 °C-4 h | 17.6 | 29.6 ± 1.4 | 1.16 (1.96) | 2.3 | |
| Fe-doping | As-solid-solution | -- | -- | 5.72 (9.45) | 1.4 |
| 450 °C-4 h | 13.0 | 18.2 ± 0.2 | 5.08 (8.32) | 2.7 | |
| 500 °C-4 h | 13.3 | 15.7 ± 1.4 | 4.83 (7.93) | 2.6 | |
| 550 °C-4 h | 8.2 | 17.5 ± 1.5 | 1.64 (2.76) | 2.2 |
| Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | ||
|---|---|---|---|---|
| Fe-free alloy | As-solid-solution | 180.4 | 113.6 | 13.5 |
| 450 °C-2 h | 260.3 | 201.1 | 15.4 | |
| 550 °C-4 h | 224.7 | 187.3 | 14.2 | |
| Fe-doping alloy | As-solid-solution | 225.4 | 126.1 | 13.7 |
| 450 °C-2 h | 315.8 | 284.2 | 14.2 | |
| 550 °C-4 h | 293.6 | 252.2 | 13.9 | |
| Fe-Free | Fe-Doping | |||
|---|---|---|---|---|
| Strength of the Cu matrix τCu matrix (MPa) | Solid solution hardening τss | Ag | 147.0 | 176.1 |
| Fe | -- | 52.1 | ||
| precipitation hardening τPre | Ag | 184 | 393 | |
| Fe | -- | 31.1 | ||
| Strength of eutectic τeut (MPa) | 9.3 | 9.7 | ||
| Total strength τtotal (MPa) | 340.3 | 662.0 | ||
| Measured strength τmeasured (MPa) | 224 | 293 | ||
| Phonon Scattering ρpho μΩ·cm | Dislocation Scattering ρdis μΩ·cm | Impurity Scattering ρimp μΩ·cm | Total Resistivity ρtotal μΩ·cm | Measured Resistivity ρmeasured μΩ·cm | ||
|---|---|---|---|---|---|---|
| Fe-free alloy | 1.64 | 0.000075 | Ag | 0.0305 | 1.671 | 2.2875 |
| Fe | -- | |||||
| Fe-doping alloy | 1.67 | 0.000075 | Ag | 0.043 | 2.588 | 2.6801 |
| Fe | 0.8748 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, E.; Zuo, X. Co-Precipitation, Strength and Electrical Resistivity of Cu–26 wt % Ag–0.1 wt % Fe Alloy. Materials 2017, 10, 1383. https://doi.org/10.3390/ma10121383
Li R, Wang E, Zuo X. Co-Precipitation, Strength and Electrical Resistivity of Cu–26 wt % Ag–0.1 wt % Fe Alloy. Materials. 2017; 10(12):1383. https://doi.org/10.3390/ma10121383
Chicago/Turabian StyleLi, Rui, Engang Wang, and Xiaowei Zuo. 2017. "Co-Precipitation, Strength and Electrical Resistivity of Cu–26 wt % Ag–0.1 wt % Fe Alloy" Materials 10, no. 12: 1383. https://doi.org/10.3390/ma10121383





